Abstract
Background
The expression of programmed death-ligand 1 (PD-L1), tumor-infiltrating lymphocytes (TILs), E-cadherin, and vimentin in lung cancer tumor microenvironment is known to impact patient survival or response to therapy. The expression of these biomarkers may also differ between primary lung tumors and brain metastatic tumors. In this study, we investigated the interaction between these biomarkers in lung tumors with or without concomitant brain metastasis and the interaction with paired brain metastatic tumors.
Methods
The study included 48 patients with stage IV epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. Sixteen of the forty-eight patients were diagnosed with brain metastasis, while the remaining thirty-two were not. All sixteen patients with brain metastasis had brain tumors. The expression of PD-L1, TILs (CD8+ T lymphocytes and FOXP3+ regulatory T lymphocytes), E-cadherin, and vimentin were evaluated using immunohistochemical (IHC) staining.
Results
Patients with brain metastasis exhibited a higher frequency of exon 19 deletion and uncommon EGFR mutations, a higher lung tumor vimentin score, worse progression-free survival (PFS), and overall survival (OS) than patients without brain metastasis. IHC staining showed no difference between paired lung and brain tumors. Patients with low PD-L1 expression had better PFS and OS. After multivariate analysis, higher body mass index, the presence of brain metastasis, bone metastasis, and uncommon EGFR mutations were correlated with worse PFS, while the presence of brain metastasis and high lung tumor E-cadherin score was associated with worse OS.
Conclusions
In patients with stage IV EGFR-mutant lung adenocarcinoma, high E-cadherin expression in the lung tumor might be associated with worse OS. Vimentin expression in the lung tumor was positively related to the risk of brain metastasis.
Similar content being viewed by others
Background
Lung cancer is a leading cause of cancer-related death worldwide despite advances in treatment [1]. In East Asia, approximately half of lung adenocarcinoma (ADC) patients have epidermal growth factor receptor (EGFR) mutations, and tyrosine kinase inhibitor (TKI) therapy is the standard treatment for advanced EGFR-mutant lung ADC [2]. Brain metastasis (BM) is more common in EGFR-mutant non-small cell lung cancer (NSCLC) than in wild-type NSCLC [3], and the prognosis is poor if patients develop BM [4, 5]. Advances in immune checkpoint inhibitors (ICIs) targeting the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway, such as pembrolizumab, improved survival compared with platinum-based chemotherapy in advanced NSCLC patients with PD-L1 expression of at least 50% and without EGFR mutation or anaplastic lymphoma kinase gene translocation [6]. A study led by Akbay and colleagues revealed that activation of the EGFR pathway resulted in PD-L1 upregulation along with an immunosuppressive tumor microenvironment (TME) characterized by a lower CD8+/CD4+ and CD8+/FOXP3+ tumor-infiltrating lymphocytes (TILs) ratio in a mouse model, and a blockade with PD-1 antibody improved survival [7]. A meta-analysis of nivolumab (CheckMate 057), pembrolizumab (KEYNOTE-010), and atezolizumab (POPLAR) confirmed that ICIs as a second-line treatment prolonged the overall survival (OS) over docetaxel in wild-type EGFR but not in EGFR-mutant advanced NSCLC patients [8]. In the TME, interaction between PD-L1, CD8+ TILs, tumor-infiltrating FOXP3+ regulatory T lymphocytes (Tregs) was also reported [9]. Among them, CD8+ TILs were associated with favorable outcomes and played an important role in cell-mediated antitumor response and were associated with favorable outcomes [10, 11], whereas tumor-infiltrating FOXP3+ Tregs were thought to have inhibitory effects on antitumor immunity and correlated to a worse prognosis in lung cancer patients [12]. In several malignancies, the CD8+/ FOXP3+ TILs ratio is also associated with improved patient survival [13]. TILs of brain metastatic tumors also have a potential prognostic value [5], and NSCLC brain metastatic tumors have a higher mutational burden and fewer T-cell clones compared with primary lung tumors [14]. Despite the presence of TILs, PD-L1 expression was found to be associated with epithelial-mesenchymal transition (EMT) in lung ADC [15]. EMT is a process in which carcinoma cells metastasize and invade organs and may contribute to drug resistance [16].
