Abstract
Background
Patients from non-small cell lung cancer (NSCLC) controlled clinical trials do not always reflect real-world heterogeneous patient populations. We designed a study to describe the real-world patient characteristics and treatment patterns of first-line treatment in patients in the US with NSCLC.
Methods
This was an observational, retrospective cohort study based on electronic medical records of US adults with locally advanced or metastatic disease in the ConcertAI Patient360 NSCLC database who initiated first-line treatment with anti-programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) therapy between July 2016 and December 2020. The analysis used patient attributes, clinical characteristics, and treatments from each patient’s medical records.
Results
A total of 2175 patients were eligible for analysis. The median age was 68 years, and 26.2% of the patients were ≥75 years old. At treatment initiation, 96.4% and 3.6% of the patients had Stage 4 and Stage 3 (B or C) NSCLC, respectively. The most common histology type was nonsquamous adenocarcinoma (66.4%), and 19.8% had Eastern Cooperative Oncology Group performance status ≥2. Immunosuppressive medications were being used by 17.7% of patients, and 11.0% were immunocompromised. Almost all patients had metastases: 64.6% had 1, 23.2% had 2, and 8.0% had ≥3 metastatic sites. Brain metastases were present in 22.9% of patients. Treatment evolution was observed with first-line standard of care shifting from single-agent immunotherapy in 2016 (90.2%) to combination immunotherapy and chemotherapy in 2020 (60.2%).
Conclusion
Between 2016 and 2020, the first-line treatment paradigm for advanced NSCLC in the US shifted from anti–PD-1/PD-L1 monotherapy to combination chemoimmunotherapy, with increasing biomarker testing. Further research in heterogeneous patient populations to characterize treatment strategies is warranted.
Similar content being viewed by others
Background
Lung cancer was the leading cause of cancer-related deaths worldwide in 2020, and approximately 70% of cases were locally advanced or metastatic disease at diagnosis, of which 80–85% were non-small cell lung cancer (NSCLC) [1,2,3].
Clinical trials in recent years have examined a variety of treatment strategies, including monotherapy and combination immunotherapy (IO) compared with chemo-therapy, the previous standard of care for advanced NSCLC [4,5,6,7,8,9,10,11,12,13,14,15]. As a result, the treatment landscape for advanced NSCLC without actionable driver mutations in EGFR or ALK has shifted from chemotherapy to IO with chemotherapy. Immunotherapies target negative immunologic regulators such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway [16].
Between 2015 and 2021, IO agents approved by the United States (US) Food and Drug Administration (FDA) as first- or second-line therapy for NSCLC without driver mutations included: the anti–PD-1 antibodies nivolumab, pembrolizumab, and cemiplimab; the anti–PD-L1 antibodies durvalumab and atezolizumab; and the anti–CTLA-4 antibody ipilimumab [17]. Use of first-line IO to treat advanced NSCLC has increased substantially in the US since the initial approvals in 2016 [18]. First-line FDA approvals occurred for IO monotherapy with pembrolizumab in October 2016 and for pembrolizumab combination therapy with chemotherapy in May 2017 [19]. Atezolizumab combination therapy was approved in December 2018 [19]. Nivolumab + ipilimumab was approved in May 2020, along with atezolizumab monotherapy [19]. Finally, cemiplimab monotherapy was approved in February 2021 [19].
Clinical trials are designed to enroll selected patients (ie, those with good performance status, adequate organ function, without certain comorbidities, and who are not immunocompromised), and treatments are administered in highly controlled settings. Therefore, it can be challenging to generalize the findings to the more clinically heterogeneous patient populations seen in practice [18]. Here we report the findings from a real-world observational study that examined the ConcertAI Patient360 NSCLC database to describe key evidence gaps related to clinical characteristics and treatment patterns in patients in the US who initiated first-line treatment with IO mono-therapy or combination therapy for advanced NSCLC from 2016 to 2020.
Methods
Study design and data source
This was a non-interventional, observational, retrospective cohort study of patients with advanced NSCLC who received treatment as documented in the Patient360 NSCLC electronic medical record (EMR) database (ConcertAI, Cambridge, MA). This database sources patient EMRs, including unstructured notes and scans, from multiple oncologic partnerships that are EMR-system agnostic. The database consists of de-identified data from patients treated at various academic (~20%) and community (~80%) oncology centers across the US (~15% in the Northeast, ~25% in the Midwest, ~40% in the South, and ~20% in the West, as defined by US Census Bureau geographic regions).
