Abstract
Background
The World Health Organization (WHO) risk assessment algorithm for vertical transmission of HIV (VT) assumes the availability of maternal viral load (VL) result at delivery and early viral control 4 weeks after initiating antiretroviral treatment (ART). However, in many low-and-middle-income countries, VL is often unavailable and mothers’ ART adherence may be suboptimal. We evaluate the inclusion of the mothers’ self-reported adherence into the established WHO-algorithm to identify infants eligible for enhanced post-natal prophylaxis when mothers’ VL result is not available at delivery.
Methods
We used data from infants with perinatal HIV infection and their mothers enrolled from May-2018 to May-2020 in Mozambique, South Africa, and Mali. We retrospectively compared the performance of the WHO-algorithm with a modified algorithm which included mothers’ adherence as an additional factor. Infants were considered at high risk if born from mothers without a VL result in the 4 weeks before delivery and with adherence <90%.
Results
At delivery, 143/184(78%) women with HIV knew their status and were on ART. Only 17(12%) obtained a VL result within 4 weeks before delivery, and 13/17(76%) of them had VL ≥1000 copies/ml. From 126 women on ART without a recent VL result, 99(79%) had been on ART for over 4 weeks. 45/99(45%) women reported suboptimal (< 90%) adherence. A total of 81/184(44%) infants were classified as high risk of VT as per the WHO-algorithm. The modified algorithm including self-adherence disclosure identified 126/184(68%) high risk infants.
Conclusions
In the absence of a VL result, mothers’ self-reported adherence at delivery increases the number of identified infants eligible to receive enhanced post-natal prophylaxis.
Similar content being viewed by others
Background
Vertical transmission (VT) of HIV remains an unacceptable 12% in many Global Plan priority countries, where 90% of the world's pregnant women are living with HIV [1]. Since implementation of Option B+ strategy, [2] antiretroviral therapy (ART) coverage among pregnant women in eastern and southern Africa increased from 84 to 92% [3]. However, the goal of eliminating new paediatric infections by 2030 [4] will likely be compromised by multiple challenges in the VT-prevention cascade, including access to adequate postnatal prophylaxis among HIV-exposed infants [1, 5,6,7,8,9,10,11].
VT prevention guidelines have evolved recently. Extended nevirapine (NVP) postnatal prophylaxis and dual or triple antiretroviral (ARV) prophylaxis have shown to halve VT among HIV-exposed infants compared with short courses of AZT or NVP alone [12,13,14,15,16,17]. The latest World Health Organization (WHO) guidelines recommend enhanced post-natal prophylaxis with daily zidovudine (ZDV) and NVP for the first 6 weeks of life for HIV-exposed infants at high-risk of HIV acquisition, followed by an additional 6 weeks if high-risk and breastfeeding. HIV-exposed infants at low-risk of VT should receive 4–6 weeks of prophylaxis with daily NVP (or twice-daily AZT) [18].
High-risk infants have mothers diagnosed with HIV at delivery and are either: 1.) not on ART 2.) started treatment within 4 weeks before delivery, or 3.) had a plasma viral load (VL) > 1000 copies/mL in the 4 weeks before delivering [18]. WHO designed an algorithm to assess HIV-exposed infants risk for HIV acquisition at delivery and to identify high-risk infants eligible for enhanced post-natal prophylaxis [19]. The implementation of the WHO algorithm is challenging due to the paucity of required information for risk stratification [20]. The ‘high-risk criteria’ assume VL result availability, adequate maternal adherence, and early and sustained viral suppression after 4 weeks of ART [18]. However, VL testing coverage and monitoring, especially during pregnancy, is challenging in low-and-middle-income countries (LMIC). Many countries have scaled VL testing via point of care or dried blood spot testing. However, in routine operating conditions, many patients who are tested either don’t receive results or they are extremely delayed [1, 21, 22]. Women with poor adherence or interrupted ART during pregnancy or breastfeeding have viral rebound and increased risk of VT [23,24,25,26]. In fact, peripartum viral suppression varies from 30 to 98% in different sub-Saharan African settings, and despite an estimated 92% ART coverage among pregnant women, nearly 30% of new pediatric infections in East and Southern Africa in 2018 were related to ART interruptions during pregnancy or breastfeeding [3, 10].
