Abstract
Background
Immune checkpoint inhibitors (ICIs) have achieved promising effects in patients with non-small cell lung cancer (NSCLC). However, not all patients with NSCLC benefit from immunotherapy. There is an urgent need to explore biomarkers that could predict the survival outcomes and therapeutic efficacy in advanced NSCLC patients treated with immunotherapy. In this study, we aimed to assess the changes in peripheral blood lymphocyte subsets and their association with the therapeutic efficacy and clinical prognosis of advanced NSCLC patients treated with immunotherapy.
Methods
A total of 276 patients with advanced NSCLC were enrolled. Peripheral blood lymphocyte subsets including CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio, NK cells, Tregs and B cells were collected before any treatment, before immunotherapy or chemotherapy, and after 4 cycles of immunotherapy or chemotherapy. T-test was used to analyze the factors influencing lymphocyte subsets and their changes before and after therapy. Logistic regression was used to plot ROC curves and analyze the relationship between lymphocyte subsets and therapeutic efficacy. Log-rank test and Cox regression model were used to evaluate the relationship between lymphocyte subsets and progression-free survival (PFS).
Results
Gender, distant metastasis, and EGFR mutation status are known to affect the proportion of peripheral blood lymphocyte subsets in patients with advanced NSCLC. The proportions of CD4+ T cells, CD8+ T cells, Tregs and B cells were found to decrease after chemotherapy as compared to the baseline. The proportion of CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio, NK cells and Tregs were higher after immunotherapy than after chemotherapy. Compared to the baseline, the effective group showed significant increase in the proportions of CD4+ T cells, CD4+/CD8+ ratio, NK cells and Tregs, and the number of CD8+ T cells was significantly lower in the peripheral blood after 4 cycles of immunotherapy. On the contrary, the ineffective group did not show any significant differences in the above parameters. Baseline CD4+ T cells and NK cells were independent predictors of immunotherapy efficacy and PFS. Baseline Tregs were independent predictor of immunotherapy efficacy.
Conclusion
Immune checkpoint inhibitors induced changes in the proportion of peripheral blood lymphocyte subsets in patients that responded well to immunotherapy. The levels of the different lymphocyte subsets could serve as valuable predictive biomarkers of efficacy and clinical prognosis for NSCLC patients treated with immunotherapy.
Similar content being viewed by others
Introduction
Lung cancer is one of the most common malignancy and the leading cause of cancer death worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for about 80% of primary lung malignancies. Due to the availability of improved therapeutics, the two-year relative survival rate of NSCLC patients has increased from 30 to 36% between 2010 and 2016 [2]. The use of immune checkpoint inhibitors (ICIs) has achieved promising efficacy and has significantly improved the clinical outcomes for NSCLC patients [3]. However, not all NSCLC patients benefit from immunotherapy. Moreover, around 4–29% of advanced NSCLC patients treated with mono-immunotherapy, have been reported to experience hyper-progression [4].
There is an urgent need to explore predictive biomarkers of efficacy and survival outcomes after immunotherapy treatment in advanced NSCLC patients, which could enable early intervention of the ongoing treatment strategies. To date, the expression of programmed cell death ligand 1 (PD-L1) has demonstrated a significant predictive role [5]. However, other biomarkers such as tumor mutation burden (TMB) and lung immune prognostic index (LIPI) have yielded conflicting results [6]. Therefore, the development of a dynamic, non-invasive and convenient approach to monitor the efficacy of ICIs is the main focus of the current research.
The theory of cancer immunoediting, including immune elimination, homeostasis and escape, states that immunity closely relates to the occurrence and progression of cancer, and is the basis of the anti-cancer effects of ICIs [7]. Lymphocytes are essential components of the human immune system. Many of the current studies focus on the tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment, a heterogeneous population including two distinct pools of effector (CD4+ T cells, CD8+ T cells, NK cells) and suppressor (Tregs) phenotypes. These lymphocytes have been shown to be correlated with the efficacy of immunotherapy [8]. However, immunoediting occurs not only locally but also systemically to a large extent. Intense lymphocyte trafficking is observed in NSCLC patients and the phenotype of immune response at the organismal level is also observed in the blood [9]. Thus, we chose to study the peripheral blood lymphocyte subsets rather than the local immune status. Though numerous studies have shown that lung cancer patients have lower levels of CD4+ T cells, CD4+/CD8+ ratio, NK cell levels, and a higher regulatory T cells (Tregs) levels as compared to the healthy population [10, 11], the prognostic value of peripheral blood lymphocyte subsets in predicting immunotherapy efficacy in advanced NSCLC patients has not been demonstrated.
Our current work aims to analyze the impact of the clinicopathological characteristics on the peripheral blood lymphocyte subsets in advanced NSCLC patients in the real world. We sought to assess the dynamic changes in the levels of peripheral blood lymphocyte subsets before and after immunotherapy, and ultimately determine their correlation with immunotherapy efficacy and the clinical prognosis in advanced NSCLC patients.