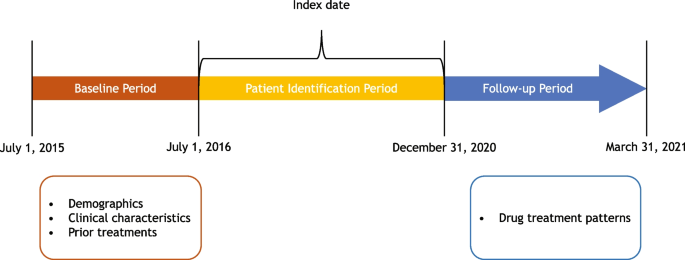
The overall study period was from July 1, 2015, to March 31, 2021 (Fig. 1). The study design included a baseline period, a patient identification period, and a follow-up period. The index date was defined as the date on which the patient initiated first-line anti–PD-1/PD-L1 therapy for locally advanced or metastatic NSCLC. The baseline period spanned from the patient’s earliest NSCLC diagnosis in the database, starting from July 1, 2015, to the index date. If more than one assessment for the same variable of interest was available within this baseline period, the assessment closest to the index date was selected. During the patient identification period (July 1, 2016, to December 31, 2020; 3 months prior to the end of the follow-up period), eligible patients with advanced NSCLC who initiated first-line systemic treatment were identified. The follow-up period began one day post-index date and ended either on the date of death or on March 31, 2021 (end date of the database).
Patients
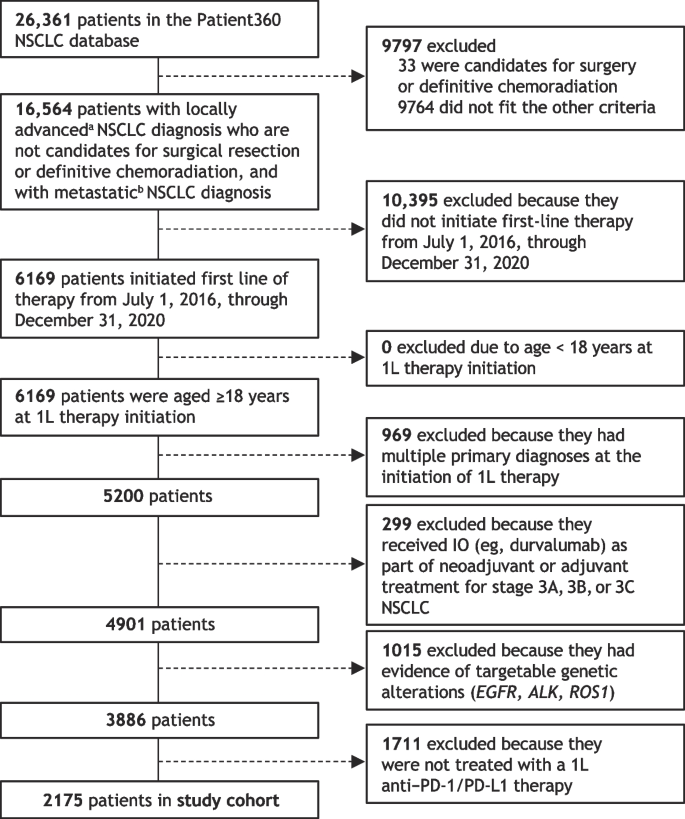
The focus of this analysis was patients with advanced NSCLC who were treated with first-line anti–PD-1/PD-L1 therapy. Patients included in the final study cohort for this analysis (Fig. 2) had to have (i) a diagnosis of locally advanced, unresectable Stage 3B and 3C, or Stage 4 metastatic NSCLC; (ii) no evidence of candidacy for surgical reconstruction or definitive chemoradiation; (iii) started first-line therapy from July 1, 2016, through December 3, 2020; (iv) age ≥18 years at the time of first-line therapy initiation; (v) absence of a multiple primary cancer diagnosis at the time of initiating first-line therapy; (vi) no evidence of IO treatment in Stage 3 (A, B, or C) NSCLC as part of neoadjuvant or adjuvant treatment; (vii) no evidence of targetable genetic alterations (eg, EGFR, ALK, ROS1); and (viii) received first-line treatment with an anti–PD-1/PD-L1 agent for NSCLC.
