Abstract
Background
Dietary carotenoids have been proven to improve intestinal disorders like inflammatory bowel disease and colon cancer, yet little is known about the link between dietary carotenoids and constipation. This study aims to examine the relationship between dietary carotenoids intake and constipation, using data from the National Health and Nutrition Examination Survey (NHANES) 2005–2010.
Methods
A total of 11,722 participants were enrolled. Chronic constipation was defined as type 1 (separate hard lumps, like nuts) and type 2 (sausage-like, but lumpy) in the Bristol stool form scale (BSFS). Carotenoids intake was obtained from the average of two 24-hour dietary recall questionnaires (if only one 24-hour was available, we used it) and divided into quartiles (Q). The prevalence of constipation was calculated across men and women individuals. The relationship between dietary carotenoids intake and constipation in men and women was assessed with weighted logistic regression and smoothed curve fitting after adjusting confounders, with results displayed as weighted odds ratio (OR) with 95% confidence intervals (95% CI). The model was further stratified by age, race, and HEI 2015 scores (with median as cutoff) among men and women.
Results
The total weighted prevalence of chronic constipation in this study was 8.08%, 11.11% in women and 5.18% in men. After multivariable adjustment, compared with the lowest intake, participants with the highest dietary lycopene intake (ORQ4 vs. Q1= 0.55, 95% CI: 0.36–0.84, p for trend = 0.01) and total lycopene intake (ORQ4 vs. Q1 = 0.52, 95% CI: 0.34–0.80, p for trend = 0.01) were negatively associated with the risk of chronic constipation in men, whereas increased dietary α-carotene intake reduced the risk of chronic constipation in women (ORQ4 vs. Q1 = 0.69, 95% CI: 0.48–0.98, p for trend = 0.04). Smoothing curve fitting further supported these results and provided evidence of dose-response effects. No association was found between other types of carotenoids and chronic constipation in men and women.
Conclusions
Increasing lycopene intake may improve bowel function in men while increased α-carotene intake may reduce the risk of chronic constipation in women. Further studies are essential to explore the role that the intake of carotenoids plays in chronic constipation.
Similar content being viewed by others
Background
Patients with chronic constipation suffer a significant burden of disease and suffer from low quality of life. According to estimates, chronic constipation affects between 10.1% and 15.3% of the adult population worldwide, with a higher prevalence observed among women compared to men [1], and its prevalence increases with age [2]. Chronic constipation is a multifactorial disease, with common risk factors such as genetic predisposition, socio-economic status, parental education, lifestyle, drugs and depression [2, 3]. Lifestyle is a modifiable risk factor for chronic constipation; in particular, the relationship between dietary components and chronic constipation has attracted much attention. Recent studies have reported that soluble fiber and microelements, such as selenium, magnesium and phosphorus have been recognized to decrease chronic constipation risk [3,4,5,6], while high dietary saturated fat or women’s relatively low energy intake is linked to increased risk [7, 8]. Therefore, the investigation of potential dietary strategies for chronic constipation holds promise for both the prevention and management of the disease.
Carotenoids, a group of natural pigments that cannot be produced by the human body and only be obtained through dietary sources, are widely found in fruits and vegetables with numerous biological activities such as antioxidant, anti-inflammatory, regulating cell growth, regulating immune function, and anti-cancer [9, 10]. Previous research findings have suggested that carotenoids intake may reduce the risk of intestinal diseases such as colon cancer and improve ulcerative colitis [11,12,13]. Furthermore, patients in remission from ulcerative colitis who consumed higher lutein and zeaxanthin reported less constipation [14]. Moreover, traditional plants that could treat constipation are rich in carotenoids, for example, Rosa spp. contains lycopene, and β-carotene is one of the main components of Aloe in treating constipation [15, 16]. Nevertheless, limited research has been conducted to investigate the impact of carotenoids on chronic constipation.
Based on NHANES data, we (1) calculated the prevalence of chronic constipation among American adults, (2) measured the association between dietary carotenoids consumption and chronic constipation, and (3) investigated the potential influence of age, gender, race and diet quality with respect to the relationship between carotenoids intake and chronic constipation.
Methods
Data source
The National Health and Nutrition Examination Survey (NHANES) is a national sample survey conducted by the National Center for Health Statistics (NCHS), aims to measure the health and nutritional status of adults and children in the non-institutionalized civilian population of the United States [4, 5, 8].
