Abstract
Background
The landscape of non-small cell lung cancer (NSCLC) therapy is rapidly changing. This analysis aimed to understand patient characteristics, diagnosis and treatment patterns in patients with metastatic NSCLC (mNSCLC) without EGFR and ALK mutations across five European countries.
Methods
Data were drawn from the Adelphi NSCLC Disease Specific Programme™, a point-in-time survey of oncologists/pulmonologists and their consulting patients in France, Germany, Italy, Spain and UK. Physicians completed record forms (RFs) for the next six consecutive consulting patients with advanced NSCLC, who then voluntarily completed questionnaires. As an oversample, physicians provided a further ten RFs specifically for patients with EGFR-wild-type mNSCLC: five patients diagnosed before March 2020 (pre-SARS-CoV-2 [COVID-19]) and five patients diagnosed from March 2020 (during COVID-19). Only EGFR-wild-type/ALK-wild-type patients were included for analysis.
Results
Mean (standard deviation [SD]) age for 1073 patients with EGFR-wild-type/ALK-wild-type mNSCLC was 66.2 (8.9) years, 65.2% were male and 63.7% had adenocarcinoma. Level of PD-L1 expression at advanced diagnosis was < 1% for 23.1% of patients, 1–49% for 40.9% and ≥ 50% for 36.0%. Most common first-line (1L) advanced treatment was chemotherapy only (36.9%), immunotherapy monotherapy (30.5%) or immunotherapy + chemotherapy (27.6%). Of 158 patients who had progressed beyond 1L therapy, the mean (SD) time-to-treatment discontinuation was 5.1 (4.3) months; 75.9% of whom completed their 1L treatment as intended. A complete response was achieved by 6.7% and a partial response by 69.2% of patients. Of 38 patients who discontinued 1L treatment early, disease progression was reported for 73.7%. Quality of life (QoL) reported by patients was generally lower than normative reference values. Of 2373 oversample patients, physicians reported management changes for 34.7% due to COVID-19, ranging from 19.6% in Germany to 79.7% in the UK. Immunotherapy was prescribed as 1L NSCLC treatment during COVID-19 for 64.2% (n = 786) of patients and pre-COVID-19, for 47.8% (n = 549).
Conclusions
Real-world treatment patterns suggest that chemotherapy use remains high despite guidelines recommending immunotherapy-based 1L treatment for mNSCLC. QoL reported by patients was generally lower than population reference values. Not implying causality, 1L immunotherapy use was higher during COVID-19 than pre-COVID-19, and the UK saw the biggest impact to patient management due to COVID-19.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer deaths globally, accounting for 18% (1.8 million) of deaths and 11.4% (2.2 million) of new cases in 2020 [1, 2]. In Europe alone, the prevalence, incidence, and death rates in 2020 were 22.4%, 21.6% and 21.4%, respectively [1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases [3, 4], with initial diagnosis most commonly in the advanced stages [5]. The overall relative 5-year survival rate for lung cancer is 23%, although this varies depending on clinical stage, and is 61% for localised, 34% for regional, and 7% for metastatic disease [5]. Diagnosis at an advanced disease stage means that the majority of lung cancers are ineligible for potentially curative surgery, unlike in the non-metastatic disease stage.
Alongside clinical understanding of the malignancy, the treatment landscape for metastatic NSCLC (mNSCLC) has evolved considerably over the past few decades; first with cytotoxic chemotherapy (from 2006), followed by targeted therapies (mainly from 2011) for patients with oncogenic driver mutations and, more recently, immunotherapy (from 2015) for patients without actionable mutations [6]. Most NSCLC tumours in Europe lack oncogenic driver mutations, rendering patients without targetable mutations ineligible to receive targeted therapies [7]. Additionally, most oncogene-driven NSCLC tumours initially responding to targeted therapies such as epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKI) and anaplastic lymphoma kinase (ALK) inhibitors eventually progress over time as they acquire drug resistance [8, 9].
The identification of mutations in the EGFR gene (EGFR-mutant [EGFR-mut]; coding for a receptor tyrosine kinase) and rearrangements in the ALKgene, found primarily in tumours of non-squamous histology, has led to the development of targeted therapies, EGFR-TKIs (e.g., erlotinib, gefitinib, afatinib, osimertinib, and dacomitinib) and ALK inhibitors (e.g., crizotinib, alectinib, ceritinib, brigatinib and lorlatinib), respectively, for patients with advanced non-squamous NSCLC [10, 11]. The discovery of targetable mutations in genes other than EGFR and ALK, such as c-ros oncogene 1 (ROS1) and v-Raf murine sarcoma viral oncogene homolog B (BRAF) has led to the development of additional targeted therapies in NSCLC, specific to each genomic mutation [11].
Immunotherapies have also been developed to treat patients with NSCLC and have shown that they may be an effective 1L treatment for patients whose tumours do not harbour oncogenic driver alterations. Programmed death-1 (PD-1) is an inhibitory T cell receptor that, when bound to its ligands PD-L1 and PD-L2, induces inhibitory messaging leading to a reduction in T-cell proliferation, cytokine production, and cytotoxic activity [12, 13], thus acting as an immunologic checkpoint. PD-L1 expression occurs in many different tumour types including lung [14, 15]. PD-1 expression on lymphocytes and its interaction with its ligands on tumour and immune cells are the basis of anti-tumour immunity and PD-1 inhibition in cancer immunotherapy [16]. For patients without oncogenic driver mutations, treatment options can be considered based on PD-L1 status [11]; immunotherapy as a monotherapy can be used for patients with tumour PD-L1 ≥ 50% and immunotherapies can be used in combination with chemotherapy in patients irrespective of PD-L1 status but mostly preferred for those with PD-L1 < 50%.
An understanding of real-world treatment patterns and outcomes can provide important context for the rapidly changing landscape of NSCLC therapy, whilst further contributing to determining the applicability of clinical trial evidence to the real-life clinical setting where patient populations are more diverse and typically have more comorbidities. The primary objectives of this analysis were to understand patient characteristics, the diagnostic landscape, and treatment patterns particularly in the first-line (1L) setting in patients with mNSCLC without EGFR and ALK mutations across Europe. Further objectives were to evaluate the burden of illness and unmet needs in patients with EGFR-wild type (WT)/ALK-WT mNSCLC in the 1L setting. The impact of SARS-CoV-2 (COVID-19) on 1L diagnostic and treatment patterns in patients with mNSCLC without EGFR and ALK mutations was also explored.
