Abstract
Background
Non-small cell cancer (NSCLC) patients with concomitant epidermal growth factor receptor (EGFR) and TP53 mutations have a poor prognosis with the treatment of tyrosine kinase inhibitors (TKIs), and may benefit from a combination regimen preferentially. The present study aims to compare the benefits of EGFR-TKIs and its combination with antiangiogenic drugs or chemotherapy in patients with NSCLC harboring EGFR and TP53 co-mutation in a real-life setting.
Methods
This retrospective analysis included 124 patients with advanced NSCLC having concomitant EGFR and TP53 mutations, who underwent next-generation sequencing prior to treatment. Patients were classified into the EGFR-TKI group and combination therapy group. The primary end point of this study was progression-free survival (PFS). The Kaplan–Meier (KM) curve was drawn to analyze PFS, and the differences between the groups were compared using the logarithmic rank test. Univariate and multivariate cox regression analysis was performed on the risk factors associated with survival.
Results
The combination group included 72 patients who received the regimen of EGFR-TKIs combined with antiangiogenic drugs or chemotherapy, while the EGFR-TKI monotherapy group included 52 patients treated with TKI only. The median PFS was significantly longer in the combination group than in the EGFR-TKI group (18.0 months; 95% confidence interval [CI]: 12.1–23.9 vs. 7.0 months; 95% CI: 6.1–7.9; p < 0.001) with greater PFS benefit in TP53 exon 4 or 7 mutations subgroup. Subgroup analysis showed a similar trend. The median duration of response was significantly longer in the combination group than in the EGFR-TKI group. Patients with 19 deletions or L858R mutations both achieved a significant PFS benefit with combination therapy versus EGFR-TKI alone.
Conclusion
Combination therapy had a higher efficacy than EGFR-TKI alone for patients with NSCLC having concomitant EGFR and TP53 mutations. Future prospective clinical trials are needed to determine the role of combination therapy for this patient population.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) mutations are the most common targetable oncogenic driver mutation in metastatic non-small cell lung cancer (NSCLC). EGFR tyrosine kinase inhibitors (TKIs) have shown superior efficacy over cytotoxic chemotherapy in patients with EGFR-mutant NSCLC [1,2,3,4]. However, these patients eventually experienced disease progression. The resistance is caused by the unique sub-molecular characteristics, including EGFR mutation subtypes and concurrent alterations [5]. TP53 mutations are the most prevalent co-alteration detected in 54.6–68.8% EGFR-mutant cases of NSCLC [5,6,7,8,9]. Patients with TP53 mutations have a low response rate and poor prognosis to EGFR-TKI therapy [10, 11].
The outcomes in patients with EGFR mutations can be improved by combining an EGFR-TKIs with an antiangiogenic agent or chemotherapy according to clinical trials suggesting the potential benefit of combination therapy [12,13,14]. EGFR/TP53 co-mutant tumors may preferentially benefit from a combination regimen. Both the RELAY and ACTIVE study reported that EGFR-TKIs with an antiangiogenic agent revealed consistent improved antitumor activity and favorable PFS in patients with TP53 and EGFR co-mutant NSCLC [13, 15, 16]. EGFR-TKI in combination with chemotherapy also demonstrated greater PFS benefit in patients with concurrent EGFR with TP53 mutations [17]. Nevertheless, these studies do not support subgroup analysis. Thus, this retrospective cohort study aimed to assess the efficacy of EGFR-TKIs alone or in combination with an antiangiogenic agent or chemotherapy as first-line therapy for patients with advanced NSCLC having concomitant EGFR and TP53 mutations. In current study, we analyzed and evaluated the status of TP53 and EGFR gene and the different types of mutations in relation to the outcomes of patients in terms of PFS, overall response rate (ORR), disease control rate (DCR), and duration of response.