Study population
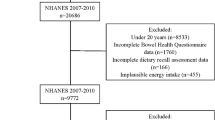
The data used in this study came from three consecutive cycles of the NHANES, which covered the years 2005–2006, 2007–2008, and 2009–2010, as they contained both carotenoids diet data and bowel health questionnaires (BHQ) data, with a total of 31,034 participants in the three cycles, of which 14,619 were included due to complete BHQ information. An additional 2897 participants were excluded because they did not fulfill the criteria for inclusion: (1) missing information on dietary carotenoids intake (n = 245); (2) self-reported chronic diarrhea (n = 1120); (3) pregnancy (n = 391); (4) self-reported colorectal cancer (n = 84); or (5) incomplete information on other variables (n = 1057). Finally, 11,722 participants were included in the final analysis (flowchart shown in Fig. 1). A total of 947 participants were diagnosed with chronic constipation, 311 of whom were men and 636 were women.
Diagnosis of constipation
Based on previous studies, chronic constipation could be assessed by the Bristol Stool Form Scale (BSFS), which was provided by the Mobile Examination Center (MEC) interview and administered to adults aged 20 years and older. Participants were told to look at a card and identify their usual or most frequent stool type by the number on it. Chronic constipation was composed of type 1 (separate hard lumps, like nuts) and type 2 (sausage-like, but lumpy) in BSFS; type 6 (fluffy pieces with ragged edges, a mushy stool) and type 7 (watery, no solid pieces) were classified as diarrhea; and type 3 (like a sausage but with cracks in the surface), type 4 (like a sausage or snake, smooth and soft) and type 5 (soft blobs with clear-cut edges) were considered to have a healthy bowel [4, 8].
Measures of dietary carotenoids
Two 24-hour dietary recall questionnaires were administered by NHANES, the first by face-to-face collection at MEC, and the second by telephone collection 3 to 10 days later. This provided dietary intakes of α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein with zeaxanthin (combined). The consumption of lycopene, as well as lutein with zeaxanthin supplements over the same two 24-hour periods was also recorded (from 2007 onwards). Intake of carotenoids was calculated using the average of two 24-hour recalls or the reported value for participants who completed one 24-hour interview. Intake of total lycopene and total lutein with zeaxanthin intake were determined from dietary and supplemental sources.
Covariates
We collected covariates associated with constipation and carotenoids intake through literature review. These variables included age, race (Mexican American, non-Hispanic black, non-Hispanic white, and other races), marital status (living alone, married or living with a partner), educational levels (below high school, high school, above high school), poverty-income ratio (PIR, < 2 or ≥ 2), body mass index (BMI), smoking, drinking, vigorous physical activity, hypertension, diabetes, depression, HEI 2015 scores and dietary intake, including energy, fat, fiber, and fluid. BMI was grouped as underweight or normal (< 25 kg/m2), overweight (25–30 kg/m2), and obese (> 30 kg/m2). Classification of smoking was as follows: Never: smoked under 100 cigarettes in their lifetime; Former: smoked over 100 cigarettes in their lifetime and smoke not at all now; and Now: smoked over 100 cigarettes in their lifetime and smoke some or all of the time. Drinking was defined if a person has had at least 12 drinks a year. The definition of vigorous physical activity varied by year. In the cycle of 2005–2006, it was defined as doing any vigorous activities for at least 10 minutes that caused heavy sweating, or significant elevations in breathing or heart rate over the past 30 days. While during 2007–2010, those who answered yes to the question ‘Work involve vigorous-intensity activities that cause significant increases in breathing or heart rate, such as carrying or lifting heavy loads, digging, or construction work for at least 10 minutes continuously’ were considered to have vigorous physical activity. Those who met any of the following criteria were considered hypertensive: (1) informed by a medical professional; (2) used anti-hypertensive medication; or (3) average systolic blood pressure of at least 130 mmHg or average diastolic blood pressure of at least 80 mmHg. The diagnostic criteria for diabetes were: (1) doctor told; or (2) glycohemoglobin HbA1c ≥ 6.5%; or (3) use of diabetes medication or insulin. Depression information from the PHQ9 questionnaire, with scores ≥ 10, was considered positive. The intake of dietary nutrients and HEI 2015 scores were obtained from two 24-hour dietary interviews (the reported value was used if it was available only).
Statistical analysis
Taking into consideration NHANES’ complex sample design, we weighted the data with appropriate weights based on NHANES guidelines (one-third of the 2005–2010 weight). Continuous variables were displayed as weighted means (SE) and comparisons between groups were tested using t-tests, and survey-weighted percentages (SE) were used to describe categorical variables, while chi-square tests were performed on comparing groups.