We retrospectively characterized post-natal prophylaxis coverage among infants living with HIV in sub-Saharan Africa and assessed whether the inclusion of readily accessible information such as mothers’ self-reported ART adherence into the WHO algorithm improved the identification of infants with perinatal HIV infection when maternal VL resulted unavailable.
Methods
Study population
This analysis was nested under a broader prospective cohort study (A prospective, observational, cohort, multicentre study of Early Anti-Retroviral Treatment in HIV- infected Infants: EARTH) within the EPIICAL project (Early treated Perinatally HIV-Infected individuals: Improving Children’s Actual Life Project). The EARTH study aimed to monitor clinical, virological and immunological features of HIV-positive, HIV early treated children during the first 4 years of age, in order to identify participants with excellent viral and immunological control. The EARTH study included 1) perinatally infected infants who initiated ART ≤90 days after diagnosis and 2) breastfed infants diagnosed with HIV ≤90 days of age and starting ART ≤90 days after diagnosis.
Standard of care for PMTCT
Lifelong ART to all pregnant women living with HIV was offered at all sites. In Mozambique, HIV-exposed infants prophylaxis consisted of 6 weeks NVP to all infants until September 2019, [19, 27] and thereafter ZDV and NVP for the first 6 weeks of life, followed by NVP for 6 weeks to all infants, as all HIV-exposed infants were considered at high-risk for VT [28]. In South Africa and Mali, enhanced post-natal prophylaxis was implemented for high-risk HIV-exposed infants throughout enrollment [19].
Data collection and analysis
The target sample size for the EARTH study was 300 children. For this analysis, we included mother-infant pairs recruited in the EARTH study from May 1st, 2018 to May 1st 2020 (N = 184) in 2 Mozambican sites, 3 South African sites and 1 site in Mali. Infants receiving an HIV diagnosis at the clinic were selected to participate along with their mothers. After obtaining informed consent, study personnel administered a study-specific questionnaire to the mother in a private room at the hospital, ensuring confidentiality. This questionnaire took 30 minutes on average and included information about sociodemographic characteristics, pregnancy and delivery, HIV diagnosis and care of the mother and a specific question on the number of doses missed by the mother during the month prior to the infant’s enrollment in the study. Suboptimal adherence was considered if > 10% of the ART doses were missed in the last month.
We retrospectively classified infants at high or low VT risk according to the WHO algorithm. We then modified the algorithm to include the maternal self-assessment of adherence to ART. For all women on ART for over 4 weeks and without a VL test result 4 weeks upon delivery, suboptimal and optimal adherence were defined as < 90% and ≥ 90% self-reported ART adherence during pregnancy, respectively. Infants of mothers with suboptimal adherence were considered high risk.
CompareGroups R package [29] was used to compare sociodemographic and clinical baseline information of mothers and their infants classified as HIV-exposed infants at high risk of VT according to the two algorithms, as described previously. The variables included were infant’s age and sex, maternal WHO stage, employment, marital status, education, and maternal health conditions or severe life events that arose at any later postnatal point during the study (change in employment, break-up, new partner, loss of home or moving/relocation, death in the family). We also included infant’s post-natal prophylaxis regimens: standard (≤6 weeks with ZDV or NVP) or extended (≥ 6 weeks with NVP or ZDV or with NVP plus ZDV).
The normality of continuous variables was tested using the Shapiro-Wilk test. The Mann-Whitney Wilcoxon test was used to compare non-normally distributed continuous variables. For categorical variables, absolute and relative frequencies were calculated, and Chi-squared tests or exact Fisher tests (frequencies< 5) were performed.
Results
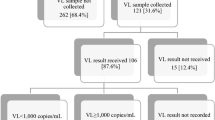
A total of 184 infants living with HIV (n = 96 male) and their mothers were studied, 112 (61%) from South Africa, 64 (35%) from Mozambique and 8 (4%) from Mali (Fig. 1). A total of 143 (78%) women were known HIV-positive and on ART at delivery. However, 29 women (16%) had their HIV diagnosis at delivery and were not on ART and 12 (7%) were known to be HIV-positive prior to delivery but were not on ART. Among them, only 17/143 (12%) had a VL result within 4 weeks before delivery, 13/17 (76%) of them had VL ≥1000 copies/ml. Therefore, most known HIV-positive women on ART (126/143, 88%) did not have a VL result. Of them, 99/126 (79%) had been on ART for over 4 weeks and 27/126 (21%) under 4 weeks. A total of 45/99 (45%) mothers reported suboptimal adherence to ART.