Patient selection and study cohort derived from the Patient360 NSCLC database. 1L, first line; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; IO, immunotherapy; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase. aStage 3 (3B, 3C) or neoplasm, secondary; bStage 4, M1 or neoplasm, metastatic
Study objectives
The study objectives were to describe the demographics, clinical characteristics, and drug treatment patterns in patients with advanced NSCLC who were treated with first-line anti–PD-1/PD-L1 therapy. This was done for the overall cohort and by year of initiation of first-line therapy (2016 to 2020) and stratified by subgroups of interest that represent clinically relevant, unmet-needs populations. Additionally, clinical characteristics were stratified by first-line therapy, ie, anti–PD-1/PD-L1 monotherapy or anti–PD-1/PD-L1 therapy combined with platinum-based chemotherapy.
Ethical considerations
The study was performed in accordance with the Declaration of Helsinki and relevant International Council for Harmonisation, Good Clinical Practice, and Good Pharmacoepidemiological Practice guidelines. As no identifiable protected health information was extracted or accessed for the conduct of this study, ethics approval was deemed unnecessary under the Code of Federal Regulations Title 45, Part 46, Section 46.104(d)(4)(ii) (45CFR46.104[d][4][ii]).
Statistical analysis
This was an observational, descriptive cohort study of real-world patients with advanced NSCLC; hence, no hypothesis was tested, and no formal sample size calculation was required. Study measures (ie, patient demographics, disease and clinical characteristics, recorded treatment) were summarized with descriptive statistics and presented as frequencies and percentages. Statistical analyses were conducted using the Palantir Foundry implementation of PySpark.
Results
Cohort disposition
From an initial population of 26,361 curated patients available in the NSCLC database, 3886 patients met all criteria and were started on first-line therapy in the study period, and 2175 of these were treated with an anti–PD-1/PD-L1 agent in the first line during the specified period and were included in the study cohort (Fig. 2).
Patient characteristics
Baseline demographics and clinical characteristics of the patients in the study cohort (n = 2175) are summarized in Table 1, overall and by year of first-line therapy initiation. In this eligible population, the median age was 68.0 years (range, 19.0 to 88.0 years), and 53.7% were male. The age group distribution of the cohort remained constant from 2016 to 2020. Most patients were White (76.8%); African Americans comprised the second largest racial group (13.2%). Current or former smokers made up 87.9% of study patients, with the proportion increasing from 83.3% of those who initiated first-line therapy in 2016 to 92.9% of those who initiated first-line therapy in 2020.
Approximately two-thirds of patients (66.4%) had nonsquamous NSCLC with adenocarcinoma histology (Table 1). Nearly all patients (96.4%) had Stage 4 disease, and 95.7% had one or more metastatic sites. Bone (29.8%), brain (22.9%), and other lung (19.9%) were the most common metastatic sites at the index date. More patients younger than 65 years (29.3%) had evidence of brain metastases than those aged 65–74 years (21.7%) and ≥75 years (15.1%) (Table 2). More than three-quarters (78.9%) of patients had visceral site(s) of metastases at the index date, and 37.6% had nonvisceral site(s) of metastases (Table 1). Almost two-thirds of patients (64.7%) with reported Eastern Cooperative Oncology Group performance status (ECOG PS) had a score of 0 or 1, and 19.8% had a score of 2 or higher.
Only one-quarter (24.4%) of patients had recorded evidence of receiving a second line of therapy (Table 1). Of those with first-line therapy starting in 2016–2017, only 31.6% received a subsequent, second line of therapy in the study period. Among patients with first-line therapy starting between 2018 and 2020, 22.4% had subsequent treatment with a second line of therapy in the study interval.
Most patients (89.0%) were not immunocompromised, defined as having human immunodeficiency virus (HIV) or taking long-term (≥30 days) immunosuppressive medications, at the index date. Nearly all patients had a negative history of HIV, hepatitis B and C, and autoimmune disease at the index date (99.8%, 100.0%, and 98.5%, respectively). A higher percentage of female patients used immunosuppressive medication at the index date compared with male patients (20.1% versus 15.7%) (data not shown). Use of immunosuppressive medication at the index date decreased each year, with initial use at 25.5% of patients in 2016, down to 13.5% in 2020.