An analysis of multivariable logistic regression was conducted to analyze the relationship between dietary carotenoids intake and chronic constipation among men and women, with results shown as weighted Odds Ratio (OR) with 95% confidence intervals (95% CI). Model 1 was not adjusted for any of the variables, while model 2 was adjusted for potential confounding including age (as a continuous variable), race, marital status, educational levels and PIR (as a continuous variable). In model 3, we further adjusted for vigorous physical activity, BMI (as a continuous variable), smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores. To better visualize the association between dietary carotenoids subclass intake and constipation risk, we performed weighted smoothed curve fitting using the generalized additive model (GAM) after fully adjusting for covariates. In addition, models were stratified by age, race and HEI 2015 scores (with median as cutoff) to explore the heterogeneity of results. All data processing was performed in R version 4.2.2 (https://www.r-project.org/). A two-sided P < 0.05 was considered statistically significant.
Results
Participants’ characteristics
Table 1 shows the participants’ baseline characteristics. According to the diagnostic criteria, 947 of the 11,722 participants screened positive for chronic constipation, 311 (5.18%) in men and 636 (11.11%) in women. Compared with participants who had healthy intestines, men with constipation were often Mexican American, having lower education levels and PIR, having lower BMI, no drinking, and were more likely to be accompanied by depression, having lower dietary intake (energy, fiber, fat, fluid) and unhealthy eating habits (lower HEI 2015 scores). Women with constipation were more likely to be non-Hispanic Black, have lower education levels and PIR, have lower BMI, were non-drinker, have lower fiber, fat, fluid intake and lower HEI 2015 scores and depression in the univariate analysis.
Association between carotenoids intake and chronic constipation among men and women
The weighted OR (95% CI) between quartiles of carotenoids intake and chronic constipation are shown in Table 2. Among the male participants in the highest quartile of carotenoids intake, the results of logistic regression showed that all carotenoids, with the exception of α-carotene, exhibited an inverse relationship with constipation in model 1 (ORQ4 vs. Q1 (95% CI): β-carotene, 0.46 (0.29–0.73); β-cryptoxanthin, 0.64 (0.43–0.96); lycopene, 0.41 (0.25–0.66); lutein with zeaxanthin, 0.46 (0.28–0.75); total lycopene, 0.39 (0.24–0.62); total lutein with zeaxanthin, 0.42 (0.26–0.69)). However, there was no statistically significant association between dietary β-cryptoxanthin intake and chronic constipation in model 2 (ORQ4 vs. Q1 = 0.70, 95% CI: 0.48–1.01). After further adjustment for other variables in Model 3, the odds of constipation were decreased across quartiles of dietary lycopene intake (ORQ4 vs. Q1 = 0.55, 95% CI: 0.36–0.84, p for trend = 0.01) and total lycopene intake (ORQ4 vs. Q1 = 0.52, 95% CI: 0.34–0.80, p for trend = 0.01).
In women, higher dietary intakes of α-carotene, β-carotene, lutein with zeaxanthin and total lutein with zeaxanthin in the fourth quartile (compared to the first quartile) were associated with a reduced risk of chronic constipation. This was observed in both model 1 (ORQ4 vs. Q1 (95% CI), α-carotene, 0.53 (0.39–0.72); β-carotene, 0.49 (0.37–0.66); lutein with zeaxanthin, 0.59 (0.44–0.80); total lutein with zeaxanthin, 0.56 (0.41–0.76)) and model 2 (ORQ4 vs. Q1 (95% CI), α-carotene, 0.60 (0.43–0.83); β-carotene, 0.57 (0.41–0.80); lutein with zeaxanthin, 0.69 (0.51–0.94); total lutein with zeaxanthin, 0.66 (0.48–0.89)). In model 3, individuals in the highest quartile of dietary α-carotene intake exhibited a 31% reduction in odds of constipation when compared to those in the lowest quartile intake (ORQ4 vs. Q1 = 0.69, 95% CI: 0.48–0.98, p for trend = 0.04).
Weighted smoothed curve fitting based on the GAM further demonstrated linear dose-response relationships between dietary lycopene intake in men and dietary α-carotene intake in women and the risk of chronic constipation (Figs. 2 and 3).