We investigated the interaction between PD-L1, TILs represented by CD8+ T lymphocytes and FOXP3+ Tregs, and EMT represented by E-cadherin and vimentin expression. In this study, we evaluated the expression of these immune biomarkers in lung tumors with or without concomitant BM and with paired brain metastatic tumors.
Methods
Participants
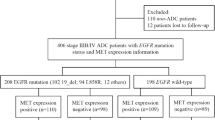
Twenty-two over 20 years old stage IV EGFR-mutant lung ADC patients with BM at diagnosis having paired lung and brain tumors were selected for the study between 2015/01/01 to 2019/12/31 from the patient database of the Department of Pathology, Kaohsiung Chang Gung Memorial Hospital, Taiwan. We also retrospectively reviewed medical records of patients over 20 years old diagnosed with stage IV lung ADC, between 2015/01/01 and 2019/12/31 at Kaohsiung Chang Gung Memorial Hospital, Taiwan. Eighty-nine stage IV EGFR-mutant lung ADC patients without BM at diagnosis were selected for propensity score matching (PSM). Eighteen patients with BM and 33 patients without BM at diagnosis were selected after PSM, but the lung tissues of three patients were not sufficient for immunohistochemical (IHC) staining. Therefore, the analysis included sixteen patients with BM and 32 without BM at diagnosis. The inclusion and exclusion criteria are described in the flow chart presented in Fig. 1.
Lung ADC was staged according to the AJCC 8th edition criteria [17]. The routine workup for lung cancer staging includes chest computed tomography, brain magnetic resonance imaging, and bone scans. Pleural effusion cytology studies and positron emission tomography were performed if needed. Progression-free survival (PFS) was defined as the period from the first day of treatment to documented disease progression or death before disease progression. Overall survival (OS) was defined as the period from the first day of treatment to death. Disease progression was determined following response evaluation criteria in solid tumors (RECIST) version 1.1 [18]. Performance status (PS) was defined based on the eastern cooperative oncology group (ECOG) criteria [19]. The follow-up time was defined as the first day of treatment to the last follow-up date and was 948.0 (603.8–1360.3) days in the median. EGFR mutation analysis was performed by real-time polymerase chain reaction using the therascreen® EGFR RGQ PCR kit (Qiagen, Hilden, Germany) or cobas® EGFR Mutation Test v2 kit (Roche Molecular systems, CA, USA) with formalin-fixed and paraffin-embedded tissue according to per manufacturers’ protocol. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB: 202000369B0D001 and 202200538B0).
Immunohistochemical staining of tissues
The hematoxylin and eosin (H&E)-stained sections of lung and brain tumors were assessed. Lung tumors were obtained only at the time of diagnosis, while brain tumors were obtained at the time of diagnosis or during the treatment course. A total of 48 formalin-fixed, paraffin-embedded lung tissue samples and sixteen brain tissues were collected and submitted for an IHC study. Using monoclonal antibodies against CD8 (rabbit, clone SP16, Thermo Fisher Scientific, Fremont, USA), FOXP3 (mouse, clone 150D, BioLegend, San Diego, USA), vimentin (rabbit, clone SP20, Thermo Fisher Scientific, Fremont, USA), and E-cadherin (mouse, clone GM016, Genemed Biotechnologies, South San Francisco, USA), an automated IHC analysis was performed by the following systems: BenchMark Ultra System (Ventana Medical Systems, Mannheim, Germany) for CD8; Leica BOND-III automated immunostainer (Leica Biosystems, Wetzlar, Germany) for FOXP3; and i6000™ Infinity System (BioGenex, CA, USA) for vimentin and E-cadherin. In addition, the anti-PD-L1 antibody clone 22C3 (Agilent/Dako, Santa Clara, USA), and a prototype IHC assay with a Dako Autostainer Link 48 platform (Agilent Technologies, Santa Clara, USA) were also used to determine the PD-L1 tumor proportion score. Slides were evaluated by two pathologists (GKH and CCH), who were blind to the clinicopathological data.