Materials and methods
Patient eligibility
We retrospectively reviewed data from patients with local advanced stage and metastatic NSCLC having concomitant EGFR and TP53 activating mutations admitted in the Tianjin Medical University Cancer Institute and Hospital(Tianjin, China) between January 2018 and June 2021. The patients who met the following inclusion criteria were included: (1) pathological confirmed, (2) harboring concomitant EGFR and TP53 mutations, as confirmed by next-generation sequencing (NGS), (3) receiving EGFR-TKIs treatment, and (4) regularly imaged for the effective analysis based on the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Patients who met the following criteria were excluded: (1) concurrently or previously diagnosed with other malignancies, (2) with insertional mutations in exon 20, and (3) with a follow-up period of no more than 6 months. Approval for a waiver of informed consent for the study was obtained from the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was not required as this is a retrospective, unicentric cohort study.
Study endpoints and assessment
PFS was defined as the primary endpoint, which represents the time between treatment initiation and the last follow-up or cancer progression. In addition, ORR, DCR, and duration of response (DoR) were deemed as secondary endpoints. We evaluated ORR through the partial response (PR) and complete response (CR) rates, while DCR was evaluated based on PR, CR, and stable disease (SD) rates. DoR was defined as the time from the first documented response to the onset of disease progression or death, whichever occurred first.
Treatment method
Patients were classified into the EGFR-TKI group and combination therapy group. The patients in EGFR-TKI group received first-, second-, or third- generation EGFR-TKI drugs only. The EGFR-TKI drugs include gefitinib, icotinib, erlotinib, afatinib, dacomitinib, osimertinib, and almonertinib. The patients in the combination group underwent EGFR-TKIs combined with anti-angiogenic drugs or chemotherapy. The antiangiogenic drugs include bevacizumab and anlotinib. The chemotherapy regimens include pemetrexed and carboplatin or cisplatin. There was no direct involvement of human tissues.
Data collection
Patient characteristics including gender, age, smoking history, Eastern Cooperative Oncology Group (ECOG) score, disease status, number of organs with metastases, metastatic sites, EGFR and TP53 mutations status, treatment status, and concomitant mutations other than EGFR and TP53 were obtained from the electronic medical record system of the Tianjin Medical University Cancer Hospital and Institute. The last follow-up was conducted on January 20, 2022. The efficacy was evaluated using the Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1). Data were considered as censored if the event had not occurred by the last follow-up time.
Next-generation target sequencing
The tissue DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Circulating cfDNA was recovered from 4 to 5 mL plasma by using the QIAamp Circulating Nucleic Acid kit (Qiagen). The tissue DNA and cfDNA were profiled using a capture-based targeted sequencing panel that consisted of 520 or 168 cancer-related genes. The captured libraries were sequenced on Illumina NovaSeq 6000 with pair-end reads with 1000X for tissue DNA and 20,000 X for cfDNA, following the manufacturer instructions (Illumina, San Diego, CA, USA).
Statistical analysis
The last follow-up in this study was conducted in January 2022. Subgroup analysis was conducted based on age, gender, smoking status, ECOG score, clinical stage, bone metastases, brain metastases, TP53 mutation types, EGFR mutation types, and other concomitant gene mutations. Differences between groups were compared using chi-square test. Kaplan–Meier (KM) curves were plotted to analyze PFS, and log-rank test was used to compare differences between groups. Univariate cox regression analysis was performed on the risk factors associated with survival. Fisher’s exact test or chi-square test was used to analyze DCR and ORR. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using stratified Cox regression. The one-year PFS rate and the corresponding 95% CIs for each treatment group were calculated using the Greenwood formula. SPSS 26.0 (IBM Corp.) was employed for statistical analysis. Two-sided p-values < 0.05 were considered statistically significant.