Materials and methods
Study population
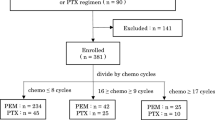
We included patients with stage IIIB or IV NSCLC who were treated with ICIs between September 2019 and July 2021 at the First Hospital of Shanxi Medical University. Pembrolizumab, nivolumab, camrelizumab and sintilimab were the different PD-1 inhibitors. The determination of the proportion of lymphocyte subsets is a routine test for patients with advanced lung cancer who require hospitalization for metronomic chemotherapy and immunotherapy, and is performed in almost all the patients on a voluntary basis as informed by the attending physician. By retrospectively reviewing the medical records of the patients, the data for peripheral blood lymphocyte subsets for different time-points, including, before any treatment, before immunotherapy or chemotherapy,and after 4 cycles of immunotherapy or chemotherapy were obtained.The inclusion criteria were as follows: (1) pathologically or cytologically diagnosed clinical stage IIIB or stage IV NSCLC; (2) Patients receiving first-line or non-first-line treatment with PD-1 inhibitors alone, or in combination with chemotherapy or anti-angiogenic drugs were included in the immunotherapy group, and patients receiving platinum-based chemotherapy with or without anti-angiogenic drugs were included in the chemotherapy group; (3) ECOG performance status between 0 and 2; and (4) availability of complete lymphocyte subsets and the follow-up data. Patients that met the following criteria were excluded: (1) suffered from other types of malignant tumors; (2) had acute infection, immunodeficiency and autoimmune disorders; and (3) received systemic corticosteroid treatment.
All the participants gave an informed consent. This study was approved by the Ethical Committee of The first Hospital of Shanxi Medical University (Ethics No. K125).
Data collection and response assessment
The clinicopathological data collected for the analysis included gender, the age at diagnosis, smoking history, pathological type, stage, distant metastasis at first treatment, the treatment regimen, and the number of treatment lines.
The data collected from the laboratory included Epidermal growth factor receptor (EGFR) gene mutation status, PD-L1 expression level, proportion of peripheral blood lymphocytes (CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio, NK cells, Tregs, and B cells) before any treatment, before immunotherapy or chemotherapy, and after 4 cycles of immunotherapy or chemotherapy. In patients that received the first-line of treatment, the proportions of peripheral blood lymphocytes before any treatment and before immunotherapy or chemotherapy were the same.
The clinical efficacy of immunotherapy was evaluated based on the computed tomography (CT) or magnetic resonance imaging (MRI) of the patient tumors. The short-term clinical efficacy according to the solid tumor response evaluation criteria (RECIST) included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients evaluated as CR, PR, and SD were classified as the effective group, and those evaluated as PD were classified as the ineffective group. The long-term efficacy was evaluated by the progression-free survival (PFS). PFS was the time from initiation of the treatment to the radiographic or clinical progression or death from any of the causes according to RECIST (RECIST-PFS).
PD-L1 immunohistochemistry assessment
Appropriate amount of tumor tissue was obtained by percutaneous lung biopsy, thoracoscopic biopsy and bronchoscopy biopsy. Sections were stained with anti-PD-L1 antibodies (PD-L1 IHC 22C3 pharmDx, Dako) on the automatic immunohistochemical staining apparatus (DAKO Link 48).The PD- L1 tumor proportion score (TPS), which is the percentage of tumor cells showing partial or complete membrane staining, was divided into PD-L1 < 50% and PD-L1 ≥ 50%. The detailed operational procedures are provided in Additional file 2.
Blood collection and flow cytometry
The proportion of the lymphocyte subsets in the peripheral blood were detected using BD FACSCanto II flow cytometry and flow antibodies. Three flow cytometry tubes were prepared for each peripheral blood specimen and labeled as Tube1, Tube2, and Tube3. CD45-PerCP-Cy5.5, CD3-FITC, CD4-APC, and CD8-PE were added to the tube1 to detect the proportion of CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+) in the peripheral blood lymphocytes. Tube 2 was added to CD16+56-PE, CD45-PerCP-Cy5.5, CD3-FITC, and CD19-AP were added to the tube 2 to detect the proportion of NK cells (CD45+CD3-CD16+CD56+) and B cells (CD45+CD3-CD19+) in the peripheral blood lymphocytes. CD45-PerCP-Cy5.5, CD4-FITC, CD25-APC, and CD127-PE were added to tube 3 to detect the proportion of Tregs (CD45+CD4+CD25HICD127LOW) in the peripheral blood lymphocytes. Finally, CD4+/CD8+ ratio was calculated by dividing the proportion of CD4+ T cells by the proportion of CD8+ T cells. According to the flow cytometry detection platform in our hospital, the normal reference ranges for the above parameters were defined as follows: CD4+ T cells (30–50%), CD8+ T cells (20–35%), CD4+/CD8+ ratio (1–2), NK cells (20–35%), Tregs (3–7%), and B cells (5.6–16%). The detailed operational procedures and related materials are provided in Additional file 1.