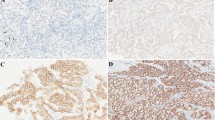
The tissue sections were analysed by light microscopy (Olympus BX43F, Tokyo, Japan) for the degree of infiltration by CD8+ and FOXP3+ T lymphocytes. The number of CD8+ and FOXP3+ cells were counted in a selected 0.238mm2 field area hotspot under 400× magnification. In the case of E-cadherin and vimentin, the staining intensity was graded as per the membranous expression: 0 equals to no expression; 1 equals to fragmented membranous and/or weak to moderate expression; 2 equals to fragmented strong or fully membranous moderate expression; and 3 equals to fully membranous strong expression. The percentage of immunoreactive positive tumor cells was graded as: 0 (no positive tumor cells), 1 (less than 10% positive tumor cells), 2 (10–50% positive tumor cells), and 3 (more than 50% positive tumor cells) [20]. An expression score was defined as the product of the percentage of immunoreactive positive tumor cells graded and the staining intensity. The score could be graded as 0, 1, 2, 3, 4, 6, or 9. The representative images of CD8, FOXP3, E-cadherin, and vimentin stains are shown in Fig. 2 and of PD-L1 expression are shown in Fig. 3.
Representative images of CD8, FOXP3, E-cadherin, and vimentin immunohistochemical staining. A to D, CD8+ cell count at the hot spot = 0, < 100/HPF, 100–200/HPF, and > 200/HPF. E to H, FOXP3+ cell count at the hot spot = 0, < 50/HPF, 50–100/HPF, and > 100/HPF. I to L, vimentin expression by tumor cells, interpreted as (0), (1+, 40%), (2+, 30%), and (3+, 90%). M to P, E-cadherin expression by tumor cells, interpreted as (0), (1+, 5%), (2+, 90%), and (3+, 100%). HPF: high-power field
Statistical analysis
Continuous variables were presented as median with interquartile range and compared using the non-parametric Mann–Whitney U-test, whereas the categorical variables were presented as frequency with percentage and compared by the Chi-square test. PFS and OS were analyzed using Kaplan–Meier curves and log-rank testing. A Cox proportional hazards regression model was used to evaluate independent factors influencing survival outcomes, and all covariates with a p-value < 0.1 were included for analysis. Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, New York, USA). Statistical significance was set at a p-value of < 0.05.
PSM was conducted to balance the differences in clinical characteristics between patients with BM at diagnosis and those without BM. Propensity scores were calculated using logistic regression analysis and covariates included sex, body height, body weight, age, and smoking habits. Patients with BM at diagnosis were matched 1:2 to patients without BM using nearest-neighbor matching with a caliper at 0.2. Standardized differences for a covariate were set at < 10.0%. PSM was performed using NCSS version 11.0.5 (NCSS LLC., Kaysville, UT, USA).
Results
Patient characteristics
A total of sixteen patients with BM and 32 without BM at diagnosis were included; the clinical characteristics and IHC staining results of lung tumors are listed in Table 1.
There was no difference in sex, age, BMI, smoking habits, ECOG PS, comorbidities, liver or bone metastasis, or first-line TKI categories between these two groups. Patients with BM at diagnosis had more frequent exon 19 deletions and uncommon EGFR mutations, less frequent L858R mutations, and worse PFS and OS than patients without BM at diagnosis. IHC stain of lung tumors from these two groups showed no difference in PD-L1 expression, CD8+ TILs, tumor-infiltrating FOXP3+ Tregs, or E-cadherin score. Patients with BM at diagnosis had considerably higher lung tumor vimentin scores than those without. Four of 32 patients without BM at diagnosis developed BM later, and the analysis of lung tumors of these four patients displayed a trend of higher vimentin scores than those 28 patients who did not develop BM during follow-up [median: 1.5 (1.0–3.5) vs. 0.5 (0.0–1.0); p-value = 0.072].
IHC staining results of paired lung and brain tumors from patients with brain metastasis at diagnosis
IHC staining results of PD-L1, CD8, FOXP3, E-cadherin, and vimentin from paired lung and brain tumors in patients with BM at diagnosis are listed in Table 2.