Results
Clinical characteristics of patients
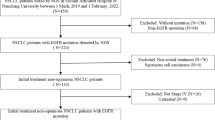
Among the 141 potentially eligible patients, we excluded six patients with insertional mutations in exon 20. Additionally, we excluded nine patients with missing follow-up information. Therefore, 124 NSCLC cases harboring EGFR and TP53 mutations were included in this study (Fig. 1). A total of 52 patients underwent EGFR-TKI treatment (EGFR-TKI group), including 31 with first-generation EGFR-TKIs, 5 with second-generation EGFR-TKIs, and 16 with third-generation EGFR-TKIs, respectively. A total of 72 patients underwent EGFR-TKIs combined with antiangiogenic drugs (22 cases) or chemotherapy (50 cases). In the EGFR-TKIs combined with antiangiogenic drugs subgroup, 12 cases were treated with anlotinib and EGFR-TKIs, and 10 with bevacizumab and EGFR-TKIs. The median chemotherapy cycle was 6 (2–34). A similar proportion of patients were present in both groups based on gender, age, smoking history, ECOG score, clinical stage, number of organs with metastases, EGFR mutations, TP53 mutations and treatment status, and concomitant mutations, except for the combination group with more patients having bone metastases than the EGFR-TKI group (p = 0.043). Twelve (23.1%) patients in the EGFR-TKIs group and 20 (27.8%) in the combination group had coexisting brain metastases, while 23 (44.2%) patients in the EGFR-TKIs group and 46 (62.5%) in combination group had coexisting bone metastases (Table 1).
Baseline genomic characteristics
EGFR mutations
Among the 124 patients with baseline tissue or plasma samples detected with gene mutations by NGS, 56 (45.2%) exon 19 deletions (19 del), 56 (45.2%) exon 21 L858R, 2 L861Q, 1 L814P, 1 E709_T710delinsD, and 7 (4.0%) compound EGFR mutations (including 2 with G719S and S768I, 1 with G719D and L861Q, 1 with G719S and E709K, 1 with 19 deletion and L858R, 1 with E709A and L858R, 1 with E709K and L858R, and 1 with 19 deletion and P848L) were observed.
TP53 mutations
Among the 124 EGFR-mutated patients, 118 cases had detailed information on TP53 mutation analysis. The TP53 mutations in 6 patients were only based on medical records without detailed information. Figure 2B shows the percentages of all exon mutations of TP53 detected in patients at baseline. The most frequent mutation in TP53 exons occurred in exon 5 and 6 (both 21.8%, n = 27), followed by exon 8 (17.7%, n = 22) and exon 7 mutation (15.3%, n = 19). Fourteen (13.1%) patients harbored exon 4 mutations. Eight (6.5%) patients harbored exon 10 mutations. Five (4.0%) patients exhibited TP53 mutation on exon 9, 2 (1.6%) on exon 3. TP53 exon 1, 2, and 11 mutations were not found in this cohort.
No statistically significant associations were observed between the type of TP53 and EGFR mutation.
Other concomitant mutations
Twenty-one patients (16.9%) harbored co-occurring lung cancer driver alterations, including one ERBB2 amplification (amp), one ERBB2 mutation, four RET mutations, five MET amp, one ROS1 amp, two MET mutations, two ALK mutations, one BRAF V600E, one BRAF mutation and one KRAS G13C, one KRAS G13D and one KRAS G12V. Among the common concomitant alterations included other EGFR alternation (17.7%, 22/124), RB1 (8.9%, 11/124), CDKN2A (5.6%, 7/124), APC (3.2%, 4/124), MYC (4.8%, 6/124), PIK3CA (5.6%, 7/124), CTNNB1 (2.4%, 3/124), TERT (3.2%, 4/124), NF2 (3.2%, 4/124), PTEN (4%, 5/124), BRAC1 (5%, 5/124), and CDK6 (5%, 5/124), as shown in Fig. 2A.
Efficacy
Progression-free survival
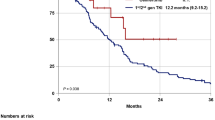
Progression events occurred in 40 (76.9%) patients in the EGFR-TKI group and in 37 (51.4%) patients in the combination group. The duration of PFS was significantly longer in the combination group than in the EGFR-TKI group (median, 18.0 months [12.1–23.9] vs. 7.0 months [6.1–7.9]; HR: 0.37; 95% CI: 0.232–0.589; p < 0.001, Fig. 3A). The estimated proportion of patients who were alive and progression-free at 6 months was 89% (95% CI: 81–95) in the combination group and 71% (95% CI: 59–84) in the EGFR-TKI group. At 12 months, the proportions were 62% (95% CI: 49–74) and 24% (95% CI: 22–25). At the time of data cutoff, 77 progression events occurred (62% maturity), representing the planned number of events and maturity. The follow-up lasted for 6–30 months with the median time of 12 months.