Statistical analysis
Quantitative data was expressed as mean ± standard deviation. T-test was adopted for comparison between groups, and paired t-tests were used to compare the before and after treatment groups. Qualitative data was expressed as the number of cases (%), and X2 test was also used for comparison between groups. In prognostic analysis, we used the median proportion of lymphocyte subsets as the threshold to define low and high levels. Prognostic multifactorial analysis was performed with logistic regression. The ROC curve was used to analyze the predictive value of T lymphocyte subsets as a biomarker for measuring the efficacy of immunotherapy NSCLC patients. Kaplan–Meier curve was drawn, and the Log Rank test was carried out to compare the differences in PFS between the different groups. Cox proportional hazard regression model was employed to analyze the risk of poor prognosis among different groups after other variables were calibrated. All statistical analyses were fulfilled with the support of SPSS software version 22.0. A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
Records of a total of 276 patients were included in this study. Of these, 153 were in the immunotherapy group and 123 in the chemotherapy group.The clinical and pathological characteristics are reported in Table 1. EGFR gene mutations were detected by next-generation sequencing (NGS) in 161 participants, of which 65 participants were EGFR-sensitive mutation positive. EGFR gene mutation participants received ICIs after EGFR tyrosine kinase inhibitor (EGFR-TKI) treatment. Immunohistochemistry was used to test the protein expression levels of PD-L1 in 130 participants, of which 44 had TPS ≥ 50%.
Factors influencing lymphocyte subsets
We collected lymphocyte subsets from patients with advanced NSCLC before any treatment and analyzed the impact of the clinicopathological features on the different lymphocyte subsets (Table 2). The proportion of CD4+ T cells (P = 0.027) was higher in females than in males. The proportion of NK cells was higher in males than in females (P = 0.007). Stage IV patients with distant metastasis had a higher proportion of Tregs (P = 0.015). Patients with EGFR mutations had lower CD8+ T cells proportion (P < 0.001) and higer CD4+/CD8+ ratio (P = 0.019). There was no effect of age, smoking, histology and PD-L1 expression on the lymphocyte subsets.
Changes in the proportion of lymphocyte subsets in different treatment groups
There were no statistical differences in the lymphocyte subsets between immunotherapy and chemotherapy groups before treatment. In the immunotherapy group, the proportions of CD4+ T cells (P < 0.001), CD4+/CD8+ ratio (P =0.012), NK cells (P = 0.002), and Tregs (P = 0.001) in the peripheral blood were significantly higher than baseline and the proportions of CD8+ T cells (P < 0.001) were significantly lower than the baseline. The proportions of CD4+ T cells (P < 0.001), CD8+ T cells (P < 0.001), Tregs (P < 0.001) and B cells (P = 0.002) in the peripheral blood were significantly lower in the chemotherapy group than in the baseline. CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio, NK cells and Tregs were higher in the immunotherapy group as compared to the chemotherapy group after treatment (Table 3).
Comparison of the lymphocyte subsets in patients from different efficacy groups
After 4 cycles of immunotherapy, out of 153 patients included in this study, 117 patients were classified as the effective group (CR 0, PR 48, SD 69) and 36 patients were classified as the ineffective group, with a treatment efficiency of 76.4%. The proportions of CD4+ T cells (P < 0.001), CD4+/CD8+ ratio (P = 0.010), NK cells (P = 0.003), and Tregs (P = 0.001) in the peripheral blood were significantly higher than the baseline, and the proportions of CD8+ T cells were significantly lower than the baseline for the effective group, as shown in Table 4. No significant differences were found regarding the proportion of CD4+ T cells (P = 0.486), CD8+ T cells (P = 0.425), CD4+/CD8+ ratio (P = 0.442), NK cells (P = 0.346), B cells (P = 0.630) or Tregs (P = 0.394) in the peripheral blood of patients as compared to the baseline, for the ineffective group.The flow image of a typical patient before and after immunotherapy are shown in Fig. 1.
Correlation between baseline lymphocyte subsets and immunotherapy efficacy
A total of 125 patients with complete data were evaluated after 4 cycles of immunotherapy, with 93 in the effective group (CR 0, PR 39, SD 54) and 32 in the ineffective group. Univariate analysis showed that the differences in PD-L1 expression, CD4+ T cells, NK cells, and Tregs were statistically significant between the two groups (P < 0.05). The proportion of PD-L1 expression (≥ 50%) was high in the effective group of immunotherapy, and the proportion of PD-L1 expression (< 50%) was high in the ineffective group. The proportion of baseline CD4+ T cells, NK cells and Tregs cells was higher in the effective group than in the ineffective group. We included these parameters into the logistic regression model and found that PD-L1 (OR: 0.315, 95% CI: 0.102–0.975, P = 0.045), CD4+ T cells (OR: 0.903, 95% CI: 0.853–0.957, P = 0.001), NK cells (OR: 0.913, 95% CI: 0.860–0.969, P = 0.003), and Tregs (OR: 0.645, 95% CI: 0.471–0.883, P = 0.006) were independent predictors of immunotherapy efficacy.