There was no significant difference observed in the expression of PD-L1, CD8+ TILs, tumor-infiltrating FOXP3+ Tregs, E-cadherin, and vimentin score between lung and brain tumors. Compared to brain tumors with low PD-L1 expression (< 1%), lung tumors with low PD-L1 expression exhibited significantly lower CD8+ TILs [median: 29.0 (4.0–89.0) vs. 125.0 (61.5–230.0); p-value = 0.011].
PD-L1 expression and association with prognosis
The clinical characteristics and IHC stain results of lung tumors stratified by PD-L1 expression are listed in Table 3. There was no difference in sex, age, BMI, smoking habits, ECOG PS, EGFR mutation type, first-line TKI categories, or proportion of brain, liver, and bone metastasis between these two groups. IHC stain of CD8+ TILs, tumor-infiltrating FOXP3+ Tregs, E-cadherin score, and vimentin score was not different between these two groups. Low PD-L1 expression patients had significantly longer PFS and OS than patients with high PD-L1 expression.
Independent factors affecting PFS and OS
Independent factors associated with PFS are listed in Table 4. Univariate analysis revealed that younger age, the presence of brain and bone metastasis, uncommon EGFR mutations, and high PD-L1 expression (≥ 1%), were all associated with worse PFS. Higher BMI, the presence of brain and bone metastasis, and uncommon EGFR mutations were found to be factors associated with poorer PFS in multivariate analysis.
Independent factors associated with OS are listed in Table 5. Both univariate and multivariate analysis revealed the presence of BM, and high E-cadherin scores were associated with worse OS. Kaplan–Meier curves of OS regarding BM and E-cadherin scores are shown in Fig. 4.
Discussion
PD-L1 expression in stage IV EGFR-mutant lung adenocarcinoma and correlation with tumor microenvironment
Previous studies by Santaniello and colleagues about the relationship between EGFR mutation and PD-L1 expression showed conflicting results; however, they concluded that it might be due to different PD-L1 evaluation methods and interpretations [9]. Activation of the EGFR pathway has been shown to induce PD-L1 expression in mouse models and NSCLC cell lines [7], and the pathway may involve yes-associated protein (YAP) [21]. In our hospital, a larger cohort showed lower PD-L1 expression in EGFR-mutant NSCLC patients than in wild-type NSCLC patients [22].
In our cohort, there was no correlation of CD8+ TILs, tumor-infiltration FOXP3+ Tregs, E-cadherin, or vimentin scores with PD-L1 expression in lung TME. Though a tendency for high PD-L1 expression in lung tumors related to higher tumor-infiltrating FOXP3+ Treg counts was observed. Activation of EGFR pathways in NSCLC was associated with decrease in CD8+ TILs [23, 24], but an increase in tumor-infiltrating FOXP3+ Tregs [25]. This difference may be due to the small population size and different patient cohorts.
PD-L1 expression in our cohort showed no correlation with E-cadherin and vimentin scores but, EMT was reported to be related to PD-L1 overexpression in lung adenocarcinoma, especially in the EGFR-mutant subgroup [15]. Asgarova et al. demonstrated that cytokine-induced EMT in lung cancer cell lines could induce PD-L1 upregulation and vimentin expression correlated with PD-L1 expression in NSCLC patients [26]. Thus, the correlation between PD-L1 expression and EMT still needs further investigation.
Although among the sixteen paired lung and brain tumors, there were no differences in IHC stain results, as listed in Table 2. Higher CD8+ TILs in brain tumors compared with lung tumors were observed, and low PD-L1 expression (< 1%) brain tumors had significantly higher CD8+ TILs than low PD-L1 expression lung tumors [median: 125.0 (61.5–230.0) vs. 29.0 (4.0–89.0); p = 0.011]. Some previous studies suggested that in NSCLC patients, brain metastatic tumors have less PD-L1 expression [27] and fewer TILs [14, 27] compared with primary tumors, but others did not [28, 29]. Notably, brain metastatic tumor PD-L1 expression was found to be strongly correlated with primary lung tumor in lung adenocarcinoma patients and no significant change was found to be affected by chemotherapy or steroid therapy. However, the majority of patients in this study were wild-type patients [29]. The different results may be due to different patient populations. In EGFR-mutant NSCLC patients, rebiopsy of the lung tumor after TKI resistance showed increased PD-L1 expression, with decreased CD8+ and FOXP3+ TIL densities [30]. This could also partially explain why our results differ from other reports because eight of sixteen patients’ brain tissues were obtained after first-line TKI treatment.