Using the WHO algorithm, 81/184 (44%) of infants with HIV were defined as high risk of VT with VL available data. Following the inclusion of the self-reported adherence risk factor applied to 99 mothers on ART greater than 4 weeks without VL test result, an additional 45 high-risk infants were identified. In total, the modified algorithm classified 126/184 (68%) infants as high risk of MTCT – assuming that infants born to mothers with suboptimal adherence are high risk (Fig. 2). The modified algorithm, identified higher number of high risk children than the WHO algorithm (p = 0.0001).
Classification of infants into high or low risk of HIV vertical transmission according to the WHO algorithm and WHO modified algorithm. The additional question was added at the last step for mothers on ART ≥4 weeks before delivery with no VL test result (in red). The WHO algorithm is described in green as well as the final classification of high-risk infants. The additional risk factor included in the modified WHO algorithm is depicted in red as well as the final classification of high-risk infants. Percentages were calculated using as denominator the number of mothers from the preceding classification step. ART = Antiretroviral treatment; VL = Viral Load
Among the 81 at high risk of MTCT according to WHO algorithm, 40% (32/81) received enhanced post-natal prophylaxis, 30% (24/81) standard post-natal prophylaxis, 22% (18/81) did not received any post-natal prophylaxis and 9% (7/81) did not have information about the post-natal prophylaxis.
In table 1, we compared clinical and sociodemographic characteristics that could have acted as confounding factors in the risk of VT, between participants classified as high-risk by the WHO algorithm and the additional participants classified as high risk when maternal adherence was incorporated into the algorithm. We did not find any differences in age, sex, maternal WHO status at delivery, maternal health, or social adverse events.
Discussion
In our study, nearly 90% of mothers of infants diagnosed with HIV did not have a VL result at delivery; a result on which the WHO algorithm relies. When applied in our cohort, the WHO algorithm identified 44% of the infected infants as high-risk for VT. When we added mothers’ self-reported ART adherence to the WHO algorithm, the proportion of high-risk infants increased to 68%, suggesting that suboptimal adherence is associated with high risk. This modification of the algorithm increased the number of infected infants being classified as high-risk and eligible for enhanced post-natal prophylaxis.
This study also showed that information is often missing when assessing VT risk assessment according to the WHO algorithm, which jeopardizes early prescription of enhanced post-natal prophylaxis [15]. Only 12% of mothers on ART had a VL result 4 weeks prior to delivery. Similarly, a cross-sectional study of programmatic data in Zimbabwe revealed that the risk of MTCT using the WHO algorithm could not be determined in 90% of HIV-exposed infants due to lack of data [20]. A more recent study of 2080 HIV-exposed infants from Zimbabwe showed that 80% of mothers did not have a VL result between 28 weeks gestation to delivery [30]. Therefore, other factors should be considered to establish the risk of VT. Our results suggest that self-reported maternal adherence to ART could be an additional clinical factor to be considered in order to improve the algorithm to assess the risk of VT in the absence of VL. We also evaluated other socio-clinical factors that could potentially affect the risk of MTCT, such as maternal WHO stage, level of education, employment status or adverse social events between the two algorithms. We didn’t find any difference among the participants newly classified as high risk of VT according to maternal ART adherence and the participants classified by the WHO algorithm as high risk of VT. However, further studies evaluating additional socio-clinical variables could inform more targeted algorithms to improve prevention of VT.
ART duration over 4 weeks, as incorporated in the algorithm, does not justify the assumption of viral suppression or the low VT risk. Although many countries have recently changed from EFV-based maternal ART to Dolutegravir, which has been shown to have superior early virologic suppression [31], adherence remains a paramount. Poor maternal adherence or interrupted ART during pregnancy and breastfeeding may cause high VL and subsequent increased transmission risk [23,24,25,26]. As such, infants are misclassified as ‘low-risk’ and do not receive appropriate e-PNP. The use of point-of-care devices for VL may be used at delivery and provide results in under 90 minutes, however, these devices are not widely available in LMIC [32]. Modification of the existing WHO risk evaluation algorithm to include a maternal self-reported adherence may significantly benefit countries without a consolidated laboratory network capacity to ensure optimal VL monitoring. Investigating the mothers’ self-reported ART adherence at delivery is an easy and low-cost intervention to guide nurses in identifying high-risk infants eligible for enhanced post-natal prophylaxis in the absence of VL result.