Methods
Survey design
Data were drawn from the Adelphi NSCLC Disease Specific Programme (DSP)™, a multinational, point-in-time survey of physicians and their patients. Data were collected for the main sample from July 2020 to November 2020. A retrospective oversample was also conducted as part of this DSP from May 2021 to August 2021.
The DSP methodology has been previously published and validated [17,18,19,20], with studies across many different disease areas implemented globally. The survey included a physician survey and workload questionnaire, a physician-reported electronic patient record form, and a voluntary patient-reported questionnaire. Physicians and their patients were recruited from five European countries (France, Germany, Italy, Spain, and the United Kingdom [UK]).
Survey population
For the main sample, physicians (oncologists/pulmonologists) were included in the study if they were actively involved in the management and systemic treatment of patients with mNSCLC and consulted at least three patients with mNSCLC in a typical month. Patients aged 18 years or over with a physician-confirmed diagnosis of mNSCLC (stage IIIb–IV) and not part of any clinical trial were eligible for inclusion in the main sample analysis.
For the oversample, oncologists/pulmonologists were included in the study if they were actively involved in the management and systemic treatment of patients with EGFR-WT mNSCLC and had a clinical workload of at least five patients with EGFR-WT mNSCLC (recurrent or de novo) diagnosed between March 2020 (a date where all five European countries were in lockdown due to COVID-19) up to when data collection ended (August 2021), and at least five patients with EGFR-WT mNSCLC diagnosed in the six months prior to March 2020. Patients aged 18 years or over with a physician-confirmed diagnosis of EGFR-WT mNSCLC and not part of any clinical trial were eligible for inclusion in the retrospective oversample.
Participant selection and data collection
Patients in the analyses include two cohorts, randomly sampled patients (the main patient sample) and an additional retrospectively captured set of patients (oversample). The main sample focused on mNSCLC, providing data to reflect current clinical practice at the time of survey. For the main sample, a geographically representative sample of oncologists and pulmonologists were recruited. Physicians meeting the inclusion criteria and willing to participate first completed an attitudinal survey regarding the management and treatment of patients with mNSCLC. Physicians were then asked to complete a patient record form for their next six consulting patients with mNSCLC who met the patient eligibility criteria. As patients consult at random, the patient sampling method is considered to generate a patient sample representative of the typical mNSCLC consulting population.
For the oversample, physicians provided information retrospectively on 10 patients with EGFR-WT mNSCLC: five patients diagnosed during the pre-COVID-19 period (defined as 1 September 2019 to 29 February 2020; prior to when all five European countries went into lockdown due to the COVID-19 pandemic) and five patients diagnosed during the COVID-19 pandemic (1 March 2020 to the time of data collection). This was in order to investigate the effects of COVID-19 and ‘lockdown’ on the treatment and management of mNSCLC.
For both samples, physicians completed an electronic patient record form for each patient who met the inclusion criteria, with data extracted from patient medical records. Data included patient demographics and clinical characteristics, diagnostic tests/assessments, biomarker status at advanced stage diagnosis, prior treatment history and associated outcomes, healthcare resource use (HCRU), and hospitalisations.
For the main sample, physicians invited the same patients for whom they completed an electronic patient record form to complete a voluntary patient-reported questionnaire. As the oversample was retrospective, these patients did not complete these questionnaires. The patient-reported questionnaire collected data on patient demographics, disease burden, and quality of life (QoL). QoL was measured using the EQ-5D-5L [21, 22], and the Functional Assessment of Cancer Therapy (FACT), including the FACT-General (FACT-G) and FACT-Lung (FACT-L) [23, 24]. The EQ-5D-5L French value set was used for all countries to remove bias in cross-country comparisons due to country differences in value sets [25].
Using a checkbox, patients provided informed consent to take part in the survey. Data were collected in such a way that patients and physicians could not be identified directly. Physician and patient data were pseudo-anonymized. A code was assigned when data were collected. Upon receipt by Adelphi Real World, data were pseudo-anonymized again to mitigate against tracing them back to the individual. Data were aggregated before being shared with the subscriber and/or for publication.
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines [26] and as such it did not require ethics committee approval. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [27], and Health Information Technology for Economic and Clinical Health Act legislation [28].
Analysis
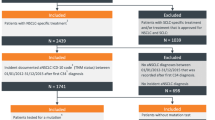
Analyses were performed separately on the main sample and the retrospective oversample. Main sample data were analysed as aggregated values and by country (France, Germany, Italy, Spain, UK). They were also stratified by line of therapy (1L only presented), by biomarker status (PD-L1 expression ≥ 50%, 1–49% and < 1%), by 1L mNSCLC treatment, and by EGFR and ALK biomarker status (only EGFR-WT/ALK-WT patients are included in this analysis). Analyses of the retrospective oversample were stratified by patients diagnosed pre-COVID-19 (up to six months prior to March 2020) and during the COVID-19 pandemic (from March 2020 to time of data collection) (patients with tumour ALK mutations were excluded from this analysis). Patient selection and sample sizes are shown in Fig. 1.
Patient selection and sample sizes. Legend. ALK, anaplastic lymphoma kinase; aNSCLC, advanced non-small cell lung cancer; COVID-19, SARS-CoV-2; EGFR, epidermal growth factor receptor; EGFR-WT/ALK-WT, (i.e., no sensitising EGFR mutation or ALK translocation; wild type); mNSCLC, metastatic non-small cell lung cancer
Data were summarized using descriptive analyses. Means and standard deviations (SD) were calculated for continuous variables, and frequency and percentages were calculated for categorical variables. Continuous variables were compared using t-tests or Mann–Whitney tests, depending on the distribution. Categorical variables were compared using Fisher’s exact tests for variables with two categories and Chi square tests for variables with more than two categories. Ordinal categorical variables were compared using Mann–Whitney tests. A p-value of less than 0.05 was taken as indicating between-group statistically significant differences. All analyses were performed using the software package IBM SPSS Data Collection Survey Reporter Version 7.5 and STATA® Version 16 (StataCorp LP, College Station, USA).