Epithelial-mesenchymal transition manifested by vimentin expression and risk of brain or other distant metastases
EMT plays a crucial role in lung cancer progression and metastasis. It is characterized by decreased E-cadherin expression and vimentin overexpression [31], and also participates in the mechanism of TKI resistance in EGFR-mutant lung adenocarcinoma [32]. In our cohort, lung tumors from patients with initial BM showed significantly higher vimentin expression than lung tumors from patients without initial BM. But this is not the case with bone or liver metastasis. Vimentin expression in NSCLC has been linked to future metastasis [33], and pathologic stage and N status [34]. Jeevan and colleagues demonstrated that the EMT/MET pathway is crucial for BM from lung adenocarcinoma [35]. Our study validated the role of EMT in lung cancer BM.
Factors associated with patient outcome
In our cohort, higher BMI and the presence of brain and bone metastasis were independently associated with unfavorable PFS, while the common EGFR mutation was independently associated with better PFS. The association of BMI and lung cancer prognosis differs between studies and race, sex, smoking habits, and lung cancer subtypes [36]. In our study, higher BMI became significantly associated with poorer PFS by multivariate analysis, this may be due to different patient population since our study focused on patients with EGFR-mutant lung adenocarcinoma and relatively small population size. BM [4, 5] and bone metastasis [37] also adversely affect patient survival, as observed in our patient cohort. Uncommon EGFR mutations showed varied responses to TKIs [38]. NSCLC patients harboring common EGFR mutations had a better response to TKIs and a better prognosis than rare EGFR mutations [39], as occurred in our cohort.
Although patients with higher PD-L1 expression had a worse outcome in our cohort, it was not statistically significant according to multivariate analysis with PFS (p = 0.090) and OS (p = 0.408). Indeed, advanced EGFR-mutant lung adenocarcinoma patients with higher PD-L1 expression had worse PFS [40, 41], OS, and a lower frequency of secondary T790M mutation [41].
The presence of BM and high E-cadherin expression were both independent factors associated with worse OS in our study. As summarized in a meta-analysis [42], low E-cadherin expression was associated with poor prognosis in NSCLC patients as well as a group of NSCLC patients treated with chemoradiotherapy [43]. The presence of aberrant E-cadherin expression or loss of E-cadherin expression was associated with worse outcomes in other cancers such as melanoma [44], gastric cancer [45], and colorectal cancer [46]. Although the patient cohort is different, our study showed the opposite result that a high E-cadherin score correlates with poor OS. Indeed, there was evidence that cancers with high E-cadherin expression showed aggressive behavior and an unfavorable outcome, such as, in a subgroup of human brain glioblastoma, E-cadherin expression was associated with aggressive behavior and could be blocked by shRNA in a cell line study [47]. Despite E-cadherin expression in IHC stain, cleaved E-cadherin fragments (soluble E-cadherin) may have an oncogenic effect, increase tumor cell motility and survival, and play a role in EGFR and Wnt/β-catenin pathway signaling [48]. Elevated serum soluble E-cadherin levels were found to be associated with disease invasiveness and a poor outcome in several cancers [48]. Additionally, erlotinib and gefitinib could reduce E-cadherin expression in human papillomavirus 16-positive and -negative cell lines [49]. Taken together, it is unclear whether high E-cadherin expression leads to high soluble E-cadherin after TKI treatment. In other cancers, the correlation of serum soluble E-cadherin and E-cadherin expression in IHC stain was studied, which was not compatible with bladder cancer [50] and hepatocellular carcinoma [51]. Also, studies on breast, gastric, and colorectal cancers have stated that serum soluble E-cadherin level is inversely correlated with E-cadherin expression in tissues [52], but whether this correlation applies to EGFR-mutant lung adenocarcinoma needs further investigation.