Weighted smooth curve fitting among men
The weighted smooth curve fitting is used to display the association between dietary lycopene (2 A), total lycopene (2B) intake and log-transformed odds ratio of chronic constipation based on the generalized additive model (GAM) among men. Adjusting for all covariates, including age, race, marital status, educational levels and PIR, vigorous physical activity, BMI, smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores. The red line represents the fitted line and the blue line refers to the 95% confidence intervals
Weighted smooth curve fitting among women
The weighted smooth curve fitting is used to display the association between dietary α-carotene and log-transformed odds ratio of chronic constipation based on the generalized additive model (GAM) among women. The covariates of age, race, marital status, educational levels and PIR, vigorous physical activity, BMI, smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores were adjusted. The red line represents the fitted line and the blue line refers to the 95% confidence intervals
Results of subgroup analysis
We observed that among men, the inverse association between the highest consumption of dietary lycopene and total lycopene and the risk of chronic constipation were particularly significant in participants of younger (ORQ4 vs. Q1 = 0.45, 95% CI: 0.25–0.81, p for trend = 0.01; ORQ4 vs. Q1 = 0.42, 95% CI: 0.24–0.75, p for trend = 0.01, respectively) and lower scores of HEI-2015 (ORQ4 vs. Q1 = 0.47, 95% CI: 0.24–0.95, p for trend = 0.04; ORQ4 vs. Q1 = 0.45, 95% CI: 0.22–0.92, p for trend = 0.04, respectively), as compared to those with the lowest consumption. No interaction was found between them (all p for interaction > 0.05) (Figs. 4 and 5). In women, the protective relationship between higher intake of α-carotene and constipation was robust in younger individuals (ORQ4 vs. Q1 = 0.51, 95% CI: 0.32–0.81, p for trend = 0.003), Mexican Americans (ORQ4 vs. Q1 = 0.33, 95% CI: 0.15–0.76, p for trend = 0.004), and those with an unhealthy diet (ORQ4 vs. Q1 = 0.47, 95% CI: 0.31–0.71, p for trend = 0.002). The interaction results indicated that the impact of α-carotene intake on chronic constipation varied according to HEI 2015 scores (p for interaction = 0.04) (Fig. 6).
The weighted stratified and interaction analysis of relationship between dietary lycopene intake and covariates in men
Age, race, marital status, educational levels, PIR, vigorous physical activity, BMI, smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores were adjusted, except for the corresponding variables
The weighted stratified and interaction analysis of association between total lycopene intake and covariates in men
The covariates include age, race, marital status, educational levels, PIR, vigorous physical activity, BMI, smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores, except for the corresponding variables
The relationship between α-carotene intake and covariates in women was assessed by weighted stratified and interaction analysis
The covariates include age, race, marital status, educational levels, PIR, vigorous physical activity, BMI, smoking, drinking, diabetes, hypertension, depression, and dietary energy, dietary fiber, fat, fluid intake, and HEI 2015 scores, except for the corresponding variables
Discussion
To our knowledge, this is the first investigation of the association between the intake of carotenoids and chronic constipation in a large, nationally representative sample of adults. The overall weighted prevalence of constipation was 8.08%, with a significantly higher prevalence among females in comparison to males. Despite being slightly lower than findings reported in previous analyses, chronic constipation continues to pose a substantial global burden of disease. Dietary and lifestyle management is an initial strategy to prevent and treat chronic constipation, therefore, there is a pressing need to enhance research efforts in this area to bolster the management of the problem.
This study discovered a noteworthy inverse relationship between the intake of lycopene and chronic constipation among males (ORQ4 vs. Q1 = 0.55, 95% CI: 0.36–0.84 for dietary lycopene, ORQ4 vs. Q1 = 0.52, 95% CI: 0.34–0.80 for total lycopene), whereas the intake of α-carotene was negatively linked to constipation among females (ORQ4 vs. Q1 = 0.69, 95% CI: 0.48–0.98). Meanwhile, higher lycopene intake in men exhibited a more protective effect in younger (ORQ4 vs. Q1 = 0.45, 95% CI: 0.25–0.81 for dietary lycopene; ORQ4 vs. Q1 = 0.42, 95% CI: 0.24–0.75 for total lycopene) and lower HEI 2015 scores individuals (ORQ4 vs. Q1 = 0.47, 95% CI: 0.24–0.95 for dietary lycopene; ORQ4 vs. Q1 = 0.45, 95% CI: 0.22–0.92 for total lycopene), while the advantageous impact of α-carotene intake was more pronounced in younger women (ORQ4 vs. Q1 = 0.51, 95% CI: 0.32–0.81), Mexican Americans (ORQ4 vs. Q1 = 0.33, 95% CI: 0.15–0.76) and those with lower HEI 2015 scores (ORQ4 vs. Q1 = 0.47, 95% CI: 0.31–0.71). There was an interaction between HEI 2015 scores subgroup and α-carotene intake against constipation. This was of great interest because the management of constipation is still a serious challenge, and increasing certain carotenoids intake through dietary changes may be an effective way to prevent and treat it, especially in particular subgroups.