In our cohort, 45% of mothers on ART for over 4 weeks without a VL result reported sub-optimal adherence. The actual proportion might be higher, since self-reported adherence is a reliable method with low cost and good specificity [33] widely used in clinical practice, but tends to overestimate adherence behavior compared with other methods [9]. Low self-reported adherence during pregnancy and breastfeeding is associated with viremia and virological treatment failure [34, 35]. The high number of women who self-disclosed ART-adherence difficulties in our study suggests that in the absence of VL result, time on ART is not enough to evaluate VT risk. Effective interventions to support adherence during pregnancy must be considered.
Our findings showed that 30% of HIV-exposed infants at high-risk of HIV infection according to WHO algorithm had no access to enhanced post-natal prophylaxis, which can be partially due to the recent guideline implementation in Mozambique [28]. A study in Zimbabwe also showed low enhanced post-natal prophylaxis coverage rates among HIV-exposed infants [20]. We also found a high proportion (17%) of HIV-exposed infants who alarmingly did not receive any PNP at all. Further studies should evaluate enhanced post-natal prophylaxis compliance and algorithm feasibility in the clinical setting, including HIV-exposed children at high risk of VT who never become infected. Nevertheless, our results suggest that besides modifying the WHO algorithm and training health staff on its correct application, further efforts are needed to ensure access to timely maternal VL testing and infant enhanced post-natal prophylaxis.
Our results have implications for policy, practice and public health. This study provided a comprehensive evaluation of the WHO risk assessment algorithm for VT. On one hand, we identified that the coverage of enhanced postnatal prophylaxis among infants with high risk of VT transmission was not optimal. On the other hand, our results suggested that adding maternal adherence to ART could increase the coverage of enhanced postnatal prophylaxis in high-risk infants, thus reinforcing WHO recommendations in clinical practice. The application of this simple measure would be especially beneficial in settings with less structural capacity to obtain viral load results. It would allow the allocation of limited resources towards prevention measures for children at high risk and inform interventions to improve VT prevention.
This study has several limitations. First, we included only infants confirmed to have HIV infection, and retrospectively reviewed their VT risk classification. For this reason, we could not calculate the specificity of the two algorithms. Second, the mothers’ ART adherence was self-reported at recruitment and recall bias, as well as social desirability bias, may have led to over-reporting of good adherence. We defined 1 month prior to study enrollment as the time interval for observing the number of missed doses in order to improve the validity of past reporting. We based this on the fact that a shorter time frame allows the respondent to more easily recall an event rather than having to recall a behavior over a large period of time. However, further studies evaluating a standardized method and definition of self-reported adherence would be important to generalize our results on ART adherence and validate the inclusion of the self-reported adherence to ART in the algorithm in other contexts. Third, we didn’t find difference in the confounders factors analyzed, however other potential confounding factors such as type of infant feeding, home delivery or maternal drug resistance have not been accounted for. Fourth, the relatively small sample size can compromise the generalizability of the results. Further studies with larger sample size are needed to validate this results in populations from different settings.
In conclusion, incorporation of maternal self-reported adherence in the WHO algorithm for VT risk assessment improves the identification of infants eligible for enhanced post-natal prophylaxis and should be considered in the algorithm. A study including HIV-exposed uninfected infants is needed to assess whether the modified WHO algorithm adequately identifies infants who do not need enhanced post-natal prophylaxis and remain HIV-free after breastfeeding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
UNAIDS. (Joint United Nations Programme on HIV/AIDS), “Communities at the centre,”; 2019. https://doi.org/10.2307/j.ctt1t898kc.12.
World Heath Organization (WHO), “WHO | Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants,” 2010. https://www.who.int/hiv/pub/mtct/antiretroviral2010/en/ (Accessed 21 Oct 2020).
UNAIDS, “Start Free stay free AIDS free 2019,” 2019.
UNAIDS (Joint United Nations Programme on HIV/AIDS), “on the Fast-Track To an Aids-Free Generation,” 2016. http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf.