Kaplan-Meier curves of progression-free survival (PFS) of patients with different therapies. (A) Patients with EGFR-TKI only and combination therapy. (B) Patients with 1st and 2nd generation EGFR-TKI, 3rd generation EGFR-TKI and combination therapy. (C) Patients with 1st ,2nd and 3rd generation EGFR-TKI in EGFR-TKI group. (D) Patients with EGFR-TKI combined with antiangiogenic drugs and EGFR-TKI combined with chemotherapy
Varying PFS benefit from combined therapy was observed across all predefined subgroups compared with the EGFR-TKI group (Fig. 4). Treatment option was the only independent factor for PFS in the univariate and multivariate Cox models (Supplementary Table 1).
In the EGFR-TKI group, the PFS of patients treated with first-, second-, and third-generation EGFR-TKIs (30, 7, 15 patients respectively) had no difference (median, 6.2 months [5.6–6.8] months vs. 9.0 months [8.0–10] months vs. 14.0 months [2.2–25.8] months; p = 0.295), as shown in Fig. 3C. Third-generation EGFR-TKIs had no superiority over other TKIs when first- and second-generation EGFR-TKIs were combined into one group (median, 14.0 months [2.2–25.8] months vs. 6.4 months [5.7–7.0] months; p = 0.193), as shown in Fig. 3B. In the combination group, we compared the PFS of patients treated with EGFR-TKIs combined with antiangiogenic drugs or EGFR-TKIs combined with chemotherapy and found no difference between two subgroups (median, 18 months [14.1–22.0] vs. 19.9 months [12.5–27.3]; HR: 0.88; 95% CI: 0.446–1.775; p = 0.122), as shown in Fig. 3D.
Responses in different EGFR subtypes
Regarding the association between the type of EGFR mutation and therapy response, patients with 19 deletion or L858R mutations both achieved a significant PFS benefit after combination therapy compared with EGFR-TKIs alone, and PFS was similar between 19 deletions, L858R, and other mutations (Fig. 5).
Kaplan-Meier curves of progression-free survival (PFS) of patients according to EGFR mutations. (A) Patients with EGFR exon 19 del. (B) Patients with exon 21 exon L858R mutation. (C) Patients with 19 del, L858R and other mutations in EGFR-TKI group. (D) Patients with 19 del, L858R and other mutations in combination therapy group
Analysis among TP53 subtypes
Considering the different TP53 mutations, patients with exon 4 or 7 (4/7) mutations had shorter PFS than patients harboring non-exon 4/7 mutations in EGFR-TKI group (Fig. 6A). However, in the combination therapy group, the patients achieved a similar PFS benefit regardless of exon 4/7 mutations in TP53 (Fig. 6B). A greater PFS benefit was observed in TP53 exon 4/7 mutations subgroups. No significant difference was observed in patients stratified by other TP53 mutation type (Fig. 6C and D),
Kaplan-Meier curves of progression-free survival (PFS) of patients according to TP53 mutations. (A) Patients with TP53 exon 4/7 vs. non exon 4/7 in EGFR-TKI group. (B) Patients with TP53 exon 4/7 vs. non exon 4/7 in combination therapy group. (C) Patients with TP53 exon 8 vs. non exon 8 in EGFR-TKI group. (D) Patients with TP53 exon 8 vs. non exon 8 in combination therapy group
Genomic predictors of PFS outcome
BRAC1 mutation had a significantly shorter PFS than wild-type BRAC1 (median, 7.0 [95% CI: 4.4–9.6] months vs. 14.0 [95% CI: 10.7–17.3] months; p = 0.007; Fig. 7A). MYC amplification tend to be associated with shorter PFS (median, 6.5 [95% CI: 4.9–8.1] months vs. 13 [95% CI: 10.0–16.0] months; p = 0.133), especially in the combination therapy group (median, 7.0 [95% CI: 5.9–8.1] months vs. 18.0 [95% CI: 14.3–21.7] months; p = 0.036; Fig. 7B).