The EQ-5D-5L utility index assessed health status with regard to mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D visual analogue score (VAS) ranges from 0 to 100, where higher scores indicate better quality of life (QoL) and the EQ-5D-5L index total score (French value set) and domain scores range from 0.00 to 1.00, where higher scores indicate better QoL [21, 22] Data for EQ-5D-5L and EQ-5D VAS were compared with normative reference values (EQ-5D, France-specific time to trade off value set 0.892; VAS, overall mean of the total for the five European countries, 77.8) [25]. The minimal clinically important differences (MCID) for (UK based) EQ-5D utility index and EQ-5D VAS are 0.082 and 0.07, respectively [29, 30].
The FACT-G is designed to measure the physical, social, emotional, and functional well-being domains of QoL in patients with cancer [24, 31]. The FACT-G serves as a foundation upon which questions are added to address specific concerns or problems, e.g., to lung cancer FACT-Lung (FACT-L) with its lung cancer subscale and trial outcomes index. The ranges of possible total scores are 0–108 in FACT-G and 0–136 in FACT-L, with higher scores corresponding to a better QoL. Data for FACT-G were compared with normative reference values [32]. The MCID for FACT-L for advanced NSCLC is 2–3-point difference on the lung cancer subscale [33].
Results
Main sample
Physician-reporting and patient-reporting populations
From the Adelphi NSCLC DSP, 248 oncologists/pulmonologists (France: n = 48, Germany: n = 50, Italy: n = 50, Spain: n = 50, UK: n = 50) provided data for a total of 1564 eligible patients with mNSCLC and a total of 598 matched patients also completed the voluntary patient-reported questionnaire (Fig. 1). For this analysis, 1073 patients with EGFR-WT/ALK-WT mNSCLC were included (France: n = 264 [24.6%], Germany: n = 152 [14.2%], Italy: n = 201 [18.7%], Spain: n = 226 [21.1%], UK n = 230 [21.4%]). Patient-reported questionnaires were completed by 262 matched patients (France: n = 41 [15.6%], Germany: n = 57 [21.8%], Italy: n = 46 [17.6%], Spain: n = 83 [31.7%], UK: n = 35 [13.4%]).
Patient demographics and clinical characteristics
Patient characteristics for the total mNSCLC sample who were EGFR-WT/ALK-WT (n = 1073) are shown in (Table 1). At the time of data collection, patients’ mean age (SD) was 66.2 (8.9) years and 65.1% were male.
At data collection, 30.5% and 69.5% of patients had stage IVa and IVb mNSCLC, respectively. Adenocarcinoma (63.7%) and squamous cell carcinoma (33.1%) were the most common histological types. Patients most frequently had metastases to the contralateral lung (47.2%).
Patients most frequently presented with cough (59.3%), fatigue (44.1%), and dyspnoea (42.3%). Hypertension and chronic pulmonary disease were the most frequently cited comorbid conditions experienced by patients with mNSCLC. The majority of patients had a current Eastern Cooperative Oncology Group performance status (ECOG PS) score of 1 (59.6%).
Of the 1073 patients that had EGFR-WT/ALK-WT mNSCLC, the majority had undergone a biopsy (89.0%), blood tests (85.7%), a computerized tomography (CT) scan (83.6%) and fluorodeoxyglucose-positron emission tomography (57.7%) to aid mNSCLC diagnosis (Supplementary Table 1). Tests that were used most frequently to aid diagnosis were also used most commonly for disease monitoring.
Overall, 1021 patients were tested for PD-L1 status; data were available for 1010 of those patients. The majority of patients (n = 1010/1073, 95%) were tested for PD-L1 status at advanced diagnosis (Table 2). PD-L1 expression ≥ 50% and PD-L1 expression of 1–49% were found in 36.0% and 40.9% of all 1010 patients, and 40.3% and 37.6% of 865 patients receiving 1L at the time of data collection, respectively.
Treatment of patients with EGFR-WT/ALK-WT mNSCLC
Among the total EGFR-WT/ALK-WT mNSCLC population (n = 1073), advanced treatment was mostly chemotherapy only (39.2%) followed by immune-oncology monotherapy (IO; 35.0%) (Table 3).
Of 158 patients who had progressed beyond 1L therapy (i.e. 2L +), the mean (SD) time to 1L treatment discontinuation of 5.1 (4.3) months. The full course of 1L treatment was completed as intended by 75.9% of patients, and a complete response was achieved by 6.7% and a partial response by 69.2% of patients. Disease progression was reported for 73.7% of 38 patients who discontinued their 1L treatment early.
Demographic and clinical characteristics by 1L treatment group of patients with EGFR-WT/ALK-WT mNSCLC
To stratify patients by treatment, 1L treatments were grouped by class. Among the 1L treatment groups (total population n = 1086), 39.6% of patients were receiving chemotherapy only, 30.7% were receiving IO, 25.0% IO + chemotherapy, 2.8% chemotherapy combination (multiple chemotherapy drugs), and 1.4% were receiving targeted therapy at the time of data collection (Table 4). The majority of these patients (≥ 55.6%) had adenocarcinoma. There were few patients in the ‘other’ treatment group (n = 7) and this group generally showed different disease characteristics from the other groups.
No differences were observed in age, gender and disease stage according to 1L treatment, compared with the total population of EGFR-WT/ALK-WT mNSCLC patients (Table 4). There was a significant difference between all treatment groups in the proportions of patients with brain metastases (p = 0.0077). ECOG PS significantly differed between all treatment groups (p < 0.0001); the majority of patients in all treatment groups (chemotherapy only, IO, IO + chemotherapy, chemotherapy combination, and targeted therapy) had a PS of 1,excluding the ‘other’ treatment group (PS ≥ 3 in three of seven patients). The proportions of patients with each common symptom were similar among all treatment groups, with the exception of loss of appetite (p = 0.0146), chest pain (p = 0.0029) and weak limbs (p = 0.0019). There was a difference between groups in comorbid hypertension (p = 0.0047), chronic pulmonary disease (p = 0.007), and diabetes without chronic complications (p = 0.0024); current Charlson comorbidity index was ≤ 0.8 for most treatment groups and 1.9 for the ‘other’ patient group (p < 0.0001 all treatment groups).