Hodgson I, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014;9(11). https://doi.org/10.1371/journal.pone.0111421.
Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis. PLoS Med. 2014;11(2). https://doi.org/10.1371/journal.pmed.1001608.
Kalk E, Slogrove A, Speert DP, Bettinger JA, Cotton MF, Esser M. HIV sero-conversion during late pregnancy - when to retest. Southern African J HIV Med. 2013;14(2):90–2. https://doi.org/10.7196/SAJHIVMED.903.
De Schacht C, et al. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in southern Mozambique. J Int AIDS Soc. 2014;17(1):18808. https://doi.org/10.7448/IAS.17.1.18808.
Nachega JB, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–52. https://doi.org/10.1097/QAD.0b013e328359590f.
Abuogi LL, et al. Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J Virus Erad. 2018;4(Suppl 2):33–9 Accessed 17 June 2020. Available: http://www.ncbi.nlm.nih.gov/pubmed/30515312.
Lain MG, Chicumbe S, Couto A, Karajeanes E, Giaquinto C, Vaz P. High proportion of unknown HIV exposure status among children aged less than 2 years: An analytical study using the 2015 National AIDS Indicator Survey in Mozambique. PLoS One. 2020;15(4). https://doi.org/10.1371/journal.pone.0231143.
Coovadia HM, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9812):221–8. https://doi.org/10.1016/S0140-6736(11)61653-X.
Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infants of breastfeeding mothers with HIV-1 infection for prevention of HIV-1 transmission (HPTN 046): 18-month results of a randomized, double-blind, placebo-controlled trial. J Acquir Immune Defic Syndr. 2014;65(3):366–74. https://doi.org/10.1097/QAI.0000000000000052.
Kumwenda NI, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–29. https://doi.org/10.1056/NEJMoa0801941.
Nielsen-Saines K, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366(25):2368–79. https://doi.org/10.1056/NEJMoa1108275.
Beste S, et al. Optimal antiretroviral prophylaxis in infants at high risk of acquiring HIV: a systematic review. Pediatr Infect Dis J. 2018;37(2):169–75. https://doi.org/10.1097/INF.0000000000001700.
Lallemant M, et al. Perinatal antiretroviral intensification to prevent intrapartum HIV transmission when antenatal antiretroviral therapy is initiated less than 8 weeks prior to delivery. JAIDS J Acquir Immune Defic Syndr. 2020. https://doi.org/10.1097/QAI.0000000000002350.
World Health Organization, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2016.
“WHO | HIV diagnosis and ARV use in HIV-exposed infants: a programmatic update.” https://www.who.int/hiv/pub/paediatric/diagnosis-arv-infants/en/ (Accessed 19 June 2020).
Komtenza B, et al. Identifying high or low risk of mother to child transmission of HIV: how Harare City, Zimbabwe is doing? PLoS One. 2019;14(3):e0212848. https://doi.org/10.1371/journal.pone.0212848.
S. Swannet et al., “Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs,” Int Health, vol. 9, no. 4, pp. 206–214, Jul. 2017, doi: https://doi.org/10.1093/inthealth/ihx021.
M. S. M. Arpadi, S. Shiau, E. P. De Gusmao, and A. Violari, “Routine viral load monitoring in HIV-infected infants and children in low- and middle-income countries: Challenges and opportunities: Challenges,” Journal of the International AIDS Society, vol. 20, no. Suppl 7. Wiley Blackwell, pp. 32–36, Nov. 01, 2017, doi: https://doi.org/10.1002/jia2.25001.
J. R. Millar et al., “HIGH-FREQUENCY failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal NON-ADHERENCE,” EClinicalMedicine, vol. 22, p. 100344, May 2020, doi: https://doi.org/10.1016/j.eclinm.2020.100344.
Myer L, et al. Frequency of Viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis. 2016;p. ciw792:Dec. https://doi.org/10.1093/cid/ciw792.
E. Bobrow, L. Mugwaneza, Placidie; Ndayisaba, Gilles; Ndatimana, Dieudonne; Gill, Michelle; Hoffman, Heather; Baribwira, Cyprien; Guay, and A. Asiimwe, “Maternal Viral Load in the Context of PMTCT B+ Within the Kabeho Study in Kigali | Request PDF.” https://www.researchgate.net/publication/299347124_Maternal_Viral_Load_in_the_Context_of_PMTCT_B_Within_the_Kabeho_Study_in_Kigali (Accessed 19 June 2020).