Age and gender are important risk factors for the occurrence of constipation [17, 18]. In our study, the prevalence of constipation was found to be significantly higher among women than men (more than twice as high), but we did not observe an increase in prevalence with age, and these results could be supported by previous findings from NHANES [8]. Furthermore, we observed that the protective effect of lycopene was more significant among younger men, potentially attributed to its significantly impaired bioavailability in older individuals [19]. Liu et al. [20] noted that a higher overall dietary quality has the potential to mitigate the risk of chronic constipation, but in our results, the protective impact of lycopene among men and α-carotene among women against constipation was more pronounced in individuals with lower HEI 2015 scores, suggesting that higher carotenoids intake may be affiliated with specific dietary components regardless of overall quality of the diet. Further research is necessary to validate these findings.
Lycopene, which is mainly found in tomatoes and its products [21], has powerful anti-inflammatory and free radical scavenging effects [22, 23], and its implications for intestinal function are being intensively studied. Several possible reasons could explain the protective association between dietary lycopene intake and chronic constipation. First, it could alleviate intestinal inflammation and inhibit oxidative stress. Animal studies have suggested that lycopene could not only suppress the expression of pro-inflammatory factors like IL-1β, IL-6, IL-8 and TNF-α, but also stimulate the production of anti-inflammatory cytokines (such as TGF-β, IL-10), so as to reduce intestinal inflammation [24, 25]. Under conditions of oxidative stress, the system exhibited an overproduction of reactive oxygen species (ROS), resulting in detrimental effects on the intestinal mucosa and subsequent impairment of intestinal function. Lycopene effectively reduced the adverse effects of drugs and radiation on oxidative stress markers such as myeloperoxidase (MPO), superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) in intestine, thereby maintaining intestinal health [24, 26, 27].
Secondly, lycopene has the potential to uphold normal intestinal barrier function. The barrier is comprised of four components, namely the mechanical, immune, chemical, and microbial barrier. Several tight junction proteins are involved in forming the intestinal mechanical barrier, and they could regulate intestinal permeability by connecting the cellular gaps. Wang et al. [24] suggested that lycopene significantly inhibited the disruption of tight junction proteins induced by sulfamethoxazole (SMZ) by increasing the transcriptional levels of Claudin1, Claudin4, Occludin and ZO-1, indicating that lycopene could regulate the expression of tight junction proteins to maintain the intestinal epithelial barrier; similar results were found in a series of animals studies [25, 26, 28]. Moreover, secretory IgA (sIgA) serves as the first defenses of intestinal mucosal immunity, effectively binding to pathogens and preventing them from damaging the intestine. IL-4, IL-12 and IFN-γ are important immunomodulatory cytokines that could reflect the status of both humoral and cellular immunity [29]. In an animal model of intestinal ischemia-reperfusion, it has been documented that the preoperative administration of lycopene limited the loss of intestinal IgA and sIgA levels, as well as decreased bacterial translocation [30]. Pan et al. [31] observed that lycopene pretreatment reduced the decline in intestinal mucosal sIgA levels induced by cyclophosphamide and stimulated the secretion of IL-4, IL-12 and IFN-γ by activating the TLR4-MyD88/TRIF-TRAF6 signaling pathway. These results imply that lycopene could potentially recover the normal immune function of the mucosa.
Thirdly, lycopene could regulate intestinal bacteria. For example, Bifidobacterium and Lactobacillus, which are widely recognized probiotics, exhibit substantial advantages in the treatment of gastrointestinal and systemic disorders [32, 33]. Lycopene could alter the composition of gut microbiome in dextran sodium sulfate (DSS) treated colitis mice by enhancing the relative abundances of Bifidobacterium and Lactobacillus and decreasing the relative abundances of the Proteobacteria [28]. In vitro Fermentation Model, an increasing abundance of Bacteroidetes and Proteobacteria was observed following lycopene treatment in comparison to the blank control group [34]. In addition, bacterial metabolites could have an impact on intestinal function. Short-chain fatty acids (SCFAs) are important metabolites produced by intestinal microorganisms that play a crucial role in preserving normal colon function, as well as maintaining the typical morphology and features of colonic epithelial cells. Zhao et al. [28] showed that lycopene could increase the levels of SCFAs such as acetate, iso butyric acid, valeric acid, and isovaleric acid in colitis mice. These results support a positive role for carotenoids in intestinal health.
Consequently, lycopene may promote intestinal health and ultimately ameliorate chronic constipation by rectifying intestinal microbial disorders, mitigating inflammation and oxidative stress, and maintaining normal intestinal mucosal epithelial barrier function.