1L Treatment by histology of patients with EGFR-WT/ALK-WT mNSCLC
There were differences in the 1L treatment received (p < 0.0001) by patients according to the histology of their NSCLC (squamous cell carcinoma, adenocarcinoma, large cell carcinoma and ‘other’) (Table 5); chemotherapy only was most commonly received by patients with adenocarcinoma, squamous cell carcinoma and large cell carcinoma. The next most common treatment was IO for patients with squamous cell carcinoma and large cell carcinoma, and IO + chemotherapy for patients with adenocarcinoma. Treatment response and reason for discontinuation were similar between patient histology groups. The majority of patients who completed 1L treatment achieved a partial response and the most common reason for discontinued treatment was disease progression.
Disease characteristics and 1L treatment of patients with EGFR-WT/ALK-WT mNSCLC by PD-L1 status
In patients with EGFR-WT/ALK-WT mNSCLC (n = 1021), prevalence of PD-L1 < 1%, 1–49% and ≥ 50% expression was 22.9%, 41.1%, and 35.9% patients, respectively (Table 6).
In the 1L treatment setting, the majority of patients with PD-L1 expression of < 1% (62.4% of 234 patients) and 1–49% (52.9% of 420 patients) received chemotherapy only; the majority of patients with PD-L1 ≥ 50% received IO (80.9% of 367 patients).
The median time to 1L treatment discontinuation in the 1L PD-L1-tested EGFR-WT/ALK-WT mNSCLC population who had progressed beyond 1L treatment was 4.0 months (3.9, 4.0, and 4.5 months for patients with PD-L1 expression of < 1%, 1–49%, and ≥ 50%, respectively). The full course of 1L treatment was completed as intended by 77.0% patients (80.4%, 77.1% and 66% of patients with PD-L1 expression of < 1%, 1–49%, and ≥ 50%, respectively), a complete response was achieved by 8.2% of patients and a partial response by 70.9% of patients. No response was reported for twice as many patients with PD-L1 expression of < 1% (31.7%) versus 1–49% (16.0%) and ≥ 50% (16.7%). For the overall 1L EGFR-WT/ALK-WT mNSCLC population for who reasons for early 1L treatment discontinuation were reported (n = 40), disease progression was given as a reason in 70.0% of patients, with no statistical difference between the PD-L1 expression groups.
Quality of life of patients with EGFR-WT/ALK-WT mNSCLC receiving 1L
Patient-reported EQ-5D VAS, EQ-5D utility index, and FACT are reported in Table 7. For the overall population of 260 patients with EGFR-WT/ALK-WT mNSCLC who completed a patient-reported questionnaire, patient-reported EQ-5D VAS mean (SD) score was 67.3 (16.5), which was lower than the mean normative reference value for France (76.8) [25]. Mean (SD) VAS scores ranged from 60.7 (19.86) for France to 71.2 (16.94) for Spain.
Patient mean (SD) EQ-5D-5L utility score was 0.86 (0.17), which was in line with the mean normative reference value for France (0.87) [25]. Mean EQ-5D-5L utility scores ranged from 0.77 (0.29) for France to 0.90 (0.12) for UK. The MCID between patients in France and patients in Germany, Italy, Spain and the UK for EQ-5D utility index was > 0.082, and was > 0.7 between patients in all evaluated countries for EQ-5D VAS.
Patient mean (SD) FACT-G score was 62.8 (15.5), which was noticeably lower than the reported mean US population normative reference value of 80.1 [32]. Mean (SD) FACT-G scores ranged from 57.4 (17.40) for France to 65.1 (15.41) for Germany. Patient mean (SD) FACT-L score was 80.0 (18.8). Mean FACT-L scores ranged from 72.7 (21.4) for France to 83.0 (18.2) for Germany. The MCID was > 2 points for FACT-Lung Cancer Subscale score between patients in France and patients in Germany, Spain and the UK, but not between patients in Germany, Italy, Spain and the UK.
Oversample
Physician and patient populations
For the retrospective oversample, 252 oncologists/pulmonologists (France, n = 51; Germany, n = 50; Italy, n = 50; Spain, n = 50, UK, n = 51) completed retrospective patient record forms for 2537 patients with EGFR-WT mNSCLC (France: n = 504, [19.9%], Germany: n = 501 [19.7%], Italy: n = 501 [19.7%], Spain: n = 515 [20.3%], UK: n = 516 [20.3%]. Of these, 2373 patients were also ALK-WT (EGFR-WT/ALK-WT, France: n = 479 [20.2%], Germany: n = 479 [20.2%], Italy: n = 460 [19.4%], Spain: n = 491 [20.7%], UK: n = 464, [19.6%]).
The retrospective oversample analysis was based around the emergence of COVID-19 in Europe and examined effects of the virus and ‘lockdown’ on the treatment and management of mNSCLC. The pre-COVID-19 period was defined as patients diagnosed from 1st September 2019 to 29th February 2020, and the period during COVID-19 was defined as patients diagnosed from 1st March 2020 (a date where all five European countries were in lockdown due to COVID-19) up to when data collection ended (August 2021). The total sample for analysis included 2373 EGFR-WT/ALK-WT patients; 1148 patients diagnosed in the pre-COVID-19 population and 1225 patients diagnosed in the population sampled during COVID-19 (Fig. 1).
Demographics and clinical characteristics of patients with EGFR-WT/ALK-WT mNSCLC
Patient characteristics for the EGFR-WT/ALK-WT mNSCLC population split by the period in which patients were diagnosed (pre-COVID-19 and during COVID-19) are shown in Table 8.
At the time of most recent consultation, patients’ mean age was 66.4 (8.9) years and 1584 (66.8%) were male. At the time of mNSCLC diagnosis, 935 (39.4%) had stage IVa disease and 1438 (60.6%) had stage IVb disease. Adenocarcinoma (n = 1513; 63.8%) and squamous cell carcinoma (n = 786; 33.1%) were the most prevalent NSCLC histological types. The most common comorbid conditions at time of data collection across the COVID cohorts were hypertension (41.8%), dyslipidaemia (21.1%), and chronic pulmonary disease (20.4%). Characteristics of patients diagnosed pre-COVID and during COVID-19 seemed to be similar.
The majority of patients had undergone a biopsy (n = 2090; 88.1%), blood tests (n = 2058; 86.7%), a CT chest scan (n = 1955; 82.4%), and bronchoscopy (n = 1541; 64.9%) during mNSCLC diagnosis in both diagnosis periods. PD-L1 status at mNSCLC diagnosis was established in 2239 (94.9%) of 2373 patients, and of these 2239 patients who had their PD-L1 expression level determined, 19.7% had an expression < 1%, 46.4% had an expression level of 1%-49% and 34% had an expression level of ≥ 50%. The PD-L1 test result was obtained prior to treatment initiation in 2141 (95.1%) patients. There seemed to be no notable differences in PD-L1 parameters between the pre- and during COVID cohorts.
COVID-19 status of patients with EGFR-WT/ALK-WT mNSCLC
A total of 1268 patients with EGFR-WT/ALK-WT mNSCLC had at least one COVID-19 test; these patients had undergone a mean (SD) of 5.8 (9.1) COVID-19 tests, ranging from 2.7 (2.9) in Spain to 11.8 (17.3) in Germany (Table 9). The majority of patients had tested negative on their most recent COVID test (n = 1231; 95.7%), ranging from 90.0% (n = 261) in Spain to 99.6% (n = 268) in Germany. Over half (n = 727; 56.5%) of patients had taken their most recent test more than two weeks prior to the consultation, and 241 (18.7%) patients had tested within the last two weeks.
At data collection, 1787 (75.3%) of patients were considered to be negative for COVID-19. The last COVID-19 test could have been any time up to the day of data collection, and so the lower percentage of tests considered to be negative was due to more patients having an unknown COVID-19 status at the point of consultation. A total of 40 (1.7%) patients had a current confirmed case of COVID-19 at time of data collection.
Impact of COVID-19 on treatment and management mNSCLC
Physicians reported that the management of 34.7% (n = 823) of patients had been impacted as a result of the COVID-19 pandemic (Table 10). The impact on management affected 78.7% (n = 365) of patients in the UK, 32.4% (n = 159) in Spain, 22.8% (n = 109) in France, 20.9% (n = 96) in Italy, and 19.6% (n = 94) in Germany.
For the total population of patients with EGFR-WT/ALK-WT mNSCLC (n = 2373), a reduction in frequency of consultation was reported for 14.9% (range: 8.1% in France to 22.2% in the UK). Additionally, there was a move to video/telephone consultations for 20.0% of patients, which varied widely between countries; from 0.4% of patients in Germany to 63.4% of patients in the UK.
For their most recent consultation, the majority of patients (87.7%) were seen face-to-face with their physician, ranging from 92.7% of patients in Spain, 96% in Germany, 96.3% in Italy, and 97.3% of patients in France. However, the face-to-face consultation rate was 55.2% for patients in the UK, where 33.6% of patients had telephone consultations and 11.0% had consultations by video/online links. In France, Germany, Italy, and Spain, telephone and video/online consultations were held with < 5% and ≤ 2% of patients, respectively.
Treatment of patients with EGFR-WT/ALK-WT mNSCLC diagnosed pre- and during COVID-19
Of 2372 patients with EGFR-WT/ALK-WT mNSCLC, pembrolizumab (53.5%) and carboplatin (45.4%) were the most frequent 1L therapies (either as monotherapy or in combination) used both pre-COVID-19 (n = 1147) and during COVID-19 (n = 1225). Between the patients diagnosed pre- and during COVID-19, there was little changes in use of the majority of mNSCLC therapies. 1L immunotherapy, either as monotherapy or combination therapy, was prescribed in 64.2% of the population diagnosed during COVID-19 and 47.8% of patients diagnosed pre-COVID-19; the between-group difference was mostly observed in immunotherapy combination therapy (Table 11).
Specifically, treatment use of pembrolizumab-based treatment was 60.9% of patients diagnosed during COVID-19 and 45.7% of patients diagnosed pre-COVID-19. Conversely, cisplatin was used by 22.8% and 17.2% of patients diagnosed pre-COVID-19 and during COVID-19, respectively, and pemetrexed was used by 38.5% and 34.4% of patients, respectively.
Discussion
This analysis of real-word patient data evaluated the characteristics and the current diagnostic landscape of patients with EGFR/ALK mNSCLC across five European countries, and the impact of COVID-19 on the treatment and management of this population. Immunotherapy is considered the standard approach for most patients with newly diagnosed EGFR-WT/ALK-WT mNSCLC and tumour PD-L1 ≥ 50% [11]. However, although there was indication that use of chemotherapy was being replaced by immunotherapies, chemotherapy-based regimens were frequently prescribed as 1L treatment.
With the evolving 1L treatment landscape and the introduction of immunotherapy, we found approximately similar usage rates of chemotherapy only and IO only, with one quarter of patients treated with IO + chemotherapy, in the current 1L setting.
PD-1 inhibitors play an important role in the treatment of patients with mNSCLC and, alongside their development, predictive biomarker testing for tumour genomic aberrations in such genes as EGFR or ALK, and PD-L1 expression have become mandatory in most European countries [11]. The likelihood of clinical benefit from anti-PD-1/PD-L1 agents in the 1L and 2L setting is related to the extent of PD-L1 expression on tumour cells [34]. The mandatory treatment threshold of PD-L1 expression for pembrolizumab is ≥ 50% in 1L and ≥ 1% in second line [11]. This analysis demonstrated that over one-third of patients with EGFR-WT/ALK-WT mNSCLC had PD-L1 expression of ≥ 50%. The majority of biomarker results of patients were received before initiation of 1L treatment and therefore it may be assumed that these results were available to inform the 1L treatment prescription. PD-L1 ≥ 50% is a reimbursement criterium in prescribing IO (pembrolizumab) for a number of markets [35], and therefore would play a significant role in informing 1L treatment.
Anti-PD-1/PD-L1 treatments are considered to be the cornerstone of 1L therapy for patients with aNSCLC lacking a targetable driver alteration, prescribed as monotherapy for patients with aNSCLC with tumour cell PD-L1 expression ≥ 50%, and typically as combination regimens with platinum-doublet chemotherapies for patients with low or absent PD-L1 expression [36].