ORR, DOR, and DoR
In the EGFR-TKI group, 23 (44.2%) of patients showed a partial response (PR), and 23 (44.2%) had SD; in the combination group, 40 (55.6%) had PR, and 29 (40.3%) had SD. The patients in two groups had comparable ORRs [44.2% [95% CI: 30.3–58.2%] vs. 55.6% [95% CI: 43.8–67.3%; OR, 0.97; p = 0.275]). Furthermore, 46 (88.5%, 95% CI: 79.5–97.6%) patients in the EGFR-TKIs and 69 (95.8%, 95% CI: 91.1–100%) patients in the combination group achieved disease control (p = 0.164). The median duration of response was significantly longer in the combination group than in the EGFR-TKI group (median, 13.2 [95% CI:10.4–16.2] months vs. 9.0 [95% CI: 7.0–11.1] months; p = 0.039) (Table 2).
Discussion
In the present study, we selected patients with EGFR and TP53 co-mutation and retrospectively analyzed the PFS in patients with different treatment options. To the best of our knowledge, this study was the first to analyze optimal treatment options for EGFR and TP53 co-mutant NSCLC patients in a relatively large case series. We found that for patients with concomitant concurrence of EGFR and TP53 mutations, the duration of PFS was significantly longer with EGFR-TKI treatment combined with antiangiogenic drugs or chemotherapy than with EGFR-TKIs only, regardless of whether first-, second-, or third-generation EGFR-TKI was used. These data were even more significant for patients with TP53 exon 4 or 7 mutations.
The p53 protein regulates cellular responses to various cellular stress signals by inducing cell-cycle arrest, senescence, or apoptosis [18]. The complete loss of TP53 function can accelerate the transformation potential of driver oncogenes in lung cancers [19]. TP53 mutations play a role in predicting poor prognosis of patients with advanced NSCLC [20, 21]. TP53 mutations is an unfavorable prognostic factor in patients receiving first-, second-, and third-generation EGFR-TKI therapy with EGFR mutant advanced NSCLC [7, 11, 22, 23]. TP53 mutation status can be used to select treatment for patients with EGFR mutated lung cancer. In the present study, we also compared the PFS of patients treated with first-, second-, and third-generation EGFR-TKIs and found comparable PFS among the treatments with lower PFS compared with the efficacy of EGFR TKIs reported in the literature.
The role of TP53 in angiogenesis has also been established, and multiple studies have shown that the presence of TP53 mutation is associated with the upregulation of the VEGF pathway and may predict clinical sensitivity to antiangiogenic therapies in several tumor types [24, 25]. Clinical studies have also shown that for patients with concomitant TP53 mutation, the combination regimen of EGFR-TKIs and antiangiogenic drugs improved the PFS compared with TKI only [16, 26]. The combination of chemotherapy and EGFR-TKIs also eliminated this heterogeneity of TP53 co-mutation [17]. In our study, PFS was significantly longer with EGFR-TKI treatment combined with antiangiogenic drugs or chemotherapy than with EGFR-TKIs only. We also compared the PFS of patients treated with antiangiogenic drugs or chemotherapy and found that the efficacy of the two regimens is comparable.
EGFR mutation types exhibit different biology after treatment with EGFR-TKIs therapy, with improved outcomes in patients harboring exon 19 deletions compared with L858R [27,28,29]. For patients with EGFR/TP53 co-mutation, higher ORR and PFS was observed in the subgroup of patients with exon 19 deletion with respect to patients with L585R mutation [16, 23]. In the present study, patients with 19 deletion or L858R mutations both achieved a significant PFS benefit after combination therapy compared with EGFR-TKI alone, but no difference was observed between 19 deletion and L858R. The survival advantage of 19 deletion may be neutralized by the negative effects of TP53 mutations.
