Abstract
Background
Breast cancer chemoresistance is attributed to a wide variety of mechanisms, including autophagy. Transcription factor EB (TFEB) has been recently identified and characterized as one major regulator of autophagy and lysosomal genesis.
Objective
This study aims to evaluate the prognostic impact of TFEB and its pathway in breast cancer chemoresistance.
Methods
This retrospective study analyzes the expression of TFEB, CARM1, SIRT1, and Beclin-1 and the methylation of PITX2 in breast carcinoma. A group of breast cancer patients treated with chemotherapy, who relapsed within 12 months from treatment initiation, were compared to a sub-cohort of chemo-treated patients who did not recur within 12 months of follow-up. The expression of TFEB, CARM1, SIRT1, and Belcin-1 was analyzed using immunohistochemistry or RT-PCR on formalin-fixed paraffin-embedded samples. PITX2 methylation was tested with the diagnostic CE-marked kit Therascreen PITX2 RGQ PCR. In the final model, 136 cases of chemo-treated breast cancer were included.
Results
A higher TFEB and Beclin-1 expression correlate with shorter survival in patients with chemo-treated invasive breast cancer (respectively HR 3.46, CI.95 1.27–9.47, p < 0.05 and 7.11, CI.95 2.54–19.9). TFEB, CARM1, and SIRT1 are positively correlated with Beclin-1. The protein expression of SIRT1 is significantly associated with TFEB and CARM1 so that a very low SIRT1 expression (lower than the first quartile of the H-score distribution) correlates with a low expression of TFEB and CARM1 and with longer survival. SIRT1 seems to have a lower H-score in the basal-like and HER2-enriched tumors than the luminal subtypes. Beclin-1 and TFEB seem to have a higher H-score in the basal-like and HER2-enriched tumors than the luminal subtypes. PITX2 methylation analysis was feasible only in 65% of the selected samples, but no significant differences between cases and controls were found, and there was also no correlation with the expression of the TFEB pathway.
Conclusions
TFEB, SIRT1, and Beclin-1 seem to have a potential prognostic significance in patients with chemo-treated breast cancer, likely because of their role in the regulation of autophagy. In addition, no correlation between TFEB and PITX2 methylation was found, likely because they perform two different roles within the autophagy process.
Similar content being viewed by others
Highlights - what this paper adds
• A high TFEB expression correlates with shorter survival in patients with chemo-treated invasive breast cancer;
• A high Beclin-1 expression correlates with shorter survival in patients with chemo-treated invasive breast cancer;
• TFEB, CARM1, and SIRT1 are positively correlated with Beclin-1 expression.
• Low SIRT1 expression correlates with a low expression of TFEB and CARM1 and with longer survival;
• PITX2 methylation analysis was feasible only in 65% of the selected samples, but no significant differences between cases and controls were found;
• No correlation between TFEB and PITX2 methylation was found;
• TFEB, SIRT1, and Beclin-1 seem to have a potential prognostic significance in patients with chemo-treated breast cancer.
Background
Despite the remarkable progress in both diagnosis and treatment, breast carcinoma continues to be a leading cause of cancer-related death among women worldwide. It is the second biggest killer out of all malignancies in the female gender, after lung and bronchial neoplasia [1, 2]. In particular, metastatic breast carcinoma remains a major challenge for the breast specialist, and unfortunately, its incidence has remained at a fairly consistent level over recent years [1]. Breast cancer comprises multiple subtypes that present different prognostic outcomes and various molecular patterns [3, 4]. The breast cancer molecular subtypes include luminal A, luminal B, luminal HER2, HER2 enriched, and basal-like [5]. The basal-like subtype presents commonly high tumor grading, and adverse outcomes (they do not express estrogen, progestogen receptors, or HER2) [3, 4]. Meanwhile, Luminal A and B show the most favorable outcomes and express hormonal receptors [6].
As in other cancer histotypes, the role of autophagy and chemoresistance in breast cancer is a critical issue. Drug resistance can be attributed to a wide variety of mechanisms. Pre-clinical studies using chemical inhibitors of autophagy or siRNA to downregulate autophagy genes demonstrate the role of autophagy in the chemotherapy sensitization of cancer cells [7,8,9,10]. Among the transcription factors that regulate autophagy, the transcription factor EB (TFEB) was identified and characterized in 2009 as the main regulator of autophagy and lysosomal genesis, as well as an element capable of regulating autophagy by coordinating the expression of genes acting at all stages of the autophagy process [11,12,13,14]. The co-activator-associated arginine methyltransferase 1 (CARM1) has a transcriptional coactivator role in autophagy and lysosomal genes through TFEB [15]. Furthermore, the CARM1 dependent histone arginine methylation is a fundamental nuclear event in autophagy [15]. A deacetylase involved in epigenetic changes, SIRT1, also influences the expression of autophagy-related genes [16, 17]. SIRT1 is known to be a significant prognostic factor in many cancers [18], and a high SIRT1 expression is known to be an unfavorable prognostic factor in breast cancer [19, 20]. In addition, Beclin-1 is a known marker of autophagy [21, 22].