α-Carotene (pro-vitamin A) is more abundant in carrots and pumpkins [32]. Similar to lycopene, it also has anti-inflammatory and antioxidant properties, but its influence on intestinal function has received limited investigation. A multi-ethnic cohort study reported that participants consuming carotenoids-rich fruits and vegetables may exhibit a higher alpha diversity of intestinal flora [36]. Similarly, the levels of dietary and plasma concentrations of α-carotene in pregnant women were positively associated with the alpha diversity of their fecal microbiota. Furthermore, the beta diversity of the gut microbiota displayed significant variations in relation to reported consumption of carotenoid-containing foods and the plasma levels of α-carotene. However, these findings may be driven by enhanced overall diet quality or elevated fiber intake, rather than a specific impact of carotenoids [37]. Therefore, further examinations regarding its impacts and potential mechanisms on gut function are warranted.
In this study, we observed that the protective effects of lycopene and α-carotene on constipation exhibit gender differences. Hormonal and metabolic variations between men and women may contribute to these disparities. First, Women’s hormonal fluctuations throughout the menstrual cycle can impact gastrointestinal motility and transit time [38], which may mask or diminish the effects of lycopene on constipation, while hormones like estrogen may interact with α-carotene [39, 40], leading to a distinct association. Second, men and women exhibit differences in the absorption, metabolism, and utilization of carotenoids [41], which may influence their effects on gastrointestinal health. We did not observe the associations between constipation and other carotenoids in this study which could be attributed to several factors, including differences in their structure, environmental polarity, absorption, metabolism, bioavailability, and interactions with gastrointestinal health [41,42,43].
There are several strengths of this research. Primarily, it is the first study to assess the association between dietary carotenoids intake and constipation among both genders, effectively addressing a void in existing research in this field. Furthermore, we utilized large sample data from NHANES with proper sample weights, which can be representative of the non-institutional population of the United States. Additionally, we conducted adjustments for potential confounding variables that would be linked to carotenoids and constipation, thereby enhancing the credibility of our study findings. Consequently, our research outcomes hold promise in offering novel insights into dietary strategies for managing constipation.
The study also has some limitations. First, NHANES collected cross-sectional data, so the causal relationship between increasing dietary carotenoids intake and constipation cannot be determined. Second, recall bias is unavoidable because dietary data are acquired from 2-day dietary questionnaires. Third, we only calculated the carotenoids in dietary and supplements, without taking into account their bioavailability. Fourth, the potential influence of medication usage, such as antidepressants, on the occurrence of constipation was not accounted for due to limited access to complete data. As a consequence, it is necessary to conduct more basic and clinical studies in the future to confirm what we found and investigate the mechanisms involved.
Conclusions
Overall, we found that dietary lycopene intake was a protective factor against constipation among men, whereas α-carotene intake was negatively associated with the prevalence of constipation among women. These results may offer people the opportunity to better manage their disease by changing their diet.
Data Availability
The datasets generated and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- BSFS:
-
Bristol stool form scale
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BHQ:
-
Bowel health questionnaires
- MEC:
-
Mobile Examination Center
- BMI:
-
Body mass index
- PIR:
-
Poverty-income ratio
- HEI:
-
Healthy eating index
- SE:
-
Standard errors
- GAM:
-
Generalized additive model
References
Barberio B, Judge C, Savarino EV, Ford AC. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. lancet Gastroenterol Hepatol. 2021;6(8):638–48.
Forootan M, Bagheri N, Darvishi M. Chronic constipation: a review of literature. Medicine. 2018;97(20):e10631.
Bharucha AE, Wald A. Chronic constipation. Mayo Clin Proc. 2019;94(11):2340–57.
Wang C, Zhang L, Li L. Association between Selenium Intake with chronic constipation and chronic diarrhea in adults: findings from the National Health and Nutrition Examination Survey. Biol Trace Elem Res. 2021;199(9):3205–12.
Zhang L, Du Z, Li Z, Yu F, Li L. Association of dietary magnesium intake with chronic constipation among US adults: evidence from the National Health and Nutrition Examination Survey. Food Sci Nutr. 2021;9(12):6634–41.
Zhao X, Wang L, Quan L. Association between dietary phosphorus intake and chronic constipation in adults: evidence from the National Health and Nutrition Examination Survey. BMC Gastroenterol. 2023;23(1):24.
Taba Taba Vakili S, Nezami BG, Shetty A, Chetty VK, Srinivasan S. Association of high dietary saturated fat intake and uncontrolled diabetes with constipation: evidence from the National Health and Nutrition Examination Survey. Neurogastroenterol Motil. 2015;27(10):1389–97.
Yang S, Wu XL, Wang SQ, Guo XL, Guo FZ, Sun XF. Association of Dietary Energy Intake with Constipation among Men and Women: results from the National Health and Nutrition Examination Survey. Front Nutr. 2022;9:856138.
Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174(11):1290–324.
Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–16.