Overall, only 60.1% of study patients had evidence of testing for RET-, BRAF-, or MET-targetable genetic alterations, and 8.2% of all patients had one or more positive test results (Table 1). More patients who initiated first-line therapy between 2018 and 2020 had evidence of testing (64.2%) than those who initiated first-line therapy from 2016 to 2017 (45.6%). Almost three-quarters (72.0%) of study patients had a PD-1/PD-L1 expression test recorded, and 45.0% of study patients had a qualitative positive test result recorded. However, only 18.1% of patients with a positive PD-1/PD-L1 test had a result reported numerically. The proportion of patients tested for PD-1/PD-L1 expression increased from 53.9% in 2016 to 79.2% in 2020, and the proportion of study patients who had a positive result increased from 33.3% to 47.2% over the same period.
Treatment patterns
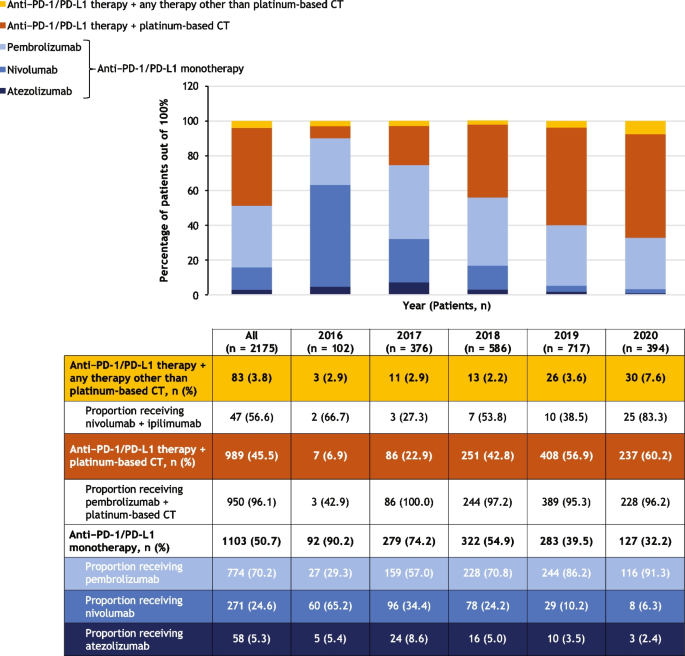
Of the 2175 study cohort patients, 102 initiated first-line therapy in 2016, 376 in 2017, 586 in 2018, 717 in 2019, and 394 in 2020. First-line treatment patterns are summarized in Fig. 3.
Treatment patterns overall and stratified by year of first-line therapy initiation. The graph shows the numbers of patients who received the treatments indicated in the legend. The table shows the numbers and percentages of patients who received each treatment regimen, along with proportions of patients receiving different types of anti–PD-1/PD-L1 monotherapy, pembrolizumab plus platinum-based chemotherapy, and nivolumab plus ipilimumab, which are shown below the relevant regimen category. During 2020, the COVID-19 pandemic may have impacted routine clinical care. Percentages reported for subcategories are proportions in the respective category, not the whole. CT, chemotherapy; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1
The most common first-line treatment overall was anti–PD-1/PD-L1 monotherapy (in 50.7% of 2175 patients), followed by anti–PD-1/PD-L1 therapy in combination with a platinum-based chemotherapy (45.5%) and anti–PD-1/PD-L1 therapy in combination with any other therapy (3.8%) (Fig. 3). The proportion of patients initiating first-line treatment with anti–PD-1/PD-L1 monotherapy decreased from 90.2% of the 102 patients in 2016 to 32.2% of the 394 patients in 2020, while the proportion of patients initiating first-line anti–PD-1/PD-L1 combined with platinum-based chemotherapy increased from 6.9% to 60.2%. PD-1/PD-L1 expression levels influence treatment selection, and PD-1/PD-L1 expression data was limited at the time of the data cutoff.
Most patients who initiated treatment with an anti–PD-1/PD-L1 monotherapy in this study cohort received pembrolizumab (70.2% of the 1103 patients who received monotherapy), followed by nivolumab (24.6%), then atezolizumab (5.3%) (Fig. 3). Use of pembrolizumab increased from 29.3% of the 92 patients treated with anti–PD-1/PD-L1 monotherapy in 2016 to 91.3% of the 127 monotherapy-treated patients in 2020, whereas the use of nivolumab declined from 65.2% in 2016 to 6.3% in 2020. Pembrolizumab was consistently the most commonly used anti–PD-1/PD-L1 mono-therapy across patient subgroups including age group, sex, number of metastatic sites, evidence of brain metastasis, ECOG PS, smoking status, and immunocompromised status (Table 3). It was the most common anti–PD-1/PD-L1 monotherapy, having been administered to 73.2% of 691 patients with nonsquamous and 61.4% of 267 patients with squamous NSCLC histology.