In addition, our ROC working curves demonstrated that the AUC areas of baseline CD4+ T cells, NK cells, and Tregs predicted the efficacy of immunotherapy in NSCLC patients as 0.690, 0.634, and 0.722, respectively (Fig. 2). Corresponding parameter cut-off values for maximum AUC areas were calculated from the maximum Youden index, with a cut-off value of 33.450 for CD4+ T cells (sensitivity = 0.656, specificity = 0.719), 12.950 for NK cells (sensitivity = 0.817, specificity = 0.469), and 4.850 for Tregs (sensitivity = 0.882, specificity = 0.531).
Correlation between the baseline lymphocyte subsets and PFS
Among the 125 patients with NSCLC, the median PFS was 9 months (95% CI: 7.65–10.35). We used the median proportion of lymphocyte subsets as the threshold to define low and high levels. The proportion of CD4+ T cells was 34.30%, CD8+ T cells was 31.80%, CD4+/CD8+ ratio was 1.07, NK cells was 18.90%, Tregs was 6.10%, and B cells was 7.20%. Univariate analysis showed that gender, PD-L1 expression, CD4+ T cells, CD4+/CD8+ ratio and NK cells were associated with PFS. Age, smoking, pathology, distant metastasis, CD8+ T cells, Tregs, and B cells were not associated with PFS (Table 5). We performed multivariate Cox regression analysis factoring in the variables such as the gender, PD-L1 expression, CD4+ T cells, and NK cells. The results from these analyses revealed that PD-L1 expression (HR: 0.428, 95% CI: 0.245–0.748, P = 0.003), CD4+ T cells (HR: 0.454, 95% CI: 0.271–0.760, P = 0.003), and NK cells (HR: 0.491, 95% CI: 0.296–0.813, P = 0.006) were independent predictors of PFS. Kaplan–Meier survival curve analysis of the correlation between the proportion of CD4+ T cell, NK cells and PFS is shown in Fig. 3.
Discussion
Although immunotherapy has achieved impressive results in the management of advanced NSCLC patients, reliable biomarkers for predicting therapeutic efficacy are still limited. Our current work focused on the changes in the composition of peripheral blood lymphocyte subsets before and after immunotherapy to assess their potential role as predictive biomarkers of immunotherapy efficacy and survival outcomes for patients with advanced NSCLC. The results showed that the proportions of CD4+ T cells, CD8+ T cells, Tregs and B cells decreased after chemotherapy as compared to the baseline. The proportions of CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio, NK cells and Tregs were higher after immunotherapy than after chemotherapy administration. Compared to the baseline, the proportions of CD4+ T cells, CD4+/CD8+ ratio, NK cells and Tregs in the peripheral blood of the immunotherapy effective group were significantly higher and the proportion of CD8+ T cells was significantly lower. In contrast, there were no statistically significant differences in these biomarkers in the ineffective group. Higher proportions of baseline CD4+ T cells and NK cells were associated with effective immunotherapy and a longer PFS, and higher proportions of baseline Tregs were associated with effective immunotherapy as well. The ROC curves further confirmed the predictive role of CD4+ T cells, NK cells, and Tregs on the efficacy of immunotherapy. Moreover, we analyzed the factors that could affect lymphocyte subsets, with the goal of determining the changes in the proportion of peripheral blood lymphocyte subsets with different clinicopathological characteristics. This would enable the identification of the population of patients that might benefit from immunotherapy. Results showed that NK cells were higher in males; CD4+ T cells were higher in females;the proportion of Tregs was higher in stage IV patients with distant metastasis; and the proportion of CD8+ T cells was lower and CD4+/CD8+ ratio was higher in patients with EGFR mutations. This is consistent with the findings of Mazzaschi et al. [12,13,14].
As an integral component of the anti-tumor immunity, peripheral blood CD4+ T cells help regulate and promote priming, migratory potential, and killing activity of cytotoxic T lymphocytes (CTLs) [15]. Our study showed that the proportion of CD4+ T cells increased in the patients within the effective immunotherapy group as compared to the baseline, while the change in CD4+ T cells in the ineffective group was not statistically significant. This suggests that patients in the effective group obtained a durable antitumor response, suggesting a continuous recruitment of antitumor T cells from the peripheral blood [16]. In addition, we found that higher levels of CD4+ T cells before immunotherapy were associated with better efficacy and a longer PFS. Similar conclusions were reached by previous studies. Ottonello et al. [17] found that high levels of CD4+ T cells were associated with a longer overall survival (OS) and PFS in advanced NSCLC patients treated with nivolumab. The value of certain CD4+ T cell subsets in predicting the efficacy of immunotherapy and patient survival has also been discovered now. A high proportion of memory CD4+T cells can be used to identify the clinical beneficiaries before the start of immunotherapy [18]; Hiroshi and colleagues identified a CD62Llow CD4+ T effector memory subset, and reported that the responders had significantly higher proportion of this effector subset prior to PD-1 blockade [15]. Another small-scale study in NSCLC patients treated with nivolumab showed that higher ratios of systemic central memory and effector subsets in the total populations of CD4+ T cells was associated with immunotherapy benefit [19]. A strong expansion of the highly differentiated CD28− CD4+ T lymphocytes (CD4THD) was significantly associated with a hyper-progressive disease (HPD) [20]. In conclusion, CD4+ T cells play an important role in anti-tumor immunity, and dynamic monitoring of CD4+ T cells during immunotherapy could help to evaluate the prognosis of patients and screen for possible patient groups that might particularly benefit. In addition, Miren et al. found that PD-1/LAG-3 co-signaling induces dysfunctional proliferation of systemic CD4+ T cells, which in turn hampers the helper functions over peripheral CD8+ responses [18]. Combined blockade of PD1/LAG-3 may further improve the efficiency of immunotherapy in the future.