Classifying TP53 mutations has become increasingly important, because mutants in different exons exhibit different biological effects and clinical implications [9, 11, 16, 23]. Potential optimization strategies for certain subgroups of patients with baseline EGFR/TP53 co-mutated status must be explored. Mutations in the exon 4 or 7 of TP53 in patients with NSCLC were correlated with worse PFS than in patients with other exons [9]. We compared the PFS of patients with 4 or 7 of TP53 mutations and other TP53 mutations and found PFS benefits tended to favor the combination group in the subgroup of exon 4 or 7 mutations. Canale and Zhao H [11, 16, 23] reported a shorter median PFS in patients with EGFR mutant NSCLC with TP53 exon 8 mutations compared with other exon subsets. In the present study, patients with exon 8 of TP53 mutations achieved a similar PFS benefit in the EGFR-TKIs group and the combination group, and had similar median PFS to the overall patient population with the same therapy group.
Co-occurring genomic alterations such as RB1 mutation, PTEN mutation, and MDM2 and CDK4/6 amplification are associated with worse PFS in patients with EGFR mutation-positive NSCLC [30, 31]. However, in our EGFR and TP53 co-mutation cohort, these alternations were not associated with PFS. BRAC1 mutation and MYC amplification predicted poor prognosis for EGFR-TKI therapy or combination therapy. The amplification and overexpression of MYC were found in approximately 10% NSCLC [32]. MYC amplification was correlated with chemotherapy resistance in lung cancers [33]. The enforced expression of ectopic MYC partially protects sensitive EGFR mutant cells from undergoing osimertinib-induced apoptosis and decreases cell survival [34]. APC alternations are also associated with shorter PFS in patients treated with gefitinib [26], which was consistent with our study. Low BRAC1 expression levels were associated with increased PFS after platinum-based chemotherapy [35]. Our study was the first to report that BRAC1 was associated with unfavorable PFS after EGFR-TKI treatment or combination therapy. Nevertheless, considering the small number of BRAC1 and MYC mutated patients, the study was not powered for subgroup analysis. Thus, the results of the analysis of BRAC1 and MYC alternations should be interpreted with caution.
The present study was limited by its retrospective nature, the use of a single accrual center, and lack of data validation from external multicenter, which restricted our ability to investigate other sources of potential bias. Considering that we included patients that received different first-, second-, third-generation EGFR-TKIs treatment and different combination regimens, and the TP53 mutations in six patients were only recorded based on medical records without detailed information, the generalizability of our study findings must be considered. In addition, this study did not include the analysis of adverse effects because of the incomplete medical records. Therefore, further prospective studies conducted in larger cohorts are required to validate the efficacy of combination therapy observed in the study.
Conclusion
This study was the first to present survival outcomes to compare the benefits of EGFR-TKIs and its combination with antiangiogenic drugs or chemotherapy in EGFR and TP53 co-mutation patients. In comparison with EGFR-TKI monotherapy, EGFR-TKIs combined with antiangiogenic drugs or chemotherapy significantly improved PFS in patients with advanced NSCLC having concomitant EGFR and TP53 mutations.
Data Availability
The datasets during the current study are not publically available due to patient confidentiality but can be obtained from Haiyan Sun upon sufficient and reasonable request.
References
Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. Journal of the National Cancer Institute2017, 109(6).
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin oncology: official J Am Soc Clin Oncol. 2013;31(27):3327–34.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, et al. Overall survival with Osimertinib in untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al. Osimertinib as First-Line treatment of EGFR mutation-positive Advanced Non-Small-Cell Lung Cancer. J Clin oncology: official J Am Soc Clin Oncol. 2018;36(9):841–9.
Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas VR, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49(12):1693–704.
Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, Zhang L. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(5):739–42.
Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, Kris MG, Hellmann MD, Li BT, Somwar R, et al. Concurrent alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin cancer research: official J Am Association Cancer Res. 2018;24(13):3108–18.
Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, Chang MT, Ni A, Kundra R, Jonsson P, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7(6):596–609.
Li XM, Li WF, Lin JT, Yan HH, Tu HY, Chen HJ, Wang BC, Wang Z, Zhou Q, Zhang XC, et al. Predictive and prognostic potential of TP53 in patients with Advanced Non-Small-Cell Lung Cancer treated with EGFR-TKI: analysis of a phase III Randomized Clinical Trial (CTONG 0901). Clin Lung Cancer. 2021;22(2):100–9. e103.
Hou H, Qin K, Liang Y, Zhang C, Liu D, Jiang H, Liu K, Zhu J, Lv H, Li T, et al. Concurrent TP53 mutations predict poor outcomes of EGFR-TKI treatments in chinese patients with advanced NSCLC. Cancer Manage Res. 2019;11:5665–75.
Canale M, Petracci E, Delmonte A, Chiadini E, Dazzi C, Papi M, Capelli L, Casanova C, De Luigi N, Mariotti M, et al. Impact of TP53 mutations on Outcome in EGFR-Mutated Patients treated with first-line tyrosine kinase inhibitors. Clin cancer research: official J Am Association Cancer Res. 2017;23(9):2195–202.
Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et al. Gefitinib alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin oncology: official J Am Soc Clin Oncol. 2020;38(2):115–23.
Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–35.
Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin oncology: official J Am Soc Clin Oncol. 2020;38(2):124–36.
Nakagawa K, Nadal E, Garon EB, Nishio M, Seto T, Yamamoto N, Park K, Shih JY, Paz-Ares L, Frimodt-Moller B et al. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research2021.
Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, Cui J, Miao L, Zhang L, Yuan X, et al. Apatinib Plus Gefitinib as First-Line treatment in Advanced EGFR-Mutant NSCLC: the Phase III ACTIVE Study (CTONG1706). J Thorac oncology: official publication Int Association Study Lung Cancer. 2021;16(9):1533–46.
Yang Z, Chen Y, Wang Y, Wang S, Hu M, Zhang B, Han B. Efficacy of EGFR-TKI Plus Chemotherapy or Monotherapy as First-Line treatment for Advanced EGFR-Mutant Lung Adenocarcinoma patients with co-mutations. Front Oncol. 2021;11:681429.
Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883.
Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483(7391):613–7.
Jiao XD, Qin BD, You P, Cai J, Zang YS. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, an analysis based on cBioPortal data base. Lung Cancer. 2018;123:70–5.
Gu J, Zhou Y, Huang L, Ou W, Wu J, Li S, Xu J, Feng J, Liu B. TP53 mutation is associated with a poor clinical outcome for non-small cell lung cancer: evidence from a meta-analysis. Mol Clin Oncol. 2016;5(6):705–13.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–6.
Canale M, Petracci E, Delmonte A, Bronte G, Chiadini E, Ludovini V, Dubini A, Papi M, Baglivo S, De Luigi N et al. Concomitant TP53 Mutation Confers Worse Prognosis in EGFR-Mutated Non-Small Cell Lung Cancer Patients Treated with TKIs. Journal of clinical medicine2020, 9(4).
Li AM, Boichard A, Kurzrock R. Mutated TP53 is a marker of increased VEGF expression: analysis of 7,525 pan-cancer tissues. Cancer Biol Ther. 2020;21(1):95–100.
Wheler JJ, Janku F, Naing A, Li Y, Stephen B, Zinner R, Subbiah V, Fu S, Karp D, Falchook GS, et al. TP53 alterations correlate with response to VEGF/VEGFR inhibitors: implications for targeted therapeutics. Mol Cancer Ther. 2016;15(10):2475–85.
Cheng Y, Ma L, Liu Y, Zhu J, Xin Y, Liu X, Wang Y, Zhang T, Yang C, Wang S, et al. Comprehensive characterization and clinical impact of concomitant genomic alterations in EGFR-mutant NSCLCs treated with EGFR kinase inhibitors. Lung Cancer. 2020;145:63–70.
Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin cancer research: official J Am Association Cancer Res. 2006;12(13):3908–14.
Li H, Zhang X, Cao J, Su P, Lian J, Song X, Yang W, Han S, Xi Y, Wang Y. Exon 19 deletion of epidermal growth factor receptor is associated with prolonged survival in brain metastases from non-small-cell lung cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(12):9251–8.
Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin cancer research: official J Am Association Cancer Res. 2006;12(3 Pt 1):839–44.
Kim Y, Lee B, Shim JH, Lee SH, Park WY, Choi YL, Sun JM, Ahn JS, Ahn MJ, Park K. Concurrent genetic alterations predict the progression to Target Therapy in EGFR-Mutated Advanced NSCLC. J Thorac oncology: official publication Int Association Study Lung Cancer. 2019;14(2):193–202.
Sitthideatphaiboon P, Teerapakpinyo C, Korphaisarn K, Leelayuwatanakul N, Pornpatrananrak N, Poungvarin N, Chantranuwat P, Shuangshoti S, Aporntewan C, Chintanapakdee W, et al. Co-occurrence CDK4/6 amplification serves as biomarkers of de novo EGFR TKI resistance in sensitizing EGFR mutation non-small cell lung cancer. Sci Rep. 2022;12(1):2167.
Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495–509.
Knapp DC, Mata JE, Reddy MT, Devi GR, Iversen PL. Resistance to chemotherapeutic drugs overcome by c-Myc inhibition in a Lewis lung carcinoma murine model. Anticancer Drugs. 2003;14(1):39–47.
Zhu L, Chen Z, Zang H, Fan S, Gu J, Zhang G, Sun KD, Wang Q, He Y, Owonikoko TK, et al. Targeting c-Myc to Overcome Acquired Resistance of EGFR Mutant NSCLC cells to the third-generation EGFR tyrosine kinase inhibitor, Osimertinib. Cancer Res. 2021;81(18):4822–34.
Papadaki C, Sfakianaki M, Ioannidis G, Lagoudaki E, Trypaki M, Tryfonidis K, Mavroudis D, Stathopoulos E, Georgoulias V, Souglakos J. ERCC1 and BRAC1 mRNA expression levels in the primary tumor could predict the effectiveness of the second-line cisplatin-based chemotherapy in pretreated patients with metastatic non-small cell lung cancer. J Thorac oncology: official publication Int Association Study Lung Cancer. 2012;7(4):663–71.
Acknowledgements
The authors thank all patients who participated in this study and their families. We also thank the whole project team members who worked on this study.
Funding
This work was supported by The National Natural Science Foundation of China (grant number 81803914 to Z.Z. Jiang).
Author information
Authors and Affiliations
Contributions
Study concepts: Zhansheng Jiang and Zhanyu Pan; study design: Zhansheng Jiang, Haiyan Sun and Zhanyu Pan; definition of intellectual content: Haiyan Sun and Peng Ren; literature research: Lan Lan, Zhuchen Yan, and Yinli Yang; clinical studies: Haiyan Sun, Peng Ren, Lan Lan, Zhuchen Yan, Bin Wang, Cong Wang, Yanwei Li, Ling Li, Yu Zhang, Yanyang Li, and Zuolin Wang; statistical analysis: Haiyan Sun and Yongzi Chen; manuscript preparation: Haiyan Sun and Peng Ren; manuscript editing and review: Zhansheng Jiang and Zhanyu Pan. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved from the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital (protocol number bc2022050). Informed consent from the participants was waived by Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital as the current study satisfied all of the requirements for the waiver of informed consent. The research was retrospective data analysis of previously collected medical records and involved no more than minimal risk to the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, H., Ren, P., Chen, Y. et al. Optimal therapy for concomitant EGFR and TP53 mutated non-small cell lung cancer: a real-world study. BMC Cancer 23, 198 (2023). https://doi.org/10.1186/s12885-023-10637-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10637-4