While the extent of tumour cell PD-L1 expression is critical to treatment selection, in patients whose 1L treatments do not follow guidelines for PD-L1 expression, many clinical factors such as comorbidities, performance status, or contraindications are considered when making the treatment decision. Patient preferences might also be relevant, in addition to factors such as progression-free survival, treatment delays, tumour-associated symptoms, treatment-related side effects, and out-of-pocket costs [37, 38].
Our analysis also demonstrated that QoL was impaired in patients with EGFR-WT/ALK-WT mNSCLC, including relative to normative reference values, and particularly for patients in France. Patients in France also differed from patients in other countries in terms of MCIDs for EQ-5D utility index and for FACT-Lung Cancer Subscale scores (excluding Italy). A real-world outcomes study of patients with mNSCLC who received IO or IO + chemotherapy showed that patient QoL (European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [QLQ-30]) was similar between those on IO and IO + chemotherapy and not related to weeks on these treatments. Indirect comparison with clinical trial data showed that global QoL scores were worse than those 1L single-agent IO, alongside higher than expected symptom burden [39]. KEYNOTE-024 indicated improved QOL in patients prescribed pembrolizumab compared with platinum-doublet chemotherapy [40].
Additional to being the most common causes of cancer-related death worldwide [41], lung cancer is one of the most prevalent tumour types among patients with cancer who also have COVID-19 [42]. Patients with lung cancer are particularly vulnerable to COVID-19 infection, likely because abnormalities in their respiratory epithelium enable rapid entry of the virus into the lungs [42]. In this analysis of the impact of the COVID-19 pandemic, there were minimal differences in the demographics and clinical characteristics of patients with EGFR-WT/ALK-WT mNSCLC diagnosed in the pre-COVID-19 period and diagnosed during the COVID-19 period.Potential delays in diagnosis during the pandemic may be suggested by the greater difference between the frequency of a stage IVb and stage IVa diagnosis within the group diagnosed during COVID-19 than that found within the pre-COVID-19 group, although there was no apparent change in the tests and assessments used within the two diagnostic periods.
Nevertheless, COVID-19 had a substantial impact on patients’ management across European countries, with at least one area of management impacted for over one-third of all patients. The greatest impact appeared to be in the UK, where management was affected for approximately 80% of patients, specifically fewer consultations and tests/investigations, a move to video and telephone consultations from the usual face-to-face appointments, and prescribed treatment changes. These impacts could have been a result of the recommendations and strict guidelines of the National Health Service in the UK during the pandemic compared to the other countries. Patients in Germany appeared to be overall least impacted by the pandemic with regards to their mNSCLC treatment and management, particularly with minimal change in method of consultation and few treatment changes.
The differences in impact to patient management across Europe was likely to reflect the pressures that COVID-19 placed on health care systems and healthcare delivery. In the UK, COVID-19 has resulted in remote consultations becoming the new standard for patients with lung cancer, with this means of communication likely to remain a vital part of the diagnostic pathway [43]. In a Dutch survey, 30% of 2664 patients with cancer reported consequences for their oncological management, of which conversion from hospital visit to consultation by phone or video was most frequently reported [44]. Clinicians have had to balance the risk of delaying evaluation and management against those of exposing patients to COVID-19 in hospital settings and exposing healthcare professionals to asymptomatic patients. Moreover, the disruption from COVID-19 exposure and resource reallocation, as a result of the pandemic, have led to the development of new recommendations to replace current guidelines for clinicians managing patients with lung cancer such as delays in evaluation and treatment in specific cases [45].
Decision making in the treatment of patients with lung cancer during the COVID-19 pandemic has also presented challenges as to whether to offer, modify, postpone or cancel treatments [46]. While few changes to the prescribed treatment were observed, there seemed to be a small shift towards use of immunotherapy at 1L in those diagnosed during COVID-19 period from the pre-COVID period. Chemotherapy and immunotherapy have previously been reported to be the most frequently adjusted treatments during the pandemic [44].
Other factors may also contribute to the apparent changes in treatment patterns during the COVID-19 pandemic. Increased prescribing of immunotherapy may have been associated with recent advances in 1L immunotherapy. In November 2020, within the period used to define the COVID-19 cohort for this analysis, the European Medicines Agency approved nivolumab plus ipilimumab with two cycles of chemotherapy for 1L treatment of mNSCLC, in adults whose tumours have no sensitising EGFR mutation or ALK translocations [47]. Moreover, guidelines for treatment made during the COVID pandemic recommend priorities for patients with metastatic disease, including use of 1L chemotherapy, IO + chemotherapy, and IO to improve prognosis, cancer-related symptoms, and QoL [48]. Anti-PD-(L)1 scheduled treatment cycles may also be modified/delayed to reduce clinical visits. Findings from several registries indicate that patients treated with immunotherapy alone have equivalent or better outcomes than those receiving other cancer treatments [46]. As such, immunotherapy has mainly been continued, but with the use of longer cycle options where available, and chemotherapy-based regimens have been used only when necessary [43]. In addition, when it comes to the implementation of new therapeutic strategies such as immunotherapy there is usually a delay between reimbursement and regulatory approval which impacts the timing of real-world implementation of new strategies. This delay can yield unexpected results when it comes to characterisation of real-world treatment use in clinical practice, particularly in this case the use of immunotherapy. Together, these findings suggest that the increase in use of 1L immunotherapy in our study would have occurred regardless of the COVID-19 pandemic, due to evolving treatment landscape.
Strengths and limitations
The DSP approach to collecting data has limitations, including its point-in-time design, which prevents any conclusions about causal relationships but allows for identification of significant associations. The DSP is not based on a true random sample of physicians; while minimal inclusion criteria governed the selection of the participating physicians, participation was influenced by willingness to complete the survey. Patients participating in the surveys may not reflect the general mNSCLC population, as patients who visit more frequently may be more severely affected, require more monitoring, treatment adjustments, or have more emergency visits than those who do not consult their physician as frequently. They also represent a pragmatic sample that may not be representative of the overall population of physicians treating NSCLC. Patient diagnosis and response to treatment was based on the judgement and diagnostic skills of the respondent physician, as there was no formalized diagnostic or response checklist, although this is entirely consistent with the decisions made by physicians in real-world clinical practice. Within the main sample, patients were recruited prospectively at the time of consultation, and the oversample was collected retrospectively. The quality of these data depends on the accurate reporting of information by physicians and patients, and therefore may be subject to recall bias, however data were collected at time of consultation and physicians had access to historical medical records, which is expected to reduce this potential for bias.