CD8+ T cells can expand and differentiate into cytotoxic T lymphocytes (CTL) that infiltrate tumors through peripheral blood migration and play an important role in antitumor immunity through the direct killing of tumor cells [21]. We found that the proportion of CD8+ T cells in the immunotherapy effective group was reduced as compared to the baseline, which is consistent with the findings of Minglei et al. [22]. This is in contradiction with the systemic expansion of CD8+ T cells after PD-1 blockade therapy in lung cancer patients [23]. We evaluated three explanations for this phenomenon. Firstly, patients with lung cancer treated with PD-1 inhibitors have a highly specific subset of proliferating CD8+ T cells in the peripheral blood, expressing effector-like phenotypes (HLA-DR+, CD38+, Bcl-2lo), costimulatory molecules (CD28, CD27, ICOS), and high levels of PD-1. Approximately 70% of the patients showed active proliferation of this CD8+T cells subset (PD-1+CD8+ T cells) after ICI treatment. This population was more likely to respond to PD-1 inhibitors and promote a longer PFS [24]. High baseline levels of PD-1+CD8+ T cells promotes a longer OS after nivolumab treatment in NSCLC patients [17, 25]. Secondly, the CD28/B7 pathway plays an important role in the proliferation of CD8+T cells after PD-1 blockade therapy in lung cancer patients. However, many human CD8+ TILs do not express CD28, which implies that they are less responsive to CD28 mediated proliferation of CD8+ T cells upon PD-1 blockade [26]. Thirdly, non-optimal timing of blood sampling might contribute as well. Induction of CD8+ T-cell response in the peripheral blood by blocking the PD-1 pathway is transient and detected only during the first 4 weeks after treatment initiation, however, these cells then migrate to the tumor sites [24]. Accordingly, the CD4+/CD8+ ratio was higher after immunotherapy than at the baseline. The CD4+/CD8+ ratio is a marker of cell-mediated immunity in cancer patients, and its reduction is related to a low immunological function [27]. This proves that the immunosuppression status of patients in the effective immunotherapy group was improved.
Toshiko's research in the previous years confirmed that the blockade of PD-L1 leads to the activation of NK cells and enhances the direct antitumor function of NK cells [28]. Myeong et al. also indicated that NK cell activity can be used as a biomarker to predict the response to immunotherapy in NSCLC patients [29]. Our research corroborated it in that the NK cells proportion increased in the patients in the effective immunotherapy group as compared to the baseline, and a higher baseline NK cells proportion is correlated with better efficacy and a longer PFS. This is consistent with the research done by Giulia and Peng et al. [12, 30]. In summary, the positive role of NK cells in tumor immune monitoring has been confirmed by more and more studies. Nowadays, medicines that modulate the proliferative activity and function of NK cells have been developed for the purpose of producing synergistic antitumor efficacy in combination with ICIs [31, 32]. Our results will further advance this field of research, as we show that higher baseline NK cell levels in patients with advanced NSCLC associates with improved immunotherapy efficacy.
Tregs play a crucial immunosuppressive role in patients with cancer. Our research showed that Tregs of patients in the clinical benefit group increased as compared to the baseline. This may be due to the Treg-NK interaction. Tregs selectively express membrane-bound transforming growth factor-β, down-regulate the expression of NKG2D on NK cells in vitro and in vivo and inhibit NK cells effector functions as a means of achieving immune homeostasis in organisms [33]. Giulia et al. also observed that following PD-1 blockade, the rise in NK cells in the clinical benefit group was accompanied by a significant increase in the number and proliferation of Tregs [12]. The current study also showed that a high Tregs proportion at baseline predicts a good immunotherapeutic response, in agreement with the findings of Jose et al. They observed an inverse correlation between the number of tumor Tregs and the number of peripheral Tregs in the mouse tumor model [34]. This means that ICIs treatment improves local immune microenvironment in the tumor. However, this study did not find a correlation between Tregs and long-term survival. Analyzing the role of circulating Treg subsets, Athanasios et al. reported for the first time that at the baseline, high levels of naive and effector Tregs were associated with a longer OS, while a high frequency of terminal effector Tregs was associated with a longer PFS and OS [35]. Jinyan et al. provided evidence that FOXA1+ Treg subsets promoted tumor growth and were associated with a poor response to lung cancer treatment [36]. Our next study will focus on the correlation between peripheral blood Treg subsets and long-term survival of patients treated with immunotherapy, in order to provide novel and reliable targets for inhibiting Tregs.