Chetty T, Newell ML, Thorne C, Coutsoudis A. Viraemia before, during and after pregnancy in HIV-infected women on antiretroviral therapy in rural KwaZulu-Natal, South Africa, 2010–2015. Trop Med Int Heal. 2018;23(1):79–91. https://doi.org/10.1111/tmi.13001.
Republica de Moçambique. Ministério da Saúde, “Tratamento Antiretroviral e Infecções Oportunistas do Adulto, Adolescente, Grávida e Criança,” 2016. http://www.misau.gov.mz/index.php/guioes#.
Ministério da Saúde (MISAU). Comité TARV., “Circular normas clínicas actualizadas,” 2019. https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/Circular_Normas_Clínicas_Actualizadas_29_11_19.pdf (Accessed 22 Sept 2020).
“R: The R Project for Statistical Computing.” https://www.r-project.org/ (Accessed 19 June 2020).
Mafaune HW, et al. Effectiveness of maternal transmission risk stratification in identification of infants for HIV birth testing: lessons from Zimbabwe. J Acquir Immune Defic Syndr. 2020;84(1):S28–33. https://doi.org/10.1097/QAI.0000000000002373.
“WHO recommends dolutegravir as preferred HIV treatment option in all populations.” https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations (Accessed 15 July 2021).
Drain PK, et al. Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response. Clin Microbiol Rev. 2019;32(3). https://doi.org/10.1128/CMR.00097-18.
Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics. 2010;28(12):1097–107. https://doi.org/10.2165/11537400-000000000-00000.
Atanga PN, et al. Using a composite adherence tool to assess ART response and risk factors of poor adherence in pregnant and breastfeeding HIV-positive Cameroonian women at 6 and 12 months after initiating option B+. BMC Pregnancy Childbirth. 2018;18(1):418. https://doi.org/10.1186/s12884-018-2058-9.
Phillips TK, et al. Decreases in self-reported ART adherence predict HIV viremia among pregnant and postpartum south African women. J Acquir Immune Defic Syndr. 2019;80(3):247–54. https://doi.org/10.1097/QAI.0000000000001909.
Acknowledgements
We thank all the patients and families for their participation in this cohort, and the staff members who cared for them; the Ministry of Health of Mozambique and South Africa for supporting the implementation of the study. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. We acknowledge support from the Spanish Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program”. CISM is supported by the Government of Mozambique and the Spanish Agency for International Development (AECID).
Members of the EPIICAL Consortium are: Paolo Rossi,12, 17 Carlo Giaquinto,17 Silvia Faggion,17 Daniel Gomez Pena,17 Inger Lindfors Rossi,17 William James,17 Alessandra Nardone,17 Paolo Palma,11 Paola Zangari,11 Carla Paganin,11 Eleni Nastouli,10 Moira Spyer,10 Anne-Genevieve Marcelin,20 Vincent Calvez,20 Pablo Rojo,4 Alfredo Tagarro,4 Sara Dominguez,4 Maria Angeles Munoz,21 Caroline Foster,22 Savita Pahwa,23 Anita De Rossi14, Mark Cotton,7 Nigel Klein,9 Deborah Persaud 24, Rob J De Boer,25 Juliane Schroeter,25 Adriana Ceci,13 Viviana Giannuzzi,13 Kathrine Luzuriaga,26 Nicolas Chomont,27 Nicola Cotugno,12 Louise Kuhn,5 Andrew Yates,5 Avy Violari,6 Kennedy Otwombe,6 Paula Vaz,3 Maria Grazia Lain,3 Elisa López-Varela,1, 2 Tacilta Nhamposssa,1 Denise Naniche,2 Ofer Levy,28 Philip Goulder,29 Mathias Lichterfeld,30 Holly Peay,31 Pr Mariam Sylla,16 Almoustapha Maiga16.
1Centro de Investigação em Saúde de Manhiça (CISM), Maputo, Mozambique.
2ISGlobal, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain.
3Fundação Ariel Glaser Contra o SIDA Pediátrico, Maputo, Mozambique.
4Pediatrics Department, Pediatric Research and Clinical Trials Unit (UPIC), Fundación para la Investigación Biomédica del Hospital 12 de Octubre, Instituto de Investigación Sanitaria Hospital 12 de Octubre (IMAS12), Madrid, Spain.