Limitations
There are a few limitations to our study. Our study may have been limited due to the small population size. Additionally, we obtained eight of the sixteen patients brain tissue samples after TKI treatment, which could affect inflammation status. For example, Isomoto et al. demonstrated that TKI treatment altered the TME by expressing PD-L1, CD8+ TILs, or tumor-infiltrating FOXP3+ Tregs [30]. The inhomogeneous spatial distribution of PD-L1 [53] and TILs [54] in NSCLC patients tumors could also affect our interpretation since our specimens were from a partial tumor biopsy.
Conclusions
Our study revealed the possible role of E-cadherin and vimentin expression in EGFR-mutant lung adenocarcinoma. In patients with stage IV EGFR-mutant lung adenocarcinoma, high E-cadherin expression in the lung tumor might be associated with worse OS, and vimentin expression in the lung tumor was positively related to the risk of brain metastasis. E-cadherin expression might be a useful biomarker in evaluating prognosis and vimentin expression in evaluating risk of brain metastasis. Further studies are recommended to clarify the role of these biomarkers in the pathogenesis of EGFR-mutant lung adenocarcinoma.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ADC:
-
Adenocarcinoma
- BM:
-
Brain metastasis
- ECOG:
-
Eastern Cooperative Oncology Group
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- ICIs:
-
Immune checkpoint inhibitors
- IHC:
-
Immunohistochemical
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- RECIST:
-
Response evaluation criteria in solid tumors
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- PS:
-
Performance status
- PSM:
-
Propensity score matching
- TILs:
-
Tumor-infiltrating lymphocytes
- TKI:
-
Tyrosine kinase inhibitor
- TME:
-
Tumor microenvironment
- Tregs:
-
Regulatory T lymphocytes
References
Yang CY, Liao WY, Ho CC, Chen KY, Tsai TH, Hsu CL, et al. Association between programmed death-ligand 1 expression, immune microenvironments, and clinical outcomes in epidermal growth factor receptor mutant lung adenocarcinoma patients treated with tyrosine kinase inhibitors. Eur J Cancer. 2020;124:110–22.
Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4(2):156–64.
Kelly WJ, Shah NJ, Subramaniam DS. Management of brain metastases in epidermal growth factor receptor mutant non-small-cell Lung Cancer. Front Oncol. 2018;8:208.
Hsiao SH, Lin HC, Chou YT, Lin SE, Kuo CC, Yu MC, et al. Impact of epidermal growth factor receptor mutations on intracranial treatment response and survival after brain metastases in lung adenocarcinoma patients. Lung Cancer. 2013;81(3):455–61.
Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1):e1057388.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to Immune escape in EGFR-Driven Lung Tumors. Cancer Discov. 2013;3(12):1355–63.
Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. 2017;12(2):403–7.
Santaniello A, Napolitano F, Servetto A, De Placido P, Silvestris N, Bianco C, et al. Tumour Microenvironment and Immune Evasion in EGFR Addicted NSCLC: hurdles and possibilities. Cancers (Basel). 2019;11(10):1419.
Kinoshita T, Muramatsu R, Fujita T, Nagumo H, Sakurai T, Noji S, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27(11):2117–23.
Donnem T, Hald SM, Paulsen E-E, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell Density—A Promising supplement to TNM staging in non–small cell Lung Cancer CD8+ T cells in NSCLC. Clin Cancer Res. 2015;21(11):2635–43.
Akimova T, Zhang T, Negorev D, Singhal S, Stadanlick J, Rao A, et al. Human lung tumor FOXP3+ Tregs upregulate four “Treg-locking” transcription factors. JCI Insight. 2017;2(16):e94075.
Rech AJ, Vonderheide RH. Dynamic interplay of oncogenes and T cells induces PD-L1 in the tumor microenvironment. Cancer Discov. 2013;3(12):1330–2.