Max, Leenders Anke M., Leufkens Peter D., Siersema Fränzel J.B., van Duijnhoven Alina, Vrieling Paul J.M., Hulshof Carla H., van Gils Kim, Overvad Nina, Roswall Cecilie, Kyrø Marie-Christine, Boutron-Ruault Guy, Fagerhazzi Claire, Cadeau Tilman, Kühn Theron, Johnson Heiner, Boeing Krasimira, Aleksandrova Antonia, Trichopoulou Eleni, Klinaki Anna, Androulidaki Domenico, Palli Sara, Grioni Carlotta, Sacerdote Rosario, Tumino Salvatore, Panico Marije F., Bakker Guri, Skeie Elisabete, Weiderpass Paula, Jakszyn Aurelio, Barricarte José, María Huerta Esther, Molina-Montes Marcial, Argüelles Ingegerd, Johansson Ingrid, Ljuslinder Timothy J., Key Kathryn E., Bradbury Kay-Tee, Khaw Nicholas J., Wareham Pietro, Ferrari Talita, Duarte-Salles Mazda, Jenab Marc J., Gunter Anne-Claire, Vergnaud Petra A., Wark H. Bas, Bueno-de-Mesquita Plasma and dietary carotenoids and vitamins A C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition International Journal of Cancer 2014;135(12):2930–2939 https://doi.org/10.1002/ijc.v135.12, https://doi.org/10.1002/ijc.28938
Carotenoids and their role in cancer prevention Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2020;1865(11):158613 https://doi.org/10.1016/j.bbalip.2020.158613
Lycopene Lutein and Zeaxanthin May Reduce Faecal Blood Mucus and Pus but not Abdominal Pain in Individuals with Ulcerative Colitis Nutrients 2016;8(10):613 https://doi.org/10.3390/nu8100613
Glabska D, Guzek D, Zakrzewska P, Lech G. Intake of Lutein and Zeaxanthin as a possible factor influencing gastrointestinal symptoms in caucasian individuals with Ulcerative Colitis in Remission Phase. J Clin Med 2019, 8(1).
Ayati Z, Ramezani M, Amiri MS, Sahebkar A, Emami SA. Genus Rosas: a review of Ethnobotany, Phytochemistry and traditional aspects according to Islamic Traditional Medicine (ITM). Adv Exp Med Biol. 2021;1308:353–401.
Tang R, Zhang J, Nan H, Lv R, Chen X, Liu Y, Wang X, Wang L. Exploring Molecular Mechanisms of Aloe barbadmsis Miller on Diphenoxylate-Induced Constipation in mice. Evid Based Complement Alternat Med. 2022;2022:6225758.
Dore MP, Pes GM, Bibbò S, Tedde P, Bassotti G. Constipation in the elderly from Northern Sardinia is positively associated with depression, malnutrition and female gender. Scand J Gastroenterol. 2018;53(7):797–802.
Włodarczyk J, Waśniewska A, Fichna J, Dziki A, Dziki Ł, Włodarczyk M. Current overview on Clinical Management of Chronic Constipation. J Clin Med 2021, 10(8).
Cardinault N, Tyssandier V, Grolier P, Winklhofer-Roob BM, Ribalta J, Bouteloup-Demange C, Rock E, Borel P. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur J Nutr. 2003;42(6):315–23.
Liu Q, Kang Y, Yan J. Association between overall dietary quality and constipation in american adults: a cross-sectional study. BMC Public Health. 2022;22(1):1971.
Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res. 2006;51:99–164.
Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: fantasy or reality? Proc Nutr Soc. 2017;76(2):122–9.
Pirayesh Islamian J, Mehrali H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: an overview. Cell J. 2015;16(4):386–91.
Wang Y, Zhao H, Liu Y, Li J, Nie X, Huang P, Xing M. Environmentally relevant concentration of sulfamethoxazole-induced oxidative stress-cascaded damages in the intestine of grass carp and the therapeutic application of exogenous lycopene. Environ Pollut. 2021;274:116597.
Liu A, Chen X, Huang Z, Chen D, Yu B, Chen H, He J, Yan H, Zheng P, Yu J, et al. Effects of dietary lycopene supplementation on intestinal morphology, antioxidant capability and inflammatory response in finishing pigs. Anim Biotechnol. 2022;33(3):563–70.
Li Y, Pan X, Yin M, Li C, Han L. Preventive effect of Lycopene in Dextran Sulfate Sodium-Induced Ulcerative Colitis mice through the regulation of TLR4/TRIF/NF-kappaB signaling pathway and tight junctions. J Agric Food Chem. 2021;69(45):13500–9.
Saada HN, Rezk RG, Eltahawy NA. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother Res. 2010;24(Suppl 2):204–8.