B cells activate T cells by antigen presentation [37] and the cytotoxic potential of activated B cells leads to the direct impairment of tumor development [38]. In this study, the proportion of peripheral blood B cells decreased after chemotherapy, suggesting humoral immunity was impaired. Johanna et al. reached similar conclusions to ours, and reported that 4–6 cycles of first-line chemotherapy reduced the absolute counts of B cells, independent of the subsets of B cells [16]. Ying et al. demonstrated that chemotherapy decreased the absolute counts of B cells, followed by a gradual increase, but ultimately did not reach the baseline level, suggesting that the effects of the chemotherapy on lymphocyte subsets is long-term [39]. There was no increase in the proportion of B cells after immune checkpoint inhibitor treatment. This is due to the fact that although B cells also express inhibitory surface markers such as PD-1, it is unclear whether PD-1 expression on B cells plays a role in the antitumor immune response or checkpoint inhibition therapy. In addition, several studies have revealed that B cells also play a pro-tumorigenic role, for example, the plasma-like B cells promote cancer cell growth in the advanced stage of NSCLC [40]. Also, B cells mediated suppression of activated CD8+ T cells that kill tumor cells in NSCLC patients upon ICI treatment, may decrease with age, implying that B cells may play a pro-tumorigenic role in younger NSCLC patients [18].
Previous studies have shown that chemotherapeutic agents not only kill tumor cells, but also damage the immune cells, and thus disturb the anti-tumor immune response [8, 10, 16]. Our study showed that CD4+ T cells, CD8+T cells, Tregs cells, and B cells were reduced after chemotherapy. A study by Wang et al. confirmed that proliferating T cells of all the subsets, were almost entirely depleted at day 8 following chemotherapy. Of these, Tregs were the most profoundly depleted cells at this time point [41]. This may be because paclitaxel significantly reduces all the subsets of Tregs [42]. There were no statistical differences in each of the parameters between the immunotherapy and chemotherapy groups before treatment. CD4+T cells, CD8+T cells, CD4+/CD8+, NK cells, and Tregs were all higher after immunotherapy than after chemotherapy, which implied that the overall immune status of patients was better after immunotherapy than after chemotherapy.
Although chemotherapy causes damage to immune cells, we need not worry that the anti-tumor effect of ICIs is diminished by the reduction of lymphocyte subsets in chemotherapy combination or sequential immunotherapy modalities. This is because, first of all, the reduction in lymphocyte subsets induced by chemotherapy is dynamic and transient [43]. Chemotherapy-induced increase in T cell proliferation, among the subsets of CD8+ T cells, and naive T cells, was the highest on day 6 after the treatment [44]. However, the increase in T-cell proliferation was not sufficient to restore T-cell numbers to pre-treatment levels [45]. Secondly, this proliferation of T cells is antigen-independent, termed as homeostatic repopulation. The homeostatic proliferation of T cells breaks tolerance, temporarily restoring the immune response to previously tolerated antigens [42]. Immune checkpoint inhibition can further augment anti-tumor immune responses by maintaining T cells in an activated state. Therefore, chemotherapy combined with immunotherapy or sequential immunotherapy may create a synergistic effect that augments anti-tumor immune responses [46].
We must acknowledge the limitations in our study. Firstly, our work did not obtain the absolute count of lymphocyte subsets. It would be more valuable to prospectively collect samples for analyzing the proportion and absolute count of lymphocyte subsets, in future studies. Furthermore, ICIs and treatment combinations obtained from the different pharmaceutical companies could have added further confounding factors into the analyses in our study. Finally, considering that the benefit to OS is the main goal of immunotherapy for lung cancer patients, future research must extend the observation time and treatment cycles, and monitor the changes in lymphocyte subsets longitudinally to explore their correlation with OS.
Conclusions
In summary, our results indicate that immune checkpoint inhibitors induce changes in the proportion of peripheral blood lymphocyte subsets in patients with effective immunotherapy. High level of CD4+ T cells, NK cells and Tregs can predict a better immunotherapy efficacy in NSCLC patients, and a high level of CD4+ T cells and NK cells are associated with a longer PFS. Therefore, peripheral blood lymphocyte subsets may serve as valuable markers of therapeutic efficacy and prognosis for advanced NSCLC patients.
Availability of data and materials
The datasets generated and /or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- ICIs:
-
Immune checkpoint inhibitor
- PFS:
-
Progression-free survival
- Tregs:
-
Regulatory T cells
- PD-L1:
-
Programmed cell death ligand 1
- TMB:
-
Tumor mutation burden
- LIPI:
-
Lung immune prognostic index
- TILs:
-
Tumor-infiltrating lymphocytes
- EGFR:
-
Epidermal growth factor receptor
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Disease progression
- RECIST:
-
The solid tumor response evaluation criteria
- NGS:
-
Next-generation sequencing
- EGFR-TKI:
-
EGFR tyrosine kinase inhibitor
- CTLs:
-
Cytotoxic T lymphocytes
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Johnson DB, Rioth MJ, Horn L. Immune checkpoint inhibitors in NSCLC. Curr Treat Options Oncol. 2014;15(4):658–69.
Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, Subbiah V. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6(3):181–91.
Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima LG. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499–506.
Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M, Prelaj A, Zilembo N, Ganzinelli M, Pallavicini LM, De Simone I, Colombo MP, Sica A, Torri V, Garassino MC. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev. 2019;75:39–51.
Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70(6):505–17.
Barua S, Fang P, Sharma A, Fujimoto J, Wistuba I, Rao AUK, Lin SH. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer. 2018;117:73–9.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30.
Wei Z, Zhang W, Gao F, Wu Y, Zhang G, Liu Z, Jiao S. Impact of lymphocyte subsets on chemotherapy efficacy and long-term survival of patients with advanced non-small-cell lung cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39(3):371–6.
Qiu J, Che G, Liu F, Sha X, Ju S, Ma H, Feng L. The detection and clinical significance of peripheral regulatory CD4+CD25hiCD127low T cells in patients with non-small cell lung cancer. Clin Transl Oncol. 2019;21(10):1343–7.
Mazzaschi G, Facchinetti F, Missale G, Canetti D, Madeddu D, Zecca A, Veneziani M, Gelsomino F, Goldoni M, Buti S, Bordi P, Aversa F, Ardizzoni A, Quaini F, Tiseo M. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer. 2019;127:153–63.
Dai S, Ren P, Ren J, Yang L, Li W. The relationship between lymphocyte subsets and the prognosis and genomic features of lung cancer: a retrospective study. Int J Med Sci. 2021;18(10):2228–34.
Li YD, Lamano JB, Lamano JB, Quaggin-Smith J, Veliceasa D, Kaur G, Biyashev D, Unruh D, Bloch O. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2019;68(9):1501–13.
Kagamu H, Kitano S, Yamaguchi O, Yoshimura K, Horimoto K, Kitazawa M, Fukui K, Shiono A, Mouri A, Nishihara F, Miura Y, Hashimoto K, Murayama Y, Kaira K, Kobayashi K. CD4+ T-cell immunity in the peripheral blood correlates with response to anti-PD-1 therapy. Cancer Immunol Res. 2020;8(3):334–44.
Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, Fong L, Nolan GP, Engleman EG. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487-502.e15.
Ottonello S, Genova C, Cossu I, Fontana V, Rijavec E, Rossi G, Biello F, Dal Bello MG, Tagliamento M, Alama A, Coco S, Boccardo S, Vanni I, Ferlazzo G, Moretta L, Grossi F, Mingari MC, Carrega P, Pietra G. Association between response to nivolumab treatment and peripheral blood lymphocyte subsets in patients with non-small cell lung cancer. Front Immunol. 2020;11:125.
Zuazo M, Arasanz H, Fernández-Hinojal G, García-Granda MJ, Gato M, Bocanegra A, Martínez M, Hernández B, Teijeira L, Morilla I, Lecumberri MJ, Fernández de Lascoiti A, Vera R, Kochan G, Escors D. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol Med. 2019;11(7):e10293.
Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, Cunningham M, Greenawalt D, Patel V, Yang M, Golhar R, Carman JA, Lezhnin S, Dai H, Kayne PS, Suchard SJ, Bernstein SH, Nadler SG. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. 2018;9:1613.
Arasanz H, Zuazo M, Bocanegra A, Gato M, Martínez-Aguillo M, Morilla I, Fernández G, Hernández B, López P, Alberdi N, Hernández C, Chocarro L, Teijeira L, Vera R, Kochan G, Escors D. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers (Basel). 2020;12(2):344.
Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–8.
Zhuo M, Chen H, Zhang T, Yang X, Zhong J, Wang Y, An T, Wu M, Wang Z, Huang J, Zhao J. The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer Biomark. 2018;22(3):467–76.
Fehlings M, Jhunjhunwala S, Kowanetz M, O’Gorman WE, Hegde PS, Sumatoh H, Lee BH, Nardin A, Becht E, Flynn S, Ballinger M, Newell EW, Yadav M. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J Immunother Cancer. 2019;7(1):249.
Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, Behera M, Wu H, McCausland M, Chen Z, Zhang C, Khuri FR, Owonikoko TK, Ahmed R, Ramalingam SS. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(19):4993–8.
Kim CG, Hong MH, Kim KH, Seo IH, Ahn BC, Pyo KH, Synn CB, Yoon HI, Shim HS, Lee YI, Choi SJ, Lee YJ, Kim EJ, Kim Y, Kwak JE, Jung J, Park SH, Paik S, Shin EC, Kim HR. Dynamic changes in circulating PD-1+CD8+ T lymphocytes for predicting treatment response to PD-1 blockade in patients with non-small-cell lung cancer. Eur J Cancer. 2021;143:113–26.
Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai RN, Ramalingam SS, Araki K, Ahmed R. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423–7.