The impact of COVID-19 on diagnosis and treatment in 1L mNSCLC may not have been fully apparent at the point of data collection during the pandemic; there may be longer-term impacts of COVID-19 on diagnosis and treatment. However, it was possible to explore the initial impact of COVID-19 by comparing patients diagnosed pre-COVID-19 with those diagnosed during the COVID-19 when physicians and patients across Europe were in national ‘lockdown’. Our data look at specific time periods before and during the COVID-19 pandemic, and the findings are likely to change over time with the waves of infection within countries and as health systems adapted to operating with the disease.
Conclusions
This analysis of the characteristics and the current diagnostic landscape in patients with EGFR-WT/ALK-WT mNSCLC across Europe found that IO as well as chemotherapy-based regimens were frequently prescribed as 1L treatment. The majority (~ 80%) of patients with PD-L1 expression of ≥ 50% were receiving 1L IO, which was used across all histological types investigated (squamous cell carcinoma, adenocarcinoma, large cell carcinoma). However, QoL of these patients was generally lower than normative reference values and variable across Europe, implying the need for more effective use of current treatments or novel therapies to manage patients with EGFR-WT/ALK-WT mNSCLC.
Investigating the impact of COVID-19 on the treatment of patients with EGFR-WT/ALK-WT mNSCLC, there was indication of delays in diagnosis during COVID-19, but with no apparent change in the tests and assessments used. Additionally, COVID-19 had a substantial impact on patients’ management across the five European countries. The UK was particularly affected in terms of consultations, tests/investigations, and prescribed treatment changes, while the impact on patients in Germany appeared to be relatively low. Few changes were made to prescribed treatments during COVID-19 but there was a small shift towards use of 1L immunotherapy potentially as a result of the changing therapeutic landscape.
Despite immunotherapy, current treatment for mNSCLC remains suboptimal, with response and sustained effectiveness in only the minority of patients [48]. Further investigation into characterisation of patients with mNSCLC is warranted, alongside its potential to guide treatment choice, and all novel potentially effective immune therapies for mNSCLC should be evaluated, particularly with the advent of the COVID-19 pandemic.
Availability of data and materials
All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hollie Bailey at hollie.bailey@adelphigroup.com.
References
World Health Organization. International Agency for Research on Cancer. Lung. Fact sheet 15. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf. Accessed 21 Feb 2023.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
Cancer.Net. Lung Cancer – Non-small cell: Statistics. 2021. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics. Accessed 21 Feb 2023.
Surveillance, Epidemiology, and End Results (SEER 17, 2012–2018 data) Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html Accessed 21 Feb 2023.
Chaft JE, Rimner A, Weder W, Azzoli CG, Kris MG, Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18(9):547–57.
To KKW, Fong W, Cho WCS. Immunotherapy in treating EGFR-Mutant lung cancer: current challenges and new strategies. Front Oncol. 2021;11:635007.
Tong X, Tanino R, Sun R, Tsubata Y, Okimoto T, Takechi M, Isobe T. Protein tyrosine kinase 2: a novel therapeutic target to overcome acquired EGFR-TKI resistance in non-small cell lung cancer. Respir Res. 2019;20:270.
Tabbò F, Reale ML, Bironzo P, Scagliotti GV. Resistance to anaplastic lymphoma kinase inhibitors: knowing the enemy is half the battle won. Transl Lung Cancer Res. 2020;9:2545–56.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, WHO Panel, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.; ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Originally published: Ann Oncol. 2018;29(Suppl 4):iv192-iv237; Updated published 15 September 2020. https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer Accessed 21 Feb 2023.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66.
Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7–H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119(2):317–27.
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7–H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–100.
Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, Singh S, Wong S, Garner N, Leblanc H, Bunch RT, Blanset D, Selby MJ, Korman AJ. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–56.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin. 2008;24:3063–72.
Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6:e010352.
Higgins V, Piercy J, Roughley A, Milligan G, Leith A, Siddall J, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–80.
Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81(2):288–93.
EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
EuroQol Research Foundation. EQ-5D-5L User Guide.v3.0 2019. EuroQol Research Foundation, Rotterdam. The Netherlands. https://euroqol.org/publications/user-guides/. Accessed 21 Feb 2023.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Janssen B, Szende A. Population Norms for the EQ-5D. 2013 Sep 26. In: Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D [Internet]. Dordrecht (NL): Springer; 2014. Chapter 3. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500364/. Accessed 21 Feb 2023.
European Pharmaceutical Market Research Association (EphMRA) Code of Conduct. Updated August 2022. Available from: https://www.ephmra.org/code-conduct-aer Accessed on 21 Feb 2023.
US Department of Health and Human Services. Summary of the HIPAA Privacy Rule; 2003. Available from: http://www.hhs.gov/sites/default/files/privacysummary.pdf. Accessed on 21 Feb 2023.
Health Information Technology. Health Information Technology Act. Available from: https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf. Accessed 21 Feb 2023.
Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Minimal Clinically Important Difference in EQ-5D: We Can Calculate it – But Does That Mean We Should? 2017. https://www.ispor.org/docs/default-source/presentations/1066.pdfI Accessed 21 Feb 2023.
American Thoracic Society. Quality of life resource. Functional Assessment of Cancer Therapy: Fatigue (FACT-F). https://qol.thoracic.org/sections/instruments/fj/pages/fact-f.html. Accessed 21 Feb 2023.
Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005;28(2):192–211.
Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J Clin Epidemiol. 2017;89:188–98.
Kerr KM, Hirsch FR. Programmed Death Ligand-1 Immunohistochemistry: Friend or Foe? Arch Pathol Lab Med. 2016;140:326–31.
Denis H, Bermudez E, Nowak F, Negellen S. Dahan M. Reference frameworks linked with the access to innovative therapies.
Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18:625–44.