Mansfield AS, Ren H, Sutor S, Sarangi V, Nair A, Davila J, et al. Contraction of T cell richness in lung cancer brain metastases. Sci Rep. 2018;8(1):2171.
Kim S, Koh J, Kim M-Y, Kwon D, Go H, Kim YA, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Patho. 2016;58:7–14.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–29.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
Motono N, Ueda Y, Shimasaki M, Iwai S, Iijima Y, Usuda K, et al. Prognostic impact of Sphingosine kinase 1 in Nonsmall Cell Lung Cancer. Clin Pathol. 2021;14:2632010X20988531.
Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) Pathway, Yes-Associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci. 2019;20(15):3821.
Wang CC, Huang KT, Chang HC, Tseng CC, Lai CH, Lan J, et al. Comprehensive analysis of PD-L1 in non-small cell lung cancer with emphasis on survival benefit, impact of driver mutation and histological types, and archival tissue. Thorac Cancer. 2022;13(1):38–47.
Busch SE, Hanke ML, Kargl J, Metz HE, MacPherson D, Houghton AM. Lung Cancer Subtypes Generate Unique Immune responses. J Immunol. 2016;197(11):4493–503.
Helland Ã, Brustugun OT, Nakken S, Halvorsen AR, Dønnem T, Bremnes R, et al. High number of kinome-mutations in non‐small cell lung cancer is associated with reduced immune response and poor relapse‐free survival. Int J cancer. 2017;141(1):184–90.
Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol. 2020;5(43):eaav3937.
Asgarova A, Asgarov K, Godet Y, Peixoto P, Nadaradjane A, Boyer-Guittaut M, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. 2018;7(5):e1423170.
Mansfield A, Aubry M, Moser J, Harrington S, Dronca R, Park S, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–8.
Batur S, Dulger O, Durak S, Yumuk PF, Caglar HB, Bozkurtlar E, et al. Concordance of PD-L1 expression and CD8+ TIL intensity between NSCLC and synchronous brain metastases. Bosn J Basic Med Sci. 2020;20(3):329–35.
Téglási V, Pipek O, Lózsa R, Berta K, Szüts D, Harkó T, et al. PD-L1 expression of lung cancer cells, unlike infiltrating immune cells, is stable and unaffected by therapy during brain metastasis. Clin Lung Cancer. 2019;20(5):363–9. e 2.
Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the Tumor Immune Microenvironment in EGFR mutation-positive Non-Small Cell Lung Cancer. Clin Cancer Res. 2020;26(8):2037–46.
Nam MW, Kim CW, Choi KC. Epithelial-mesenchymal transition-inducing factors involved in the progression of lung cancers. Biomol Ther (Seoul). 2022;30(3):213–20.
Sakuma Y. Epithelial-to-mesenchymal transition and its role in EGFR-mutant lung adenocarcinoma and idiopathic pulmonary fibrosis. Pathol Int. 2017;67(8):379–88.
Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81(1):117–22.
Chikaishi Y, Uramoto H, Tanaka F. The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res. 2011;31(12):4451–6.
Jeevan DS, Cooper JB, Braun A, Murali R, Jhanwar-Uniyal M. Molecular Pathways Mediating Metastases to the Brain via Epithelial-to-mesenchymal transition: genes, proteins, and functional analysis. Anticancer Res. 2016;36(2):523–32.
Jiang M, Fares AF, Shepshelovich D, Yang P, Christiani D, Zhang J, et al. The relationship between body-mass index and overall survival in non-small cell lung cancer by sex, smoking status, and race: a pooled analysis of 20,937 International lung Cancer consortium (ILCCO) patients. Lung Cancer. 2021;152:58–65.
Vicent S, Perurena N, Govindan R, Lecanda F. Bone metastases in lung cancer. Potential novel approaches to therapy. Am J Respir Crit Care Med. 2015;192(7):799–809.
Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–8.
Arrieta O, Cardona AF, Corrales L, Campos-Parra AD, Sanchez-Reyes R, Amieva-Rivera E, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87(2):169–75.
Hsu KH, Huang YH, Tseng JS, Chen KC, Ku WH, Su KY, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naive advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer. 2019;127:37–43.