Paired-like homeodomain transcription factor 2 (PITX2) works in the Wnt signaling pathway by modulating β-catenin, which in turn affects the transcription of cell cycle-associated and proliferation genes, enhancing cell proliferation [23]. The promoter methylation of the PITX2 gene can lead to gene silencing inducing changes in molecular signaling networks, and it was found to be a prognostic biomarker in breast cancer [23]. The kit for evaluating the methylation of PITX2 (a commercially available CE-marked kit: Therascreen PITX2 RGQ PCR) was found to be a reliable prognostic factor for drug resistance, and it was found suitable if applied on formalin-fixed paraffin-embedded tissue samples. In particular, the methylation of PITX2 has been found to predict sensitivity to anthracycline-based chemotherapy in breast cancer [24,25,26,27].
This study’s main objective is to assess the prognostic role of TFEB, CARM1, SIRT1, and Beclin-1 in chemo-treated breast carcinoma and compare the TFEB, CARM1, SIRT1, and Beclin-1 expression with the methylation of PITX2 in breast cancer treated with chemotherapy.
Methods
Study population
The study population comprises women affected by invasive breast carcinoma, treated by adjuvant or neoadjuvant chemotherapy, and with archived tissue specimens of the primary tumor. Tissue samples (standard archived, formalin-fixed, paraffin-embedded tissue) and clinical data were retrospectively collected at the Institute of Pathology and at the Breast Unit of our center, respectively.
In this study, all breast cancer cases treated by adjuvant or neoadjuvant chemotherapy between January 2002 and December 2016 were included in order to have at least 12 months of follow-up. All cases were included where sufficient tumor tissue was stored in paraffin blocks at the time of surgery. Consequently, all cases of complete pathological response were excluded, as the quantity of tissue from preoperative biopsy did not provide enough material for the current study. Also, the cases of poorly conserved or quantitatively insufficient tissue were excluded. Furthermore, all of the following cases were excluded from the study: those without a documented follow-up, breast cancer patients who did not undergo adjuvant or neoadjuvant treatments, and male patients.
Study design
This retrospective observational study reviews pathological archives and medical records to identify breast cancer cases treated by adjuvant or neoadjuvant chemotherapy. For the purpose of the study, two different types of sample selection from the original cohort were performed: case-cohort study; and case-control study.
Case-cohort study
The case-cohort study design model is a sampling methodology to randomly select a sample (called subcohort) from an assembled epidemiologic cohort and to use this subcohort as a comparison group for the selected cases that occur in the cohort (in this particular case, the group of breast cancer patients that relapsed within 12 months of follow up) [28]. This kind of study is particularly suitable for very numerous cohorts, where it is cost-prohibitive to provide a complete follow-up either for disease outcomes or for specific information on the whole cohort [28,29,30].
Of the 4504 women in the cohort treated for breast pathology, 894 were eligible for the present study because of chemotherapy treatment and fulfilling the required inclusion criteria. The study sample was selected from the latter 894 women. It was composed of a random group of 163 of the 894 eligible women (hereafter called the subcohort) together with all eligible women diagnosed with a breast cancer recurrence within 12 months of treatment initiation. We considered all recurrences (loco-regional recurrences or distant metastases) and adverse events (breast cancer-related death) occurring between the baseline attendance and April 30, 2017.
The final sample included 203 women: 163 belonging to the subcohort and 42 breast cancer patients who experienced recurrence within a 12-month follow-up. We factored in that about 35% of samples could potentially be unavailable for the assessment in the TMA. Power and sample size considerations were based on Cai and Zeng’s approach, and the study had > 80% power to detect HRs of 3.0, assuming alfa = 0.05 [31].
Case-control study
In the case-control sample selection, all breast cancer patients included in the tissue micro-array were considered. Cases and controls were randomly selected from the patients mentioned above.
The number of the analyzed samples was established from the maximum available resources to test PITX2 methylation. Therefore, the total number of cases was 13, and the total number of controls was similarly 13.
Clinical information
Information collected included some patient characteristics, such as the age at diagnosis, the body mass index (BMI), any familial history for breast or ovarian carcinoma, the current fertility status (pre-or post-menopausal), any use of estro-progestin therapies (with contraceptive intent in the pre-menopause or as hormone replacement therapy in the post-menopause). Tumor characteristics considered were the histotype, grading, expression of estrogen receptor (ER), progesterone receptor (PR), HER2/neu and Mib1/Ki-67, as well as any presence of multifocality/multicentricity, perivascular invasion (PVI), peritumoral lymphocytic infiltration, nodal extracapsular invasion or bunched axillary nodes [32]). Surgical and non-surgical treatments were also taken into account for data elaboration.
Pathological specimens were routinely assessed following the European guidelines [33, 34]. In particular, samples sized 30 mm or less were wholly sliced and evaluated, whereas specