Abstract
Use of nanoparticles have established benefits in a wide range of applications, however, the effects of exposure to nanoparticles on health and the environmental risks associated with the production and use of nanoparticles are less well-established. The present study addresses this gap in knowledge by examining, through a scoping review of the current literature, the effects of nanoparticles on human health and the environment. We searched relevant databases including Medline, Web of Science, ScienceDirect, Scopus, CINAHL, Embase, and SAGE journals, as well as Google, Google Scholar, and grey literature from June 2021 to July 2021. After removing duplicate articles, the title and abstracts of 1495 articles were first screened followed by the full-texts of 249 studies, and this resulted in the inclusion of 117 studies in the presented review.
In this contribution we conclude that while nanoparticles offer distinct benefits in a range of applications, they pose significant threats to humans and the environment. Using several biological models and biomarkers, the included studies revealed the toxic effects of nanoparticles (mainly zinc oxide, silicon dioxide, titanium dioxide, silver, and carbon nanotubes) to include cell death, production of oxidative stress, DNA damage, apoptosis, and induction of inflammatory responses. Most of the included studies (65.81%) investigated inorganic-based nanoparticles. In terms of biomarkers, most studies (76.9%) used immortalised cell lines, whiles 18.8% used primary cells as the biomarker for assessing human health effect of nanoparticles. Biomarkers that were used for assessing environmental impact of nanoparticles included soil samples and soybean seeds, zebrafish larvae, fish, and Daphnia magna neonates.
From the studies included in this work the United States recorded the highest number of publications (n = 30, 25.64%), followed by China, India, and Saudi Arabia recording the same number of publications (n = 8 each), with 95.75% of the studies published from the year 2009. The majority of the included studies (93.16%) assessed impact of nanoparticles on human health, and 95.7% used experimental study design. This shows a clear gap exists in examining the impact of nanoparticles on the environment.
Highlights
• While nanoparticles are beneficial in a range of applications, they pose significant threats to humans and the environment.
• Immortalised cell lines are mostly used as biomarkers to assess human health effect of nanoparticles.
• Biomarkers such as soil samples and zebrafish larvae are used to investigate the environmental effect of nanoparticles.
• This work has revealed the toxic effects of nanoparticles to include production of oxidative stress, DNA damage, apoptosis, cell death, and induction of inflammatory responses.
Similar content being viewed by others
Introduction
Importance and meaning
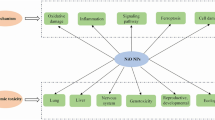
Nanoparticles are small particles ranging from 1 to 100 nm (nm) in size [1]. They are used in a wide range of applications and can be grouped into four types: 1) inorganic-based nanoparticles, 2) carbon-based nanoparticles, 3) organic/polymer nanoparticles, and 4) composite-based nanoparticles [2]. Inorganic-based nanoparticles are made up of different metal and metal oxides. Examples of metal-based inorganic nanoparticles include aluminium, silver, gold, zinc, lead, iron, cadmium, and copper, whereas examples of metal oxide-based inorganic nanoparticles include aluminium oxide, copper oxide, iron oxide, silica, zinc oxide, titanium oxide, and magnesium aluminium oxide. Carbon-based nanoparticles include fullerene, graphene, multi- and single-walled carbon nanotubes, carbon black, and carbon fibres. Organic-based nanoparticles are derived from organic materials without carbon, for example, liposome, dendrimers, cyclodextrin, and micelle, whereas composite nanoparticles are made from combinations of metal oxide-based, metal-based, organic-based, and/or carbon-based nanoparticles.
In recent years, nanoparticles have gained increasing attention due to their use in consumer products, medicine, soil, and aquatic environments. For example, nanoparticles have been used for textiles [3], water treatment [4], environmental remediation [5,6,7], cancer therapy [8], radiology [9], and cosmetics [10]. This growing attention and extensive usage of nanoparticles is due to specific novel characteristics exhibited by such particles, which results from their small size and large surface area [11]. These unique qualities, while advantageous, pose certain risks to living organisms.
The harmful effects of nanoparticles
The small sizes of nanoparticles give them the ability to permeate physiological barriers of living organisms, causing harmful biological reactions. Nanoparticles are known to enter the human body through the lung, intestinal tract, or skin, and can be toxic to the brain, cause lung inflammation and cardiac problems [12]. In fact, certain nanoparticles have been found to cause permanent cell damage through organ injury and oxidative stress, due to their size and composition. In a study by Magrez et al. [13] to assess the toxic effect of carbon-based nanoparticles on lung cancer cells, the authors reported findings suggesting that carbon-based nanoparticles cause size-dependent cytotoxicity. The level of toxicity of nanoparticles is suggested to be dependent on factors such as composition of the nanoparticle, size, surface functionality, crystallinity, and aggregation [14]. Moreover, the toxicity of a nanoparticle in an individual is dependent on the genetic make-up of that individual, which is determined by the individual's ability to adapt and respond to toxic substances.
The gaps of previous studies
There are growing concerns regarding the toxicologic effects of nanoparticles, and frequent exposure to nanoparticles is regarded as a public health threat [15]. While there is extensive evidence about the benefits of nanoparticles, as well as the potential health and environmental risks associated with its production and use, current understanding of the impact of nanoparticles exposure to human health and the environment is limited. The current review seeks to explore, through a scoping review of the current literature, the effects of nanoparticles on human health and the environment. This review is unique as it adopts a systematic scoping approach to explore the current literature on the health risks posed by the manufacture, distribution, and use of nanoparticles. Published studies in this area have mainly used a narrative literature review approach [2, 16, 17].
Objective and research questions
The objective of this review is to map the distribution of the current literature on the human and environmental impacts of nanotoxicity. Specifically, this scoping review will be guided by the following research questions:
-
1.
What is the relative distribution of the current literature on the human and environmental impact of nanotoxicity?
-
2.
Which exposure pathways and nanoparticles have been researched and which have not?
-
3.
What biomarkers have been used in assessing the human and environmental impact of exposure to nanoparticles?
Methods
This scoping review was conducted and reported in accordance with the Joanna Briggs Institute Reviewers Manual [18]. The following steps were followed:
-
1.
Defining and aligning the objectives and research questions
-
2.
Developing and aligning the inclusion criteria with the objectives and research questions
-
3.
Describing the planned approach to evidence searching and selection
-
4.
Searching for the evidence
-
5.
Extracting the evidence
-
6.
Charting the evidence
-
7.
Summarising the evidence in relation to the objectives and research questions
The Preferred Reporting of Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was used to summarise the screening process. The protocol of this review has been registered with the Open Science Framework [19].
Search strategy
The aim of the search strategy was to find both published and unpublished studies that have examined the effect of nanotoxicity on human health and the environment. Search terms consisted of a combination of key terms and concepts in the objective and research questions, using the Boolean operators, 'AND', and 'OR' as follows:
(nanomaterials OR nanoparticles OR nanostructures) AND (toxicity OR health) AND (“biomarker* of exposure” OR biomarker OR exposure) AND (human OR environment).
The search was limited to peer-reviewed articles published from the year 2000. This was to enable us to study the current literature (research conducted over the last 2 decades). The search was limited to primary studies published in the English language due to difficulties with language translation.
Table 1 below presents a list of the databases, grey literature, and search engines that were searched for eligible papers. The reference list of all included papers was also searched for additional papers on the subject matter.
For the database searches, a master search strategy was first developed using the Medline database, this was then modified for the other databases. The supplementary material file presents the Medline search history. The literature search was conducted between 1st June 2021 and 31st July 2021.
Reference management
All search results were imported into an Endnote library to help manage references and to remove duplicate articles. Once duplicates were removed, the search results were exported from Endnote into Covidence (a web-based software platform that streamlines the production of scoping/systematic reviews) for screening. The Covidence software was also useful in identifying and deduplicating articles that could not be identified by Endnote.
Selection criteria
The following criteria were used to identify eligible articles for inclusion in the review.
Inclusion criteria
Types of participants
Studies that have assessed the human and environmental impacts of nanotoxicity were considered for inclusion in this review. Human participants included children and/or adults of any age, gender, or ethnicity. Studies involving the use of animals as biomarkers for assessing the environmental impact of nanotoxicity were also considered for inclusion.
Concept
Studies that have examined the impacts of nanotoxicity as well as the biomarkers for assessing exposure to nanoparticles were eligible for inclusion in this review. While all types of nanoparticles were considered for inclusion, attention was given to studies involving metallic (oxides, pure metal) and carbonaceous (fullerenes, carbon nanotubes, and graphene) nanoparticles. This is mainly due to these particles being widely produced and used [20], therefore, they are considered the most relevant for public health.
Context
Studies from any geographical location aimed at assessing the human and/or environmental impact of nanotoxicity were considered eligible for inclusion. Studies whose full texts were in a language other than English were excluded because there were no available translators.
Study types
We included all original primary research (both quantitative and qualitative), including, but not limited to randomised controlled studies, quasi-experimental studies, surveys, retrospective and prospective cohort studies, case studies, and phenomenological studies.
Exclusion criteria
The following exclusion criteria were applied to the title and abstract, as well as the full-text review stage:
-
Irrelevant problem/focus: studies that have not examined the human and/or environmental impact of nanotoxicity, or the biomarkers for assessing exposure to nanoparticles
-
Irrelevant type of study: review reports or studies that did not contain any original research
Selection of studies
We employed a two-step screening process to assess search results for eligible studies. The first level involved screening of the titles and abstracts and was done independently by two reviewers (EK and RF). The next step was carried out independently by three reviewers (EK, RF, and SH) and involved screening of the full-texts of potentially eligible papers. Disagreements between reviewers were resolved through discussions and consensus. Where disagreements persisted, a third reviewer (TP or FVZ) was consulted.
Data charting
We developed a standardised data extraction form in the Covidence software for data extraction. The form was designed to collect the following information from included studies: year of publication, aim/objective of study, study design, country, type of nanoparticle, application of the nanoparticle, major exposure route(s), biomarker/model used, how biomarker was obtained, and study outcome(s).
The developed data extraction form was pilot-tested using 10% of the included articles before beginning the actual data extraction. Data extraction was done by one reviewer (PB, RF, or SH) and verified by another (EK, TP, or FVZ), using the Covidence software.
Data synthesis
The extracted data was first exported into Excel for editing and to check for accuracy. The edited data was then exported from Excel into SPSS (version 26) to aid with data synthesis. Descriptive statistics was used to report included studies by their characteristics and outcome measures, described below.
Characteristics of included studies
-
Year of publication: studies were grouped based on their year of publication. As stated earlier, this included studies published from the year 2000 to July 2021 (the date of completion of literature searches).
-
Country in which study was conducted: to assess the distribution of the current literature on human and environmental impact of nanotoxicity, the countries in which eligible studies were conducted were classified into six regions based on the World Bank’s classification of countries. This included: East Asia and Pacific, Europe and Central Asia, Latin America and Caribbean, Middle East and North Africa, North America, South Asia, and Sub-Saharan Africa (World Bank Group, 2018).
-
Study design: randomised controlled trial, non-randomised controlled trial, cohort study, experimental study, case control study, longitudinal study, uncontrolled before and after studies.
-
Impact/effect assessed: human health and/or environment
Outcome measures
-
Type of nanoparticle: This was divided into four groups: 1) inorganic-based nanoparticles, 2) carbon-based nanoparticles, 3) organic nanoparticles, and 4) composite-based nanoparticles two groups, metallic (oxides, pure metal) and carbonaceous (fullerenes and carbon nanotubes) particles.
-
Biomarker or model used in assessing human and/or environmental exposure: primary cell or immortalised cell line
-
Effect/impact on human health and/or the environment
A narrative synthesis was then used to further explore findings.
Results
Search results
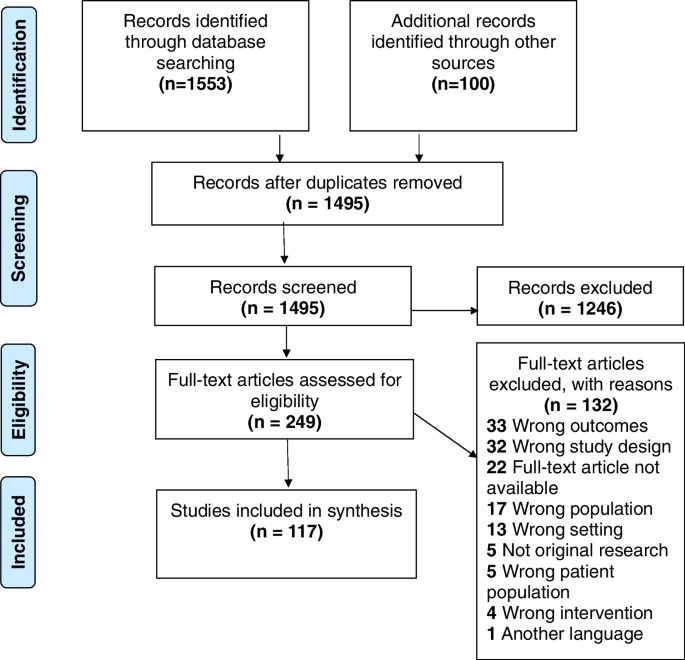
The database searches resulted in 1553 papers (presented in Fig. 1): Medline (n = 1,381); ScienceDirect (n = 0); Sage Journals Online (n = 50); Campbell Collaboration (n = 0); Cochrane Collaboration (n = 0); Embase (n = 5); Scopus (n = 6); Web of Science (n = 50); CINAHL (n = 61). Google and Google Scholar searches yielded 100 results, and no article was obtained from grey literature searches. Following removal of duplicate articles, the titles and abstracts of 1495 articles were screened to assess their eligibility for inclusion, which resulted in the exclusion of a total of 1246 articles as they did not meet the inclusion criteria. As such, the full texts of 249 articles were assessed for eligibility. Following this stage, a total of 132 articles were excluded for several reasons (see Fig. 1), whereas 117 studies qualified for inclusion in the review.
Characteristics of included studies
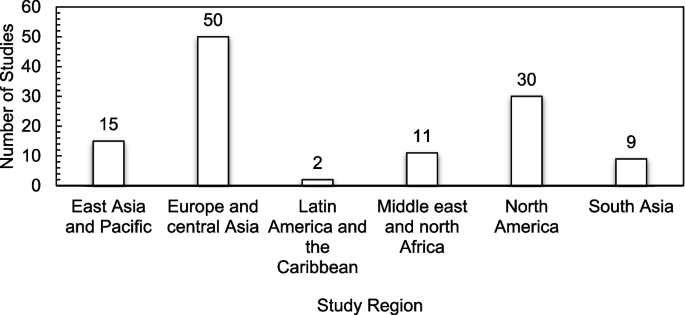
The studies included in this review originated from 23 countries across several continents, with the majority of the studies originating from Europe and Central Asia (n = 50). Nevertheless, the United Sates recorded the highest number of publications (n = 30), followed by China, India, and Saudi Arabia recording the same number of publications (n = 8). The lowest number of studies (n = 1 each) originated from Argentina, Czech Republic, Egypt, Mexico, Pakistan, Poland, and Russia. There were no studies recorded from Sub-Saharan Africa. Figure 2 presents a classification of the included studies by region.
Included studies were published between the year 2006 and 2021, with a high proportion of the articles (95.75%) published from the year 2009. However, the year 2020 recorded the highest number of publications (n = 15; 12.82%), followed by 2016 (n = 14; 11.97%). Table 2 below presents the number of publications per year.
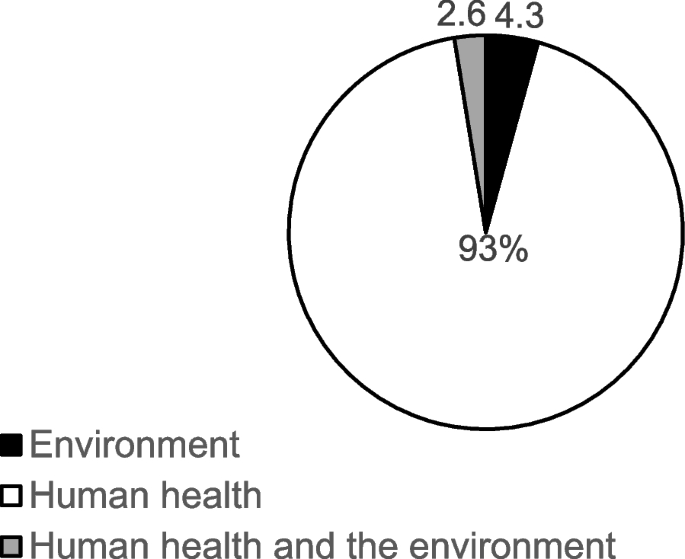
The majority of the studies used an experimental study design (n = 112, 95.7%), with only 5 (4.3%) studies employing a cross-sectional design. Regarding the type of impact/effect of nanoparticle assessed, a vast majority of the studies assessed impact on human health (n = 109), 5 of the studies assessed effects on the environment, with only 3 studies assessing both human and environmental health impact (Fig. 3).
Outcome measures
Just over 65% (n = 77) of the included studies investigated the human and/or environmental effect of inorganic-based nanoparticles. The inorganic-based nanoparticles that were investigated include, but not limited to, bismuth oxide (Bi2O3), silicon dioxide (SiO2), copper oxide (CuO), zinc oxide (ZnO), titanium dioxide (TiO2), silver (Ag), gold (Au), platinum (Pt), iron oxide (Fe2O3), cerium oxide (CeO2), cobalt oxide (Co3O4), aluminium oxide (Al2O3), molybdenum trioxide (MoO3), magnesium oxide (MgO), nickel oxide (NiO), chromium oxide (Cr2O3), tungsten oxide (WO3), yttrium oxide (Y2O3), and manganese oxide (Mn2O3).
Thirty-five (29.9%) studies reported on carbon-based nanoparticles (including single and multi-walled carbon nanotubes (SWCNTs/MWCNTs), graphene oxide (GO), and graphene nanoplatelets, GNP). Three studies [21,22,23] reported on both inorganic- and carbon-based nanoparticles; one study [24] reported on both inorganic-based and polymer nanoparticles (i.e., Titanium dioxide, terbium-doped gadolinium, and polylactic-co-glycolic acid, PLGA), whereas another study [25] investigated the effect of Poly lactic-co-glycolic acid (a polymer) nanoparticle on the environment.
The most investigated nanoparticles were ZnO (n = 25), followed by MWCNTs (n = 20), TiO2 (n = 16), CeO (n = 15), SWCNTs and Fe2O3 (n = 14), and SiO2 (n = 12). The least studied nanoparticles include Pt, Au, MgO, MoO3, WO3, Carbon Black (CB), and GNP with only one report available.
A significant number (n = 90, 76.9%) of the included studies used immortalised cell lines as the biomarker for assessing the human health effect of nanoparticles. Examples of the immortalised cell lines that were used include the human hepatocarcinoma cell line (HepG2), human (alveolar) epithelial A549 cell line with human monocyte-derived dendritic cells (MDDCs) and macrophages (MDMs), Melanoma cells and human foreskin fibroblasts, human airway epithelial (BEAS-2B) cells, human bronchial epithelium (BEAS-2B) cells, human neuroblastoma SHSY5Y cell line, human keratinocyte (HaCaT) cell line, and MCF-7 cell line, which is a human breast cancer cell line with oestrogen, progesterone and glucocorticoid receptors. Immortalised cell lines were mostly purchased/obtained from organisations such as the American Type Culture Collection (ATCC, Manassas, VA, USA).
Twenty-two studies used primary cells obtained from study participants/volunteers. Examples of the primary cells that were used as biomarkers by included studies are human bone marrow mesenchymal stem cells (hBMMSCs) taken from the iliac crest of human donors, human lymphocytes (blood), and human dermal fibroblasts which were isolated by the outgrowth method using infant foreskins obtained after circumcision. Workplace air samples have also been used to investigate workplace exposures to graphene nanoplatelets [26]. Five studies [25, 27,28,29,30] that reported on the environmental effect of nanoparticles used a variety of biomarkers, including soil samples and soybean seeds, Allium cepa bulbs, zebrafish larvae, seedlings of buckwheat, Nitrosomonas europaea KCTC 12270 bacterium (an ammonia-oxidizing bacterium) and Nitrospira moscoviensis (a nitrite-oxidizing bacterium), as well as aquatic species including Daphnia magna neonates, fish, and Carp (Cyprius carpio). The studies included in this review reported several toxicities associated with the production and application of nanoparticles. The most reported health impact of nanoparticles was found to be decreased cell viability and/or cell death (observed by twenty-nine studies). Twenty-eight studies also noted reactive oxygen species generation as a result of exposure to nanoparticles, especially to CNT (n = 7), ZnO (n = 7), SiO2 (n = 5), and TiO2 (n = 4). The third commonly observed health impact was dose-dependent oxidative stress in the biomarkers (n = 25), particularly, in cases of exposure to SiO2 (n = 5), ZnO (n = 5), Fe3O4 (n = 4), CeO2 (n = 3), and CuO (n = 3). In addition, there were sixteen reports regarding DNA damage after exposure to nanoparticles, mainly for ZnO (n = 4) and MWCNTs (n = 3). Table 3 presents a comprehensive outline of the effects (human health and the environment) reported by each of the included studies. These are further explored in the ensuing section.
Discussion
The objective of this scoping review was to ascertain the distribution of the current literature on the human and environmental impacts of nanoparticles. Specifically, in this review, we synthesised evidence regarding the exposure pathways and types of nanoparticles that have been researched and the ones that have not, as well as the biomarkers that have been used in assessing human and environmental impact of exposure to nanoparticles.
Characteristics of included studies
While the majority of studies originated from Europe and Central Asia, the United States of America (USA) alone recorded the highest number of publications. This finding is not surprising, as the USA has continuously fostered the development of nanotechnology through significant investments in research and development in this area. In 2016, the USA was projected to account for almost one-third of total global nanotechnology research funding [136]. Moreover, the USA and the European Union have over the years taken a committed approach towards enhancing the health and safety of nanoparticles [137]. As part of this commitment, annual meetings are held, where researchers discuss topics relating to nano-safety, as well as funding priorities and research needs.
While there have been some investments in nanotechnology research in African countries (including Egypt and South Africa), a recent publication by the United Nations Economic Commission for Africa (UNECA) indicates that the African continent, relative to other continents, is lagging behind with regards to nanotechnology research [138]. This assertion is consistent with the findings of this review, which found only one study originating from North Africa (Egypt), with no study conducted in Sub-Saharan Africa.
Over the past two decades, there have been increasing public awareness of nanotechnology and a growing concern about its commercial applications [139]. This has led to rapidly increasing scientific publications in this field, especially from early 2000s [140]. It is, therefore, not surprising that the studies included in this scoping review were published from the year 2006. Indeed, a literature search of nanotechnology publications by Huang et al. [140] revealed over 50,000 publications for the year 2006.
Although the included studies investigated a wide range of nanoparticles, most of them focused on inorganic-based nanoparticles (e.g., zinc oxide, titanium dioxide, copper oxide, and silica), followed by carbon-based nanoparticles (e.g., carbon-nanotubes, fullerenes, and graphene) (Table 3). This finding is consistent with previous reviews that have reported extensive investigation into the impact of inorganic-based and/or carbon-based nanoparticles [141, 142]. These nanoparticles may have gained attention due to their extensive production and usage. In addition to their use for cancer treatment, inorganic and carbon-based nanoparticles provide significant benefits in photothermal therapy, diagnosis, tissue engineering, imaging contrast agents, and sensing applications [143]. This is due to their unique physical and chemical properties (such as electrical, thermal, structural, mechanical, and optical diversity), which make them stronger, flexible, and more electrically conductible towards several biological entities [141, 144]. The advantages of, for example inorganic-based nanoparticles, including their high reactivity, small size and good capacity have been found to induce adverse harmful effects in both humans and the environment.
In this review, a number of approaches were used by included studies to assess the toxicity of nanoparticles. However, the majority of the studies applied the in vitro method, perhaps because in vitro studies are time saving and cost-effective. Nonetheless, the in vitro approach has been criticised by researchers (e.g., Bahadar et al. [145]) for producing varying results in different laboratories.
The included studies used differing methods in assessing cytotoxicity and genotoxicity: cell membrane integrity was assessed with Lactate dehydrogenase (LDH) assays [44, 57, 116]; cell viability was assessed using tetrazolium reduction assays [82, 83, 90, 116]; apoptosis was assessed using immunohistochemistry biomarkers [60, 65, 86]; electron microscopy was used to assess intracellular localisation of nanoparticles [34, 106]; and cell inflammation was estimated using chemokines biomarkers (i.e., IL-8, TNF- α, and IL-6) [146]. Compounds such as MTT, XTT, MTS, and WST-1 are used to detect viable cells [147]. However, in the current review, most of the studies employed MTT tetrazolium assays for investigating cell toxicity [47, 49, 50, 58, 116]. Similar findings have been reported by Bahadar et al. [145] who conducted a review on the toxicity of nanoparticles.
The human impact of nanoparticles
Most of the studies in this review focused on assessing the characteristics of nanoparticles, as well as the impact of nanoparticles on, particularly, human health. In recent years, there have been promising results from the application of nanoparticles to human health, especially in cancer treatment. This is due to the potential of nanoparticles to provide innovative solutions to curb the limitations of traditional treatment methods, including radiotherapy and chemotherapy [148]. Relative to conventional cancer treatment methods, nanoparticle-based drug delivery systems have been shown to have significant advantages in a) drug resistance, b) correctly targeting tumour cells, c) having good pharmacokinetics, and d) reduction of treatment side effects [149]. Notwithstanding these benefits, however, nanoparticles have potential harmful effects, and there are controversies about their safe use in humans [139]. This has undoubtedly led to the rapidly growing number of studies investigating the human health impact of nanoparticles, as was revealed in this review.
The majority of the studies (n = 90) in this review used immortalised cell lines as the biomarker for assessing human health impact of nanoparticles, and only 22 studies used primary cells as biomarkers. Immortalised cell lines have mostly been used for nano-safety studies because, relative to primary cells, they are generally less expensive, readily accessible, and easier to cultivate [150]. However, the type of cell that is used as biomarker for nano-safety studies is of great importance since this may have an impact on the general outcome of studies [151]. Cancer cell lines, for example, have a disturbed anti-apoptotic balance, and have undergone transformation in metabolism, which impacts their ability to sustain their high rate of proliferation [152]. As such, using these cells may have an impact on study findings. Nonetheless, the use of primary cells in nano-safety studies, are not without limitations. Primary cells have limited lifespan in vitro and can suffer from clonal changes.
In using immortalised cell lines, several studies [153, 154]) have reported variations in findings regarding nanoparticle-induced effects in cell lines obtained from different species or tissues. For example, Zhang et al. [153] and Mukherjee et al. [154] investigated the effect of exposure to silver nanoparticle on mammalian cells. Zhang et al. [153] used epithelial cells and microphages, and Mukherjee et al. [154] used the human dermal and cervical cell lines as biomarkers. Mukherjee et al. [154] reported nanoparticle-induced cytotoxicity such as elevated levels of oxidative stress, cell membrane damage, and glutathione depletion, whereas Zhang et al. [153] reported effects including changes in antioxidant defence and metallothionein. Moreover, while Ekstrand-Hammarstrom et al. [155] and Kermanizadeh et al. [156] have compared the effect of nanoparticles on immortalised cell lines versus primary cells of the same species and tissues, available data regarding the relative effectiveness of these two types of cells are unclear. Therefore, it is difficult to make explicit conclusions as to which of these two types of cells can be used as a reliable biomarker for nano-safety studies.
This review has revealed that humans are exposed to nanoparticles through inhalation, ingestion, or dermal route. After their exposure, nanoparticles induce toxic effects such as production of oxidative stress at the exposure site, inflammation, DNA damage, and cell death [87, 88]. For instance, exposure of human neuroblastoma (Sh-sy5y) cells to inorganic nanoparticles, such as titanium dioxide, silica dioxide, and silver are associated with induction of neurotoxicity, membrane damage, reaction oxygen specie formation, decrease in cell viability, and autophagy dysfunction [40]. Similarly, exposure to carbon-based nanoparticles such as single and multi-walled carbon nanotubes reduce cell viability, as well as induce changes in cell structure, cell cycle, and cell-to-cell interactions in human lung epithelial cells (BEAS-2B) [107].
The environmental impact of nanoparticles
The findings of this scoping review indicate a gap in the literature regarding environmental impact of nanoparticles. Out of the 117 included studies, only 5 had assessed the environmental impact of exposure to nanoparticles. This significant gap in the scientific literature has been highlighted by authors such as Bundschuh et al. [157]. The growing production and usage of nanoparticles has undoubtedly led to a diversification of emission sources into both the aquatic and soil environment. Nanoparticles enter the environment mainly through three emission scenarios: a) released during production of nano-enabled products and raw materials, b) during application, and c) following disposal of products containing nanoparticles [158]. These emissions occur either indirectly through systems such as landfills or wastewater treatment plants, or directly to the environment. Nonetheless, nanoparticles are mostly released during the application phase and following disposal [159]. Indeed, during production, only about 2% of the production volume is emitted [160]. The studies in this review used biomarkers such as soil samples and soybean seeds, zebrafish larvae, fish, and Daphnia magna neonates. This finding is in line with a previous review by Bundschuh et al. [157], which explored the effects of nanoparticles on the environment.
Limitations of the review
In this review, every effort was made to reduce bias. The search strategy was developed by experts of the review team with many years of experience in conducting systematic/scoping reviews. A comprehensive search of multiple relevant databases and other resources was conducted by one review author (EAK) and a rerun of the searches was done after 4 weeks of the initial search. Two authors (EAK and RF or PB and SH) independently screened the search results, and disagreements between reviewers were resolved by FVZ or TP.
The main limitation of this review is that the searches were limited to studies published in the English language. This may have led to the exclusion of potentially relevant papers published in other languages. Also, searches were restricted to studies published from the year 2000, which may have led to the omission of potentially relevant papers.
Conclusions
This review has provided an extensive synthesis of the current literature on the effects of nanoparticles on human health and the environment. The review has shown that while nanoparticles are beneficial in a range of applications, they pose significant threats to humans and the environment. Through the use of several biological models and biomarkers (e.g., human bronchial epithelial cells (Beas-2), soil samples, and soybean seeds), the included studies revealed the toxic effects of nanoparticles, with the most investigated nanoparticles being Zinc Oxide, MWCNTs, Titanium Dioxide, Cerium Oxide, SWCNTs, Ferric Oxide, and Silicon Dioxide. The main health impacts of nanoparticles identified in this review are decreased cell viability, cell death, reactive oxygen species generation, production of oxidative stress (dose-dependent), DNA damage, apoptosis, and induction of inflammatory responses.
This review has revealed a significant gap in the scientific literature regarding environmental impact of nanoparticles of all types. Future studies should be directed at investigating the impact of the various types of nanoparticles on the aquatic, terrestrial, and soil environment. The findings from this review have also shown limited data regarding the relative effectiveness of immortalised cell lines and primary cells as biomarkers in nano-safety studies. Future research should focus on evaluating the effectiveness of these two types of cells, in order to determine the cell that can be used as a reliable biomarker for nano-safety studies. There is also the need for future studies in this area to focus on exploring the toxic effects of Platinum, Gold, Magnesium Oxide, Molybdenum Trioxide, Tungsten trioxide, and Carbon Black nanoparticles, as findings from this review has shown that these nanoparticles are least researched. The findings of this review will be useful to policy makers and stakeholders in assessing the potential effects of nanoparticles.
Availability of data and materials
All data generated or analysed during this study are included in this published article and in the presented supplementary material file.
References
Jiao H, Barakat N. Balanced depth and breadth in a new interdisciplinary nanotechnology course. J Educ Technol Syst. 2012;40(1):75–87.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017;12(7):908–31.
Som C, Wick P, Krug H, Nowack B. Environmental and health effects of nanomaterials in nanotextiles and facade coatings. Environ Int. 2011;37:1131–42.
Bottero JY, Rose J, Wiesner MR. Nanotechnologies: Tools for sustainability in a new wave of water treatment processes. Integr Environ Assess Manag. 2006;2:391–5.
Pak T, Archilha NL, de Lima Luz, LF. Nanotechnology-Based Remediation of Groundwater. In: Kumar C, editor. Nanotechnology Characterization Tools for Environment, Health, and Safety. Berlin, Heidelberg: Springer; 2019. p. 145–65.
Pak T, Archilha NL, Al-Imari R. Application of nanotechnology in removal of NAPLs from contaminated aquifers: a source clean-up experimental study. Clean Technol Environ Pol. 2018;20:427–33.
Pak T, Luz LFDL, Tosco T, Costa GSR, Rosa PRR, Archilha NL. Pore-scale investigation of the use of reactive nanoparticles for in situ remediation of contaminated groundwater source. Proc Natl Acad Sci. 2020;117(24):13366–73.
Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: Principles and practice. Br J Cancer. 2008;99:392–7.
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2006;26:3995–4021.
Botta C, Labille J, Auffan M, Borschneck D, Miche H, Cabie M, et al. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities. Environ Pollut. 2011;159:1543–50.
Auffan M, Bottero JY, Chaneac C, Rose J. Inorganic manufactured nanoparticles: How their physicochemical properties influence their biological effects in aqueous environments. Nanomed Nanotechnol Biol Med. 2010;5:999–1007.
Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39.
Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–5.
Mancuso L, Cao G. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicol Mech Methods. 2014;24(7):449–54.
Yokel RA, MacPhail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol. 2011;6:7.
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulation. Beilstein J Nanotechnol. 2018;9:908–31.
Sahu SC, Hayes AW. Toxicity of nanomaterials found in human environment: a literature review. Toxicol Res Appl. 2017;1:2397847317726352.
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. Joanna Briggs Institute. 2020. Available from: https://doi.org/10.46658/JBIMES-20-01
Kumah EA, Zohoori V, Pak T, Harati S, Raoul FD, Boadu P. Effects of nanotechnology: A scoping review of the current literature. OSF; 2021. Available from: osf.io/w67m9.
Boczkowski J, Hoet P. What’s new in nanotechnology? Implications for public health from a brief review of the 2008 literature. Nanotoxicology. 2010;4(1):1–14.
Phuyal S, Kasem M, Rubio L, Karlsson HL, Marcos R, Skaug V, et al. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol Vitro : Int J Publishe Assoc BIBRA. 2017;44:230–40.
Shalini D, Senthilkumar S, Rajaguru P. Effect of size and shape on toxicity of zinc oxide (ZnO) nanomaterials in human peripheral blood lymphocytes. Toxicol Mech Methods. 2018;28(2):87–94.
Simon-Deckers A, Gouget B, Mayne-L’hermite M, Herlin-Boime N, Reynaud C, Carrière M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology. 2008;253(1–3):137–46.
Setyawati MI, Khoo PKS, Eng BH, Xiong S, Zhao X, Das GK, et al. Cytotoxic and genotoxic characterization of titanium dioxide, gadolinium oxide, and poly(lactic-co-glycolic acid) nanoparticles in human fibroblasts. J Biomed Mater Res, Part A. 2013;101(3):633–40.
Nishu SD, Park S, Ji Y, Han I, Key J, Lee TK. The effect of engineered PLGA nanoparticles on nitrifying bacteria in the soil environment. J Ind Eng Chem. 2020;84:297–304.
Lee JH, Han JH, Kim JH, Kim B, Bello D, Kim JK, et al. Exposure monitoring of graphene nanoplatelets manufacturing workplaces. Inhalation Toxicol. 2016;28(6):281–91.
Gaiser BK, Fernandes TF, Jepson M, Lead JR, Tyler CR, Stone V, et al. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ Health. 2009;8(1):S2.
Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res. 2015;774:49–58.
Lee S, Kim S, Kim S, Lee I. Effects of soil-plant interactive system on response to exposure to ZnO nanoparticles. J Microbiol Biotechnol. 2012;22(9):1264–70.
Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci Total Enviro. 2020;738:140240.
Ahamed M, Akhtar MJ, Khan MAM, Alrokayan SA, Alhadlaq HA. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019;216:823–31.
Eom H-J, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro. 2009;23(7):1326–32.
Bengalli R, Colantuoni A, Perelshtein I, Gedanken A, Collini M, Mantecca P, et al. In vitro skin toxicity of CuO and ZnO nanoparticles: Application in the safety assessment of antimicrobial coated textiles. NanoImpact. 2021;21: 100282.
Ferraro SA, Domingo MG, Etcheverrito A, Olmedo DG, Tasat DR. Neurotoxicity mediated by oxidative stress caused by titanium dioxide nanoparticles in human neuroblastoma (SH-SY5Y) cells. J Trace Elem Med Biol. 2020;57: 126413.
Gambardella C, Morgana S, Bari GD, Ramoino P, Bramini M, Diaspro A, et al. Multidisciplinary screening of toxicity induced by silica nanoparticles during sea urchin development. Chemosphere. 2015;139:486–95.
Oh JH, Son MY, Choi MS, Kim S, Choi AY, Lee HA, Kim KS, Kim J, Song CW, Yoon S. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol Appl Pharmacol. 2016;299:8-23. https://doi.org/10.1016/j.taap.2015.11.004.
Zhao L, Yu C, Lv J, Cui Y, Wang Y, Hou C, et al. Fluoride exposure, dopamine relative gene polymorphism and intelligence: A cross-sectional study in China. Ecotoxicol Environ Saf. 2021;209: 111826.
Ying E, Hwang H-M. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci Total Environ. 2010;408(20):4475–81.
Jing X, Park JH, Peters TM, Thorne PS. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air–liquid interface compared with in vivo assessment. Toxicol In Vitro. 2015;29(3):502–11.
Lojk J, Repas J, Veranič P, Bregar VB, Pavlin M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology. 2020;432: 152364.
Tsai S-M, Duran-Robles E, Goshia T, Mesina M, Garcia C, Young J, et al. CeO2 nanoparticles attenuate airway mucus secretion induced by TiO2 nanoparticles. Sci Total Environ. 2018;631–632:262–9.
Sramkova M, Kozics K, Masanova V, Uhnakova I, Razga F, Nemethova V, et al. Kidney nanotoxicity studied in human renal proximal tubule epithelial cell line TH1. Mut Res/Gen Toxicol Environ Mutagen. 2019;845: 403017.
Bell KJ, Lansakara TI, Crawford R, Monroe TB, Tivanski AV, Salem AK, et al. Mechanical cues protect against silica nanoparticle exposure in SH-SY5Y neuroblastoma. Toxicol In Vitro. 2021;70: 105031.
Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102.
Dankers ACA, Kuper CF, Boumeester AJ, Fabriek BO, Kooter IM, Gröllers-Mulderij M, et al. A practical approach to assess inhalation toxicity of metal oxide nanoparticles in vitro. J Appl Toxicol : JAT. 2018;38(2):160–71.
Chen R, Huo L, Shi X, Bai R, Zhang Z, Zhao Y, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–74.
Patil NA, Gade WN, Deobagkar DD. Epigenetic modulation upon exposure of lung fibroblasts to TiO 2 and ZnO nanoparticles: alterations in DNA methylation. Int J Nanomed. 2016;11:4509–19.
Mohamed BM, Verma NK, Prina-Mello A, Williams Y, Davies AM, Bakos G, et al. Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as an early indicator of cytotoxicity. J Nanobiotechnol. 2011;9:29.
Alshatwi AA, Subbarayan PV, Ramesh E, Al-Hazzani AA, Alsaif MA, Alwarthan AA. Aluminium oxide nanoparticles induce mitochondrial-mediated oxidative stress and alter the expression of antioxidant enzymes in human mesenchymal stem cells. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(1):1–10.
Baber O, Jang M, Barber D, Powers K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhalation Toxicol. 2011;23(9):532–43.
Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int J Nanomed. 2012;7:631–9.
Branica G, Mladinić M, Omanović D, Želježić D. An alternative approach to studying the effects of ZnO nanoparticles in cultured human lymphocytes: combining electrochemistry and genotoxicity tests. Arh Hig Rada Toksikol. 2016;67(4):277–88.
Gurunathan S, Jeyaraj M, La H, Yoo H, Choi Y, Do JT, et al. Anisotropic platinum nanoparticle-induced cytotoxicity, apoptosis, inflammatory response, and transcriptomic and molecular pathways in human acute monocytic leukemia cells. Int J Mol Sci. 2020;21(2):440.
Hussain S, Al-Nsour F, Rice AB, Marshburn J, Ji Z, Zink JI, et al. Cerium dioxide nanoparticles do not modulate the lipopolysaccharide-induced inflammatory response in human monocytes. Int J Nanomed. 2012;7:1387–97.
Zerboni A, Bengalli R, Baeri G, Fiandra L, Catelani T, Mantecca P. Mixture effects of diesel exhaust and metal oxide nanoparticles in human lung a549 cells. Nanomaterials (Basel, Switzerland). 2019;9(9):1302.
Zielinska E, Tukaj C, Radomski MW, Inkielewicz-Stepniak I. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: protective effect of inducible nitric oxide synthase inhibitor. PLoS ONE. 2016;11(10): e0164137.
Rajiv S, Jerobin J, Saranya V, Nainawat M, Sharma A, Makwana P, et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol. 2016;35(2):170–83.
Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol. 2013;32(4):296–307.
Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol Toxicol. 2011;27(5):333–42.
Tolliver LM, Holl NJ, Hou FYS, Lee H-J, Cambre MH, Huang Y-W. Differential cytotoxicity induced by transition metal oxide nanoparticles is a function of cell killing and suppression of cell proliferation. Int J Mol Sci. 2020;21(5):1731.
Rothen-Rutishauser B, Grass RN, Blank F, Limbach LK, Mühlfeld C, Brandenberger C, et al. Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ Sci Technol. 2009;43(7):2634–40.
Benameur L, Auffan M, Cassien M, Liu W, Culcasi M, Rahmouni H, et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology. 2015;9(6):696–705.
Gojova A, Lee J-T, Jung HS, Guo B, Barakat AI, Kennedy IM. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhalation Toxicol. 2009;21(Suppl 1):123–30.
Hildebrand H, Kühnel D, Potthoff A, Mackenzie K, Springer A, Schirmer K. Evaluating the cytotoxicity of palladium/magnetite nano-catalysts intended for wastewater treatment. Environ Pollut (Barking, Essex 1987). 2010;158(1):65–73.
Lai JCK, Lai MB, Jandhyam S, Dukhande VV, Bhushan A, Daniels CK, et al. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int J Nanomed. 2008;3(4):533–45.
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun. 2010;396(2):578–83.
Vergaro V, Aldieri E, Fenoglio I, Marucco A, Carlucci C, Ciccarella G. Surface reactivity and in vitro toxicity on human bronchial epithelial cells (BEAS-2B) of nanomaterials intermediates of the production of titania-based composites. Toxicol Vitro : Int J Publish Assoc BIBRA. 2016;34:171–8.
Dávila-Grana Á, Diego-González L, González-Fernández Á, Simón-Vázquez R. Synergistic effect of metal oxide nanoparticles on cell viability and activation of MAP Kinases and NFκB. Int J Mol Sci. 2018;19(1):246.
Pierscionek BK, Li Y, Schachar RA, Chen W. The effect of high concentration and exposure duration of nanoceria on human lens epithelial cells. Nanomed Nanotechnol Biol Med. 2012;8(3):383–90.
Rafieepour A, Azari MR, Khodagholi F, Jaktaji JP, Mehrabi Y, Peirovi H. The effect of single and combined exposures to magnetite and polymorphous silicon dioxide nanoparticles on the human A 549 cell line: in vitro study. Environ Sci Pollut Res Int. 2019;26(31):31752–62.
Ickrath P, Wagner M, Scherzad A, Gehrke T, Burghartz M, Hagen R, et al. Time-dependent toxic and genotoxic effects of zinc oxide nanoparticles after long-term and repetitive exposure to human mesenchymal stem cells. Int J Environ Res Public Health. 2017;14(12):1590.
Radeloff K, Radeloff A, Ramos Tirado M, Scherzad A, Hagen R, Kleinsasser NH, et al. Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) following long-term exposure to Very Small Iron Oxide Particles (VSOPs). Nanomaterials (Basel, Switzerland). 2020;10(4):741.
Jin M, Li N, Sheng W, Ji X, Liang X, Kong B, et al. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ Int. 2021;146: 106179.
Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol. 2014;33(2):86–97.
Fernández-Bertólez N, Costa C, Brandão F, Kiliç G, Duarte JA, Teixeira JP, et al. Toxicological assessment of silica-coated iron oxide nanoparticles in human astrocytes. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2018;118:13–23.
Gliga AR, Di Bucchianico S, Åkerlund E, Karlsson HL. Transcriptome profiling and toxicity following long-term, low dose exposure of human lung cells to Ni and NiO nanoparticles-comparison with NiCl 2. Nanomaterials (Basel, Switzerland). 2020;10(4):649.
Kennedy IM, Wilson D, Barakat AI. Uptake and inflammatory effects of nanoparticles in a human vascular endothelial cell line. Res Rep (Health Effects Institute). 2009;136:3–32.
Schanen BC, Das S, Reilly CM, Warren WL, Self WT, Seal S, et al. Immunomodulation and T helper TH1/TH2 response polarization by CeO2 and TiO2 nanoparticles. PLoS ONE. 2013;8(5): e62816.
Seker S, Elçin AE, Yumak T, Sınağ A, Elçin YM. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol Vitro : Int J Publish Assoc BIBRA. 2014;28(8):1349–58.
Könen-Adıgüzel S, Ergene S. In vitro evaluation of the genotoxicity of CeO 2 nanoparticles in human peripheral blood lymphocytes using cytokinesis-block micronucleus test, comet assay, and gamma H2AX. Toxicol Ind Health. 2018;34(5):293–300.
Henson TE, Navratilova J, Tennant AH, Bradham KD, Rogers KR, Hughes MF. In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology. 2019;13(6):795–811.
Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115(3):403–9.
Alarifi S, Ali D, Alkahtani S, Verma A, Ahamed M, Ahmed M, et al. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int J Nanomed. 2013;8:983–93.
Åkerlund E, Islam MS, McCarrick S, Alfaro-Moreno E, Karlsson HL. Inflammation and (secondary) genotoxicity of Ni and NiO nanoparticles. Nanotoxicology. 2019;13(8):1060–72.
Jiménez-Chávez A, Solorio-Rodríguez A, Escamilla-Rivera V, Leseman D, Morales-Rubio R, Uribe-Ramírez M, et al. Inflammatory response in human alveolar epithelial cells after TiO 2 NPs or ZnO NPs exposure: Inhibition of surfactant protein A expression as an indicator for loss of lung function. Environ Toxicol Pharmacol. 2021;86: 103654.
Hussain S, Garantziotis S. Interplay between apoptotic and autophagy pathways after exposure to cerium dioxide nanoparticles in human monocytes. Autophagy. 2013;9(1):101–3.
Abudayyak M, Öztaş E, Arici M, Özhan G. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere. 2017;169:117–23.
Fahmy HM, Ebrahim NM, Gaber MH. In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J Trace Elem Med Biol. 2020;60: 126481.
Božinović K, Nestić D, Centa UG, Ambriović-Ristov A, Dekanić A, de Bisschop L, et al. In-vitro toxicity of molybdenum trioxide nanoparticles on human keratinocytes. Toxicology. 2020;444: 152564.
Ahamed M, Alhadlaq HA, Alam J, Khan MAM, Ali D, Alarafi S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des. 2013;19(37):6681–90.
Pelclova D, Zdimal V, Kacer P, Fenclova Z, Vlckova S, Komarc M, et al. Leukotrienes in exhaled breath condensate and fractional exhaled nitric oxide in workers exposed to TiO2 nanoparticles. J Breath Res. 2016;10(3): 036004.
Valdiglesias V, Costa C, Kiliç G, Costa S, Pásaro E, Laffon B, et al. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ Int. 2013;55:92–100.
Verdon R, Gillies SL, Brown DM, Henry T, Tran L, Tyler CR, et al. Neutrophil activation by nanomaterials in vitro : comparing strengths and limitations of primary human cells with those of an immortalized (HL-60) cell line. Nanotoxicology. 2021;15(1):1–20.
Siddiqui MA, Ahamed M, Ahmad J, Majeed Khan MA, Musarrat J, Al-Khedhairy AA, et al. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2012;50(3–4):641–7.
Park E-J, Choi J, Park Y-K, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1–2):90–100.
Fakhar-e-Alam M, Kishwar S, Willander M. Photodynamic effects of zinc oxide nanowires in skin cancer and fibroblast. Lasers Med Sci. 2014;29(3):1189–94.
Hackenberg S, Zimmermann F-Z, Scherzed A, Friehs G, Froelich K, Ginzkey C, et al. Repetitive exposure to zinc oxide nanoparticles induces dna damage in human nasal mucosa mini organ cultures. Environ Mol Mutagen. 2011;52(7):582–9.
Andujar P, Simon-Deckers A, Galateau-Sallé F, Fayard B, Beaune G, Clin B, et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol. 2014;11:23.
Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 2012;17(8):852–70.
Senapati VA, Kumar A, Gupta GS, Pandey AK, Dhawan A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: a mechanistic approach. Food Chem Toxicol. 2015;85:61–70.
Vlaanderen J, Pronk A, Rothman N, Hildesheim A, Silverman D, Hosgood HD, et al. A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon nanotubes. Nanotoxicology. 2017;11(3):395–404.
Asghar W, Shafiee H, Velasco V, Sah VR, Guo S, El Assal R, et al. Toxicology study of single-walled carbon nanotubes and reduced graphene oxide in human sperm. Sci Rep. 2016;6:30270.
Periasamy VS, Athinarayanan J, Alfawaz MA, Alshatwi AA. Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA and BCL2L1 genes. Chemosphere. 2016;144:275–84.
Beard JD, Erdely A, Dahm MM, de Perio MA, Birch ME, Evans DE, et al. Carbon nanotube and nanofiber exposure and sputum and blood biomarkers of early effect among U.S. workers. Environ Int. 2018;116:214–28.
Zhang W, Zhang D, Tan J, Cong H. Carbon nanotube exposure sensitize human ovarian cancer cells to paclitaxel. J Nanosci Nanotechnol. 2012;12(9):7211–4.
de Gabory L, Bareille R, Daculsi R, L’Azou B, Flahaut E, Bordenave L. Carbon nanotubes have a deleterious effect on the nose: the first in vitro data. Rhinology. 2011;49(4):445–52.
Eldawud R, Wagner A, Dong C, Stueckle TA, Rojanasakul Y, Dinu CZ. Carbon nanotubes physicochemical properties influence the overall cellular behavior and fate. NanoImpact. 2018;9:72–84.
Pacurari M, Qian Y, Fu W, Schwegler-Berry D, Ding M, Castranova V, et al. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. J Toxicol Environ Health A. 2012;75(2):112–28.
Reamon-Buettner SM, Hackbarth A, Leonhardt A, Braun A, Ziemann C. Cellular senescence as a response to multiwalled carbon nanotube (MWCNT) exposure in human mesothelial cells. Mech Ageing Dev. 2021;193: 111412.
Phuyal S, Kasem M, Knittelfelder O, Sharma A, Fonseca DDM, Vebraite V, et al. Characterization of the proteome and lipidome profiles of human lung cells after low dose and chronic exposure to multiwalled carbon nanotubes. Nanotoxicology. 2018;12(2):138–52.
Witzmann FA, Monteiro-Riviere NA. Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomedicine. 2006;2(3):158–68.
Snyder RJ, Hussain S, Rice AB, Garantziotis S. Multiwalled carbon nanotubes induce altered morphology and loss of barrier function in human bronchial epithelium at noncytotoxic doses. Int J Nanomed. 2014;9:4093–105.
Snyder RJ, Verhein KC, Vellers HL, Burkholder AB, Garantziotis S, Kleeberger SR. Multi-walled carbon nanotubes upregulate mitochondrial gene expression and trigger mitochondrial dysfunction in primary human bronchial epithelial cells. Nanotoxicology. 2019;13(10):1344–61.
Yu M, Chen R, Jia Z, Chen J, Lou J, Tang S, et al. MWCNTs Induce ROS Generation, ERK Phosphorylation, and SOD-2 expression in human mesothelial cells. Int J Toxicol. 2016;35(1):17–26.
Rizk MZ, Ali SA, Kadry MO, Fouad GI, Kamel NN, Younis EA, et al. C-reactive protein signaling and chromosomal abnormalities in nanotoxicity induced via different doses of TiO 2 (80 nm) boost liver function. Biol Trace Elem Res. 2020;198(1):157–67.
Jos A, Pichardo S, Puerto M, Sánchez E, Grilo A, Cameán AM. Cytotoxicity of carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicol Vitro : Int J Published Assoc BIBRA. 2009;23(8):1491–6.
Vankoningsloo S, Piret J-P, Saout C, Noel F, Mejia J, Zouboulis CC, et al. Cytotoxicity of multi-walled carbon nanotubes in three skin cellular models: effects of sonication, dispersive agents and corneous layer of reconstructed epidermis. Nanotoxicology. 2010;4(1):84–97.
Herzog E, Byrne HJ, Davoren M, Casey A, Duschl A, Oostingh GJ. Dispersion medium modulates oxidative stress response of human lung epithelial cells upon exposure to carbon nanomaterial samples. Toxicol Appl Pharmacol. 2009;236(3):276–81.
Müller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010;7(Suppl 1):S27–40.
Basak SC, Vracko M, Witzmann FA. Mathematical nanotoxicoproteomics: quantitative characterization of effects of Multi-walled Carbon Nanotubes (MWCNT) and TiO2 Nanobelts (TiO2-NB) on protein expression patterns in human intestinal cells. Curr Comput Aided Drug Des. 2016;12(4):259–64.
Patlolla A, Patlolla B, Tchounwou P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem. 2010;338(1–2):225–32.
Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Bertke S, Beard JD, et al. Exposure assessments for a cross-sectional epidemiologic study of US carbon nanotube and nanofiber workers. Int J Hyg Environ Health. 2018;221(3):429–40.
Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, et al. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol. 2016;299:125–31.
Zhao X, Chang S, Long J, Li J, Li X, Cao Y. The toxicity of multi-walled carbon nanotubes (MWCNTs) to human endothelial cells: The influence of diameters of MWCNTs. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2019;126:169–77.
Öner D, Ghosh M, Coorens R, Bové H, Moisse M, Lambrechts D, et al. Induction and recovery of CpG site specific methylation changes in human bronchial cells after long-term exposure to carbon nanotubes and asbestos. Environ Int. 2020;137: 105530.
Luanpitpong S, Wang L, Castranova V, Rojanasakul Y. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:22.
Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch ME, Fatkhutdinova LM. Integrated analysis of dysregulated ncRNA and mRNA expression profiles in humans exposed to carbon nanotubes. PLoS ONE. 2016;11(3): e0150628.
Domenech J, Hernández A, Demir E, Marcos R, Cortés C. Interactions of graphene oxide and graphene nanoplatelets with the in vitro Caco-2/HT29 model of intestinal barrier. Sci Rep. 2020;10(1):2793.
Wang L, Stueckle TA, Mishra A, Derk R, Meighan T, Castranova V, et al. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology. 2014;8(5):485–507.
Mukherjee SP, Gupta G, Klöditz K, Wang J, Rodrigues AF, Kostarelos K, et al. Next-generation sequencing reveals differential responses to acute versus long-term exposures to graphene oxide in human lung cells. Small. 2020;16(21): e1907686.
Pérez RF, Soto Fernández AY, Bousquets Muñoz P, Sierra MI, Tejedor JR, Morales-Sánchez P, et al. No genome-wide DNA methylation changes found associated with medium-term reduced graphene oxide exposure in human lung epithelial cells. Epigenetics. 2020;15(3):283–93.
Xu Y, Luo Z, Li S, Li W, Zhang X, Zuo YY, et al. Perturbation of the pulmonary surfactant monolayer by single-walled carbon nanotubes: a molecular dynamics study. Nanoscale. 2017;9(29):10193–204.
Gasser M, Wick P, Clift MJD, Blank F, Diener L, Yan B, et al. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part Fibre Toxicol. 2012;9:17.
Di Cristo L, Grimaldi B, Catelani T, Vázquez E, Pompa PP, Sabella S. Repeated exposure to aerosolized graphene oxide mediates autophagy inhibition and inflammation in a three-dimensional human airway model. Materials Today Bio. 2020;6: 100050.
Chortarea S, Clift MJD, Vanhecke D, Endes C, Wick P, Petri-Fink A, et al. Repeated exposure to carbon nanotube-based aerosols does not affect the functional properties of a 3D human epithelial airway model. Nanotoxicology. 2015;9(8):983–93.
Sargent JF. Nanotechnology: a policy primer. Congres Res Serv. 2011;4(3/4):191–203.
Duschl A, Windgasse G. A survey on the state of nanosafety research in the European Union and the United States. J Nanoparticles Res. 2018;20(12):335.
UNECA. Towards African nanotechnology future: trends, impacts and opportunities. Addis Ababa: United Nations. Economic Commission for Africa; 2020. Available from: https://archive.uneca.org/publications/towards-african-nanotechnology-future-trendsimpacts-and-opportunities.
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112.
Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75(1):R50–7.
Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:1401.
Pandey P, Dahiya M. A brief review on inorganic nanoparticles. J Crit Rev. 2016;3(3):18.
Lohse SE, Murphy CJ. Applications of colloidal inorganic nanoparticles: from medicine to energy. J Am Chem Soc. 2012;134(38):15607–20.
Wang P, Lombi E, Zhao FJ, Kopittke PM. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 2016;21(8):699–712.
Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20(1):1–11.
Baktur R, Patel H, Kwon S. Effect of exposure conditions on SWCNT-induced inflammatory response in human alveolar epithelial cells. Toxicol Vitro : Int J Published Assoc BIBRA. 2011;25(5):1153–60.
Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9–10:1533–45.
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193.
Dadwal A, Baldi A, Narang RK. Nanoparticles as carriers for drug delivery in cancer. Artificial Cells, Nanomed Biotechnol. 2018;46(Sup2):295–305.
Kermanizadeh A, Lohr M, Roursgaard M, Messner S, Gunness P, Kelm JM, et al. Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Part Fibre Toxicol. 2014;11:56.
Joris F, Valdepérez D, Pelaz B, Soenen SJ, Manshian BB, Parak WJ, et al. The impact of species and cell type on the nanosafety profile of iron oxide nanoparticles in neural cells. J Nanobiotechnol. 2016;14:69.
Wang Y, Chen S, Yan Z, Pei M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019;9:7.
Zhang S, Zhang X, Liu H, Qu W, Guan Z, Zeng Q, et al. Modifying effect of COMT gene polymorphism and a predictive role for proteomics analysis in children’s intelligence in endemic fluorosis area in Tianjin, China. Toxicol Sci J Soc Toxicol. 2015;144(2):238–45.
Mukherjee SG, O’Claonadh N, Casey A, Chambers G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol In Vitro. 2012;26(2):238–51.
Ekstrand-Hammarström B, Akfur CM, Andersson PO, Lejon C, Österlund L, Bucht A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology. 2011;6(6):623–34.
Kermanizadeh A, Pojana G, Gaiser BK, Birkedal R, Bilanicová D, Wallin H, et al. In vitro assessment of engineered nanomaterials using a hepatocyte cell line: cytotoxicity, pro-inflammatory cytokines and functional markers. Nanotoxicology. 2013;7(3):301–13.
Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE, et al. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. 2018;30(1):6.
Tolaymat T, Genaidy A, Abdelraheem W, Dionysiou D, Andersen C. The effects of metallic engineered nanoparticles upon plant systems: An analytic examination of scientific evidence. Sci Total Environ. 2017;579(1):93–106.
Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J Nanopart Res. 2013;15:1692.
Gottschalka F, Nowack B. The release of engineered nanomaterials to the environment. J Environ Monitor. 2011;13:1145–55.
Acknowledgements
We would like to thank Daphne Silva Pino and Romana Petry for their contribution at the initial stages of this research.
Funding
This research was funded through the GRUN project (Towards the first implementation of groundwater remediation using nanotechnology in Brazil) as part of UKRI’s Global Challenges Research Fund (GCRF) and a grant from the São Paulo Research Foundation (FAPESP) (Grant 17/20308–0).
Author information
Authors and Affiliations
Contributions
Elizabeth Adjoa Kumah: Conceptualization, Methodology, Investigation, Formal analysis, Validation, Supervision, Writing—Original Draft, Writing—Review & Editing, Raoul Djou Fopa: Investigation, Data Curation, Saeed Harati: Investigation, Data Curation, Paul Boadu: Investigation, Data Curation, Formal analysis, Fatemeh Vida Zohoori: Conceptualization, Methodology, Validation, Supervision, Writing—Review & Editing, Funding acquisition, Tannaz Pak: Conceptualization, Methodology, Validation, Supervision, Writing—Review & Editing, Funding acquisition. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research presented in this article is a scoping review, as such, no ethical approval was needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
MEDLINE Search History.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kumah, E.A., Fopa, R.D., Harati, S. et al. Human and environmental impacts of nanoparticles: a scoping review of the current literature. BMC Public Health 23, 1059 (2023). https://doi.org/10.1186/s12889-023-15958-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15958-4