Yoon BW, Chang B, Lee SH. High PD-L1 expression is associated with unfavorable clinical outcome in EGFR-mutated lung adenocarcinomas treated with targeted therapy. Onco Targets Ther. 2020;13:8273–85.
Chao D, Hu G, Li Q. Clinicopathological significance and prognostic value of E-cadherin expression in non-small cell lung cancer: a protocol for systematic review and meta-analysis. Med (Baltim). 2021;100(7):e24748.
Gkogkou P, Peponi E, Ntaskagiannis D, Murray S, Demou A, Sainis I, et al. E-Cadherin and Syndecan-1 expression in patients with Advanced Non-small Cell Lung Cancer treated with Chemoradiotherapy. In Vivo. 2020;34(1):453–9.
Yan S, Holderness BM, Li Z, Seidel GD, Gui J, Fisher JL, et al. Epithelial–mesenchymal expression phenotype of primary melanoma and matched metastases and relationship with overall survival. Anticancer Res. 2016;36(12):6449–56.
Lazar D, Taban S, Ardeleanu C, Dema A, Sporea I, Cornianu M, et al. The immunohistochemical expression of E-cadherin in gastric cancer; correlations with clinicopathological factors and patients’ survival. Rom J Morphol Embryol. 2008;49(4):459–67.
Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, et al. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. 2007;50(4):453–64.
Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS ONE. 2010;5(10):e13665.
David JM, Rajasekaran AK. Dishonorable discharge: the oncogenic roles of cleaved E-Cadherin fragments. Cancer Res. 2012;72(12):2917–23.
Kramer B, Hock C, Schultz JD, Lammert A, Kuhlin B, Birk R, et al. Impact of small molecules on beta-catenin and E-Cadherin expression in HPV16-positive and -negative squamous cell carcinomas. Anticancer Res. 2017;37(6):2845–52.
Griffiths TR, Brotherick I, Bishop RI, White MD, McKenna DM, Horne CH, et al. Cell adhesion molecules in bladder cancer: soluble serum E-cadherin correlates with predictors of recurrence. Br J Cancer. 1996;74(4):579–84.
Soyama A, Eguchi S, Takatsuki M, Kawashita Y, Hidaka M, Tokai H, et al. Significance of the serum level of soluble E-cadherin in patients with HCC. Hepatogastroenterology. 2008;55(85):1390–3.
Repetto O, De Paoli P, De Re V, Canzonieri V, Cannizzaro R. Levels of soluble E-cadherin in breast, gastric, and colorectal cancers. Biomed Res Int. 2014; 2014:408047.
Haragan A, Field JK, Davies MPA, Escriu C, Gruver A, Gosney JR. Heterogeneity of PD-L1 expression in non-small cell lung cancer: implications for specimen sampling in predicting treatment response. Lung Cancer. 2019;134:79–84.
de Rodas ML, Nagineni V, Ravi A, Datar IJ, Mino-Kenudson M, Corredor G, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J Immunother Cancer. 2022;10(6):e004440.
Acknowledgements
We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work, and the National Biobank Consortium of Taiwan, Taiwan, for technical and facility support.
Funding
This work was supported by grants from Chang Gung Memorial Hospital Kaohsiung Medical Center, Taiwan (CORPG8K0041 to Y.P. Chang). The funding body has no role in the design of the study and collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YPC is the major contributor to writing the manuscript. YPC, MCL, and CCW designed the research, collected data, and data interpretation. GKH and CCH collected the specimen and performed the immunohistochemical stain and interpretation. YCC, KTH, YMC, and CYL helped revise the manuscript and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB: 202000369B0D001 and 202200538B0). The need for informed consent was waived by the Institutional Review Board of Chang Gung Memorial Hospital due to the samples and medical data were anonymized and the study was conducted in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chang, YP., Huang, GK., Chen, YC. et al. E-cadherin expression in the tumor microenvironment of advanced epidermal growth factor receptor-mutant lung adenocarcinoma and the association with prognosis. BMC Cancer 23, 569 (2023). https://doi.org/10.1186/s12885-023-10980-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10980-6