Pembrolizumab combined with platinum-based chemo-therapy was the most common combination regimen, accounting for 96.1% of the 989 study cohort patients who initiated first-line treatment with anti–PD-1/PD-L1 therapy combined with platinum-based chemotherapy (Fig. 3). Of the 83 patients (3.8% of the cohort) who initiated first-line treatment with anti–PD-1/PD-L1 therapy in combination with any other therapy, 56.6% received nivolumab plus ipilimumab.
From 2018 to 2020, the most common first-line treatment among patients younger than 65 years was anti–PD-1/PD-L1 in combination with platinum-based chemotherapy (61.1%); and in those 75 years or older, the most common regimen was anti–PD-1/PD-L1 monotherapy (56.7%) (data not shown). During the same period, 55.3% of patients with nonsquamous NSCLC were treated with anti–PD-1/PD-L1 in combination with platinum-based chemotherapy, and 40.8% received anti–PD-1/PD-L1 monotherapy (data not shown). The proportions of patients with 1, 2, or ≥3 metastatic sites treated with anti–PD-1/PD-L1 in combination with platinum-based chemotherapy were 50.9%, 54.6%, and 64.7%, respectively (data not shown). From 2018 to 2020, anti–PD-1/PD-L1 in combination with platinum-based chemotherapy was also the most commonly used regimen in patients with ECOG PS 0 or 1 (55.5%), current or former smokers (53.6%), and those who were not immunocompromised (53.9%) (data not shown).
Discussion
This retrospective, real-world cohort study using the ConcertAI Patient360 NSCLC database demonstrated that, from 2016 to 2020, the most common first-line treatment among US patients with locally advanced or metastatic NSCLC who received IO treatment was anti–PD-1/PD-L1 monotherapy, followed by anti–PD-1/PD-L1 agents combined with platinum-based chemotherapy. Nivolumab was the most common monotherapy in 2016, but after the negative CheckMate-026 trial, was overtaken by pembrolizumab in 2017, which remained the most frequently used monotherapy agent until the end of the study period in 2020, corresponding with the positive KEYNOTE-024 trial results [15, 20]. A shift in the most commonly used treatments occurred during the study period, from predominantly anti–PD-1/PD-L1 monotherapy in 2016 to combination treatment with anti–PD-1/PD-L1 agents and platinum-based chemotherapy. This was likely driven by US regulatory approval of IO-chemotherapy combination regimens and positive clinical trial results after earlier approvals of IO monotherapies [21,22,23,24,25].
Several patient characteristics in this real-world cohort differed from those in pivotal IO clinical trials [5, 9, 11, 12]. The median age of 68.0 years was numerically higher than the median of approximately 60–65 years in several clinical trials [5, 9, 11, 12, 26], and 26.2% of the patients in this study were 75 years or older. This finding is consistent with another real-world study [26], which demonstrated that patients receiving IO in the clinic are substantially older than patients studied in the trials that led to these agents’ approvals, as NSCLC trials often recruit fewer elderly patients [26]. Clinical trials also typically exclude patients with ECOG PS >1, but almost 20% of the patients in this study had ECOG PS ≥2. In our study, ECOG PS deteriorated with age, suggesting that including greater proportions of elderly patients may make clinical trials substantially more generalizable to the real-world setting. Compared with real‑world studies of patients receiving first-line treatment for metastatic NSCLC [18, 27], our study included a higher proportion of patients with an ECOG score ≥2 and a higher proportion of patients with brain metastases.
Patients with a history of autoimmune disease and those who are immunocompromised or receiving immuno-suppressive medications are also generally excluded from clinical trials of IO, but these types of patients comprised 1.5%, 11.0%, and 17.7%, respectively, of this real-world cohort and received first-line IO therapy for NSCLC. The fact that 78.9% of this study cohort had visceral metastases and 37.6% had nonvisceral metastases suggests that a substantial proportion of this cohort had dual visceral and nonvisceral metastases.