Mühlbacher AC, Bethge S. Patients’ preferences: a discrete-choice experiment for treatment of non-small-cell lung cancer. Eur J Health Econ. 2015;16:657–70.
MacEwan JP, Gupte-Singh K, Zhao LM, Reckamp KL. Non-small cell lung cancer patient preferences for first-line treatment: a discrete choice experiment. MDM Policy Pract. 2020;5:2381468320922208.
Steffen McLouth LE, Lycan TW Jr, Levine BJ, Gabbard J, Ruiz J, Farris M, et al. Patient-reported outcomes from patients receiving immunotherapy or chemoimmunotherapy for metastatic non-small-cell lung cancer in clinical practice. Clin Lung Cancer. 2020;21(3):255-63.e4.
Brahmer JR, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–9. https://doi.org/10.1016/S1470-2045(17)30690-3.
World Health Organization. Cancer. Geneva, Switzerland: WHO (2022). Available at: https://www.who.int/health-topics/cancer#tab=tab_1. Accessed 21 Feb 2023.
Lemos AEG, Silva GR, Gimba ERP, Matos ADR. Susceptibility of lung cancer patients to COVID-19: a review of the pandemic data from multiple nationalities. Thorac Cancer. 2021;12:2637–47.
Round T, L’Esperance V, Bayly J, Brain K, Dallas L, Edwards JG, et al. COVID-19 and the multidisciplinary care of patients with lung cancer: an evidence-based review and commentary. Br J Cancer. 2021;125:629–40.
de Joode K, Dumoulin DW, Engelen V, Bloemendal HJ, Verheij M, van Laarhoven HWM, et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients’ perspective. Eur J Cancer. 2020;136:132–9.
Mazzone PJ, Gould MK, Arenberg DA, Chen AC, Choi HK, Detterbeck FC, et al. Management of Lung Nodules and Lung Cancer Screening During the COVID-19 Pandemic: CHEST Expert Panel Report. Chest. 2020;158(1):406–15.
Passaro A, Bestvina C, Velez M, Garassino MC, Garon E, Peters S. Severity of COVID-19 in patients with lung cancer: evidence and challenges. J Immunother Cancer. 2021;9:e002266.
Bristol Myers Squibb. Press release. Bristol Myers Squibb Receives European Commission Approval for Opdivo (nivolumab) plus Yervoy (ipilimumab) with Two Cycles of Chemotherapy for First-Line Treatment of Metastatic Non-Small Cell Lung Cancer. 2020. https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Receives-European-Commission-Approval-for-Opdivo-nivolumab-plus-Yervoy-ipilimumab-with-Two-Cycles-of-Chemotherapy-for-First-Line-Treatment-of-Metastatic-Non-Small-Cell-Lung-Cancer/default.aspx. Accessed 21 Feb 2023.
European Society for Medical Oncology. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/lung-cancer-in-the-covid-19-era. Accessed 21 Feb 2023.
van der Hoorn IAE, Flo´ rez-Grau G, van den Heuvel MM, de Vries IJM and Piet B. Recent Advances and Future Perspective of DC-Based Therapy in NSCLC. Front Immunol. 2021;12:704776.
Acknowledgements
A substantial contribution to the analysis and critical review of the paper was made by Matthew Last, Adelphi Real World.
Medical writing and editorial assistance were provided by Sue Libretto, PhD, of Sue Libretto Publications Consultant Ltd (Hertfordshire, UK).
The FACIT and all related works are owned and copyrighted by, and the intellectual property of David Cella, Ph.D. Permission for use of the FACT questionnaire is obtained by contacting Dr. Cella at information@facit.org.
Previous publications
Bailey H, Lee A, Eccles L, Yuan Y, Khela K, Hall J, Last M, Varol N. The Evolving Diagnostic And Treatment Landscape In Metastatic Non-Small Cell Lung Cancer (mNSCLC) Across Europe – A Real World Evidence Survey. Presented at Virtual ESMO-Immuno-Oncology Congress 2021, 8–11 December 2021.
Bailey H, Lee A, Eccles L, Yuan Y, Last M, Burlison H, Forshaw C. Exploring the Real-World Impact Of COVID-19 On First Line (1L) Treatment and Management of EGFR-Wild Type (EGFR-WT) and ALK-Wild Type (ALK-WT) Metastatic Non-Small Cell Lung Cancer (mNSCLC) Across Europe. Presented at Virtual ESMO-Immuno-Oncology Congress 2021, 8–11 December 2021.
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Non-Small Cell Lung Cancer Disease Specific Programme™ (NSCLC DSP).
Bristol Myers Squibb did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Real World NSCLC DSP. The DSP is a wholly owned Adelphi Real World product. Bristol Myers Squibb is one of multiple subscribers to the DSP.
Publication of survey results was not contingent on the subscriber's approval or censorship of the journal BMC Cancer.
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception or design, or analysis and interpretation of data; drafting and revising the article; providing intellectual content of critical importance to the work described; and final approval of the version to be published, and therefore meet the criteria for authorship in accordance with the International Committee of Medical Journal Editors guidelines. In addition, all named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Using a checkbox, patients provided informed consent to take part in the survey. Data were collected in such a way that patients and physicians could not be identified directly.
The DSP uses survey techniques to collect anonymized data that is analyzed in aggregated form. Physician and patient data were pseudo-anonymized. A code was assigned when data were collected. Upon receipt by Adelphi Real World, data were pseudo-anonymized again to mitigate against tracing them back to the individual. Data were aggregated before being shared with the subscriber and/or for publication.
This research was submitted to the Western Institutional Review Board, study protocol number AG8759, which granted ethical exemption.
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines and as such it did not require ethics committee approval. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996, and Health Information Technology for Economic and Clinical Health Act legislation.
Consent for publication
Not applicable.
Competing interests
A Lee, L Eccles, Y Yuan, and N Varol are employees of Bristol Myers Squibb, and H Bailey, M Last, H Burlison, and C Forshaw are employees of Adelphi Real World.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bailey, H., Lee, A., Eccles, L. et al. Treatment patterns and outcomes of patients with metastatic non-small cell lung cancer in five European countries: a real-world evidence survey. BMC Cancer 23, 603 (2023). https://doi.org/10.1186/s12885-023-11074-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11074-z