Zhao B, Wu J, Li J, Bai Y, Luo Y, Ji B, Xia B, Liu Z, Tan X, Lv J, et al. Lycopene alleviates DSS-Induced colitis and behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J Agric Food Chem. 2020;68(13):3963–75.
Rajanna M, Bharathi B, Shivakumar BR, Deepak M, Prashanth D, Prabakaran D, Vijayabhaskar T, Arun B. Immunomodulatory effects of Andrographis paniculata extract in healthy adults - an open-label study. J Ayurveda Integr Med. 2021;12(3):529–34.
Ikiz O, Kahramansoy N, Erkol H, Kocoglu E, Firat T. Effects of Lycopene in Intestinal Ischemia Reperfusion Injury via Intestinal Immunoglobulin A. J Surg Res. 2021;267:63–70.
Pan X, Niu X, Li Y, Yao Y, Han L. Preventive Mechanism of Lycopene on Intestinal Toxicity Caused by Cyclophosphamide Chemotherapy in Mice by Regulating TLR4-MyD88/TRIF-TRAF6 Signaling Pathway and Gut-Liver Axis. Nutrients 2022, 14(21).
O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. 2016;7:925.
Zhang Z, Lv J, Pan L, Zhang Y. Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol. 2018;102(19):8135–43.
Dai Z, Li Z, Shi E, Nie M, Feng L, Chen G, Gao R, Zeng X, Li D. Study on the Interaction between four typical Carotenoids and Human Gut Microflora using an in Vitro Fermentation Model. J Agric Food Chem. 2022;70(42):13592–601.
Zhao Z, Chen J, Ci F, Pang H, Cheng N, Xing A. alpha-Carotene: a valuable carotenoid in biological and medical research. J Sci Food Agric. 2022;102(13):5606–17.
Frankenfeld CL, Hullar MAJ, Maskarinec G, Monroe KR, Shepherd JA, Franke AA, Randolph TW, Wilkens LR, Boushey CJ, Le Marchand L, et al. The gut microbiome is Associated with circulating dietary biomarkers of Fruit and Vegetable Intake in a multiethnic cohort. J Acad Nutr Diet. 2022;122(1):78–98.
Schmidt KM, Haddad EN, Sugino KY, Vevang KR, Peterson LA, Koratkar R, Gross MD, Kerver JM, Comstock SS. Dietary and plasma carotenoids are positively associated with alpha diversity in the fecal microbiota of pregnant women. J Food Sci. 2021;86(2):602–13.
Judkins TC, Dennis-Wall JC, Sims SM, Colee J, Langkamp-Henken B. Stool frequency and form and gastrointestinal symptoms differ by day of the menstrual cycle in healthy adult women taking oral contraceptives: a prospective observational study. BMC Womens Health. 2020;20(1):136.
Ombra MN, Di Santi A, Abbondanza C, Migliaccio A, Avvedimento EV, Perillo B. Retinoic acid impairs estrogen signaling in breast cancer cells by interfering with activation of LSD1 via PKA. Biochim Biophys Acta. 2013;1829(5):480–6.
Saumet A, Vetter G, Bouttier M, Antoine E, Roubert C, Orsetti B, Theillet C, Lecellier CH. Estrogen and retinoic acid antagonistically regulate several microRNA genes to control aerobic glycolysis in breast cancer cells. Mol Biosyst. 2012;8(12):3242–53.
Moran NE, Mohn ES, Hason N, Erdman JW Jr, Johnson EJ. Intrinsic and extrinsic factors impacting absorption, metabolism, and Health Effects of Dietary Carotenoids. Adv Nutr (Bethesda Md). 2018;9(4):465–92.
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430(1):37–48.
Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem. 2013;70:102–10.
Acknowledgements
The authors express their gratitude to the NHANES participants and staff members for their valuable contribution to data collection and for their efforts in making the data publicly accessible.
Funding
This work is supported by the National Natural Science Foundation of China (Grant number: 71964021) and the Foundation of The First Hospital of Lanzhou University, China (Grant number: ldyyyn2021-59).
Author information
Authors and Affiliations
Contributions
JW, WK, YoZ and YaZ conceptualized the study. JW, YaZ, ML and YW collected, analyzed and interpreted the data. JW wrote the draft manuscript. WK, YoZ and YaZ critically commented the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The First Hospital of Lanzhou University in Gansu Province did not conduct an ethical review of this study because the data was obtained from a publicly available database. All participants provided written informed consent at the time of the survey. All procedures were conducted in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Kong, W., Liu, M. et al. Association between dietary carotenoids intake and chronic constipation in American men and women adults: a cross-sectional study. BMC Public Health 23, 1597 (2023). https://doi.org/10.1186/s12889-023-16367-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16367-3