5Gertrude H. Sergievsky Center, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY, USA.
6Perinatal HIV Research Unit, Chris Hani Baragwanath Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
7Family Center for Research with Ubuntu, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
8School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
9Africa Health Research Institute (AHRI), KwaZulu-Natal, South Africa.
10Great Ormond Street Institute for Child Health (GOS ICH), University College London (UCL), London, UK.
11Research Unit in Clinical Immunology and Vaccinology, Bambino Gesu’ Children’s Hospital, 00165, Rome, Italy.
12Chair of Pediatrics, Department of Systems Medicine, University of Rome “Tor Vergata”, Rome 00133, Italy.
13Fondazione per la Ricerca Farmacologica Gianni Benzi onlus.
14Department of Mother and Child Health, University of Padova, Padova, Italy.
15Instituto Nacional de Saúde (INS), Mozambique.
16Department of Medical Biology, Gabriel Toure University Hospital, Bamako, Mali.
17PENTA Foundation. Padova, Italy.
18Pediatrics Department, Hospital Universitario Infanta Sofía; Infanta Sofia University Hospital and Henares University Hospital Foundation for Biomedical Research and Innovation (FIIB HUIS HHEN), San Sebastián de los Reyes, Madrid, Spain.
19Pediatrics Research Group, Universidad Europea de Madrid, Madrid, Spain.
20Academic Department of Pediatrics, Children’s Hospital Bambino Gesù, Rome, Italy.
21Department of Clinical Virology, University College London Hospitals (UCLH) NHS Foundation Trust, London, UK.
22Imperial College Healthcare NHS Trust (ICHT), United Kingdom.
23University of Miami Miller School of Medicine, USA.
24Johns Hopkins University, Baltimore, USA.
25University of Utrecht, The Netherlands.
26University of Massachusetts Medical School Worcester. USA.
27Centre de Recherche du Centre Hospitalier de l’Universitè de Montreal – University of Montreal. Canadá.
28The Children’s Hospital Corporation d/b/a Boston Children’s Hospital. USA.
29Umkhuseli Innovation and Research Management RF NPC. South Africa.
30The Brigham & Women’s Hospital, Inc. USA.
31Research Triangle Institute dba RTI International. USA.
Funding
This work is part of the EARTH Project, supported by the EPIICAL Consortium and funded from a research grant provided by ViiV.
All the authors involved developed this project with funds from a research grant provided by ViiV to the Pediatric European Network from the Treatment of Aids (PENTA-ID).
S.F.L. receives a pre-doctoral fellowship from the Secretariat of Universities and Research, Ministry of Enterprise and Knowledge of the Government of Catalonia and cofounded by European Social Fund. E.L.V. is supported by a Spanish Pediatrics Association (AEP) fellowship and a Ramon Areces Foundation fellowship.
Author information
Authors and Affiliations
Consortia
Contributions
AT conceptualized and designed the study. SDR, AT, and MSP performed the data management. MSP performed the statistical analysis. MGL and SFL drafted and reviewed the manuscript. AT, PR and LK were involved in the preparation and review of the final manuscript. AL, SB, ELV, KO, SD, EN, PP, NC, MS, VG, CG, AV, MC, TN, NK, NR, AJR, OB, PV, AM, AO, DN, PR, other co-authors implemented the study, enrolled participants and participated in the management of the study and the collection of data. All co-authors participated and were involved in the critical review of the final manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Mozambique National Bioethics Committee, 53/CNBS/2018; Stellenbosch University Bioethics Committee, 1832; University of Witwatersrand and AHRI Bioethics Committee, 171114, and Bamako Faculté de Médecine Bioethics Committee, 2019/53. Mothers signed an informed consent for their infants as well as their participation in the study. All methods were carried out in accordance with relevant guidelines and regulations. Data was managed according to the European General Data Protection Regulation 2016/679.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fernández-Luis, S., Lain, M.G., Serna-Pascual, M. et al. Optimizing the World Health Organization algorithm for HIV vertical transmission risk assessment by adding maternal self-reported antiretroviral therapy adherence. BMC Public Health 22, 1312 (2022). https://doi.org/10.1186/s12889-022-13543-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-13543-9