Xu J, Jiang L, Cao H, Jia Y, Wu S, Jiang C, et al. Predictive value of CD4+/ CD8+ ratio in patients with breast cancer receiving recombinant human thrombopoietin. J Interferon Cytokine Res. 2018;38(5):213–20.
Kamata T, Suzuki A, Mise N, Ihara F, Takami M, Makita Y, Horinaka A, Harada K, Kunii N, Yoshida S, Yoshino I, Nakayama T, Motohashi S. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477–89.
Choi MG, Kim YJ, Lee JC, Rho JK, Choi CM. Efficacy of natural killer cell activity as a biomarker for predicting immunotherapy response in non-small cell lung cancer. Thorac Cancer. 2020;11(11):3337–45.
Li P, Qin P, Fu X, Zhang G, Yan X, Zhang M, Zhang X, Yang J, Wang H, Ma Z. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor. Ann Palliat Med. 2021;10(3):3039–49.
Zingoni A, Fionda C, Borrelli C, Cippitelli M, Santoni A, Soriani A. Natural killer cell response to chemotherapy-stressed cancer cells: role in tumor immunosurveillance. Front Immunol. 2017;8:1194.
Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–32.
Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–38.
Kim HR, Park SM, Seo SU, Jung I, Yoon HI, Gabrilovich DI, Cho BC, Seong SY, Ha SJ, Youn JI. The ratio of peripheral regulatory T cells to lox-1+ polymorphonuclear myeloid-derived suppressor cells predicts the early response to anti-PD-1 therapy in patients with non-small cell lung cancer. Am J Respir Crit Care Med. 2019;199(2):243–6.
Kotsakis A, Koinis F, Katsarou A, Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V, Vetsika EK. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci Rep. 2016;6:39247.
Liang J, Tian C, Zeng Y, Yang Q, Liu Y, Liu Y, Wu J, Hu Y, Gu F, Zhang K, Wang Y, Zhang Y, Liu L. FOXA1+ regulatory T cells: a novel T cell subset that suppresses antitumor immunity in lung cancer. Biochem Biophys Res Commun. 2019;514(1):308–15.
DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–16.
Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–57.
Xia Y, Li W, Li Y, Liu Y, Ye S, Liu A, Yu J, Jia Y, Liu X, Chen H, Guo Y. The clinical value of the changes of peripheral lymphocyte subsets absolute counts in patients with non-small cell lung cancer. Transl Oncol. 2020;13(12):100849.
Chen J, Tan Y, Sun F, Hou L, Zhang C, Ge T, Yu H, Wu C, Zhu Y, Duan L, Wu L, Song N, Zhang L, Zhang W, Wang D, Chen C, Wu C, Jiang G, Zhang P. Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol. 2020;21(1):152.
McCoy MJ, Lake RA, van der Most RG, Dick IM, Nowak AK. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br J Cancer. 2012;107(7):1107–15.
Li JY, Duan XF, Wang LP, Xu YJ, Huang L, Zhang TF, Liu JY, Li F, Zhang Z, Yue DL, Wang F, Zhang B, Zhang Y. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res. 2014;2014:286170.
Zheng Y, Dou Y, Duan L, Cong C, Gao A, Lai Q, Sun Y. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cell Immunol. 2015;294(1):54–9.
Takahashi H, Sakakura K, Mito I, Ida S, Chikamatsu K. Dynamic changes in immune cell profile in head and neck squamous cell carcinoma: immunomodulatory effects of chemotherapy. Cancer Sci. 2016;107(8):1065–71.
Park DS, Robertson-Tessi M, Luddy KA, Maini PK, Bonsall MB, Gatenby RA, Anderson ARA. The goldilocks window of personalized chemotherapy: getting the immune response just right. Cancer Res. 2019;79(20):5302–15.
Kareva I. A combination of immune checkpoint inhibition with metronomic chemotherapy as a way of targeting therapy-resistant cancer cells. Int J Mol Sci. 2017;18(10):2134.
Acknowledgements
We thank all the members in Department of Medical Oncology of the first Hospital of Shanxi Medical University for their help and valuable opinion. In addition, we thank MJEditor (www.mjeditor.com) for his linguistic assistance in the preparation of this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YY and JMJ participated in the research design, YY wrote the main manuscript text, YY, XYW and CAL performed the data collection, YY performed data analysis and prepared Figs. 2 and 3, XYW prepared Fig. 1, YY and JMJ participated in the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As the retrospective nature of our study, we contacted patients by telephone to obtain informed consent from them and made telephone recordings. The Ethical Committee of first Hospital of Shanxi Medical University reviewed the telephone recordings and approved our study (Ethics No. K125). All methods were performed in accordance with the relevant guidelines and regulations. All participants gave informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Flow cytometry staining and analysis.
Additional file 2.
PD-L1 Immunohistochemistry.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, Y., Wang, X., Liu, C. et al. Association of lymphocyte subsets with efficacy and prognosis of immune checkpoint inhibitor therapy in advanced non-small cell lung carcinoma: a retrospective study. BMC Pulm Med 22, 166 (2022). https://doi.org/10.1186/s12890-022-01951-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-01951-x