This study has several strengths and limitations. The database provided valuable real-world data on diagnosis, clinical assessment, and recorded treatments in patient groups not typically enrolled in clinical trials, such as older patients and those with higher ECOG PS, immuno-suppression, and various types and numbers of metastatic sites.
This was a descriptive observational study (no hypothesis testing) and has several limitations. The study may have been subject to confounding if physicians preferentially prescribed certain therapies to patients who were perceived to have worse adverse effects if their underlying disease was more severe or if they had poorer overall health. The study was not designed to evaluate patient outcomes; therefore, no conclusions on prognosis may be drawn. As with all retrospective epidemiological studies, unmeasured confounding and missing data may have an impact on the descriptive estimates presented.
Data entry errors at the points of care could not be detected nor corrected during analysis. Missing data in the form of information not routinely and repeatedly captured may also have impacted the completeness, validity, and reliability of some variables (eg, PD-L1 testing). Potentially interesting data that were unavailable for analysis included the rate of transition to second-line treatment when stratified by first-line therapy and information on local radiotherapy. This study included patients who were treated during the COVID-19 pandemic, which may have impacted routine clinical care in the year 2020. Finally, patients treated at individual sites included in this study may not be representative of all patients with NSCLC across all the sites of care in the US. This study highlights the differences in patient characteristics between real-world populations and clinical trial populations, presenting difficulties in treating patients underrepresented in clinical trial populations. Incorporating more diverse, traditionally excluded patient populations will increase the generalizability of studies and provide the evidence-base required to support decision-making in routine clinical practice.
Conclusions
The initial adoption of anti–PD-1/PD-L1 monotherapy as first-line treatment for advanced NSCLC in the US quickly shifted to combination anti–PD-1/PD-L1 therapy with platinum-based chemotherapy between 2016 and 2020. This real-world study was conducted during these important inflection points for the treatment of advanced NSCLC with anti–PD-1/PD-L1 therapy. This study has emphasized the real-world patient characteristics, how they differ from clinical trial populations, and how these characteristics impact treatment patterns.
In conclusion, we have shown that evidence gaps exist for patients who are older, have ECOG PS ≥2, and are on immunosuppressive medications—patients who make up a substantial proportion of real-world patient populations. To optimize the personalized treatment of advanced NSCLC, further real-world studies will be needed to elucidate the clinical characteristics of patients with advanced NSCLC who are most likely to benefit from an evolving first-line IO treatment landscape.
Availability of data and materials
The data that support the findings of this study are available from ConcertAI but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The corresponding author may be contacted regarding potential access to the data upon reasonable request and with permission of ConcertAI.
Abbreviations
- 1L:
-
First line
- ALK :
-
Anaplastic lymphoma kinase
- BRAF :
-
Proto-oncogene B-Raf
- CT:
-
Chemotherapy
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen 4
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- EGFR :
-
Epidermal growth factor receptor
- EMR:
-
Electronic medical record
- FDA:
-
Food and Drug Administration
- HIV:
-
Human immunodeficiency virus
- IO:
-
Immunotherapy
- MET :
-
Mesenchymal epithelial transition factor
- NOS:
-
Not otherwise specified
- NSCLC:
-
Non-small cell lung cancer
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death ligand 1
- RET :
-
RET proto-oncogene
- ROS1 :
-
ROS proto-oncogene 1
- US:
-
United States
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. https://doi.org/10.4065/83.5.584.
Jones CM, Brunelli A, Callister ME, Franks KN. Multimodality treatment of advanced non-small cell lung cancer: where are we with the evidence? Curr Surg Rep. 2018;6(2):5. https://doi.org/10.1007/s40137-018-0202-0.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. https://doi.org/10.1056/NEJMoa1809697.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948.
Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. https://doi.org/10.1016/S0140-6736(21)00228-2.
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. https://doi.org/10.1200/jco.2017.74.3062.
Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic non-squamous non–small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–17. https://doi.org/10.1200/jco.19.03136.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231.
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/s0140-6736(18)32409-7.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. 2021;39(21):2339–49. https://doi.org/10.1200/jco.21.00174.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/s0140-6736(16)32517-x.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. https://doi.org/10.1016/s0140-6736(16)00587-0.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. https://doi.org/10.1200/JCO.2014.59.4358.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. v1.2022 - December 7, 2021. 2021. https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients. Accessed 25 Jan 2022.
Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. 2021;11(1):9222. https://doi.org/10.1038/s41598-021-88453-8.
FDA approval timeline of active immunotherapies. https://www.cancerresearch.org/regulatory-approval-timeline-of-active-immunotherapies. Accessed 20 Apr 2023.
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493.
FDA approves Genentech’s Tecentriq in combination with Avastin and chemotherapy for the initial treatment of metastatic non-squamous non-small cell lung cancer. 2018. https://www.drugs.com/newdrugs/fda-approves-genentech-s-tecentriq-combination-avastin-chemotherapy-initial-metastatic-non-squamous-4883.html. Accessed 28 Mar 2022.
FDA approves Opdivo (nivolumab) + Yervoy (ipilimumab) as first-line treatment of patients with metastatic non-small cell lung cancer whose tumors express PD-L1≥1%. 2020. https://www.drugs.com/newdrugs/fda-approves-opdivo-nivolumab-yervoy-ipilimumab-first-line-patients-metastatic-non-small-cell-lung-5239.html. Accessed 28 Mar 2022.
FDA approves Genentech’s Tecentriq plus chemotherapy (Abraxane and carboplatin) for the initial treatment of metastatic non-squamous non-small cell lung cancer. 2019. https://www.drugs.com/newdrugs/fda-approves-genentech-s-tecentriq-plus-chemotherapy-abraxane-carboplatin-initial-metastatic-non-5117.html. Accessed 28 Mar 2022.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. https://doi.org/10.1056/NEJMoa1810865.
O’Connor JM, Fessele KL, Steiner J, Seidl-Rathkopf K, Carson KR, Nussbaum NC, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. https://doi.org/10.1001/jamaoncol.2018.0798.
Simeone JC, Nordstrom BL, Patel K, Klein AB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol. 2019;15(30):3491–502. https://doi.org/10.2217/fon-2019-0348.
Acknowledgements
Samantha Santangelo and Micaela Genca provided medical writing support on behalf of inScience Communications (Philadelphia, PA). This work was performed in accordance with current Good Publication Practice guidelines, and was funded by Sanofi, Inc.
Funding
This work was supported by Sanofi, which was involved in the study design; data analysis and interpretation; manuscript writing; and the decision to submit to BMC Cancer.
Author information
Authors and Affiliations
Contributions
H.A.D. provided conceptualization, methodology, data curation, formal analysis, and writing of the manuscript. M.A.B. provided data curation, formal analysis, and writing of the manuscript. A.K. provided conceptualization, methodology, data curation, writing, and supervision of the manuscript. D.P.C. provided conceptualization, methodology, writing, and supervision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and relevant International Council for Harmonisation, Good Clinical Practice, and Good Pharmacoepidemiological Practice guidelines. As no identifiable protected health information was extracted or accessed for the conduct of this study, ethics approval and informed consent were deemed unnecessary under the Code of Federal Regulations Title 45, Part 46, Section 46.104(d)(4)(ii) (45CFR46.104[d][4][ii]).
Consent for publication
Not applicable.
Competing interests
H.A.D. was an employee of Sanofi at the time of the study and holds stock in the company. M.A.B. received consulting fees from Sanofi. A.K. is an employee of Sanofi and holds stock in the company. D.P.C. has received funding from clinical trial grants from Genentech and Merck Sharp & Dohme to The Ohio State University; received consulting fees from Bristol Myers Squibb (BMS), BMS KK, Boehringer Ingelheim, Curio Science, Genentech/Roche, GI Therapeutics (Intellisphere), GlaxoSmithKline (GSK), Janssen, Mirati, Novartis, Novacure, OncoCyte, OncoHost, Roche China, and Seattle Genetics; received honoraria from AstraZeneca and BMS; participated in Data Safety Monitoring Boards for European Organisation for Research and Treatment of Cancer (EORTC), AbbVie, and Lilly; and participated in advisory boards for Amgen, Arcus Biosciences, AstraZeneca, Cantargia, Daiichi Sankyo, EMD Serono/Merck, Flame Biosciences, Gritstone Oncology, GSK, Lilly, Regeneron, Sanofi, and Seattle Genetics.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Divan, H.A., Bittoni, M.A., Krishna, A. et al. Real-world patient characteristics and treatment patterns in US patients with advanced non-small cell lung cancer. BMC Cancer 24, 424 (2024). https://doi.org/10.1186/s12885-024-12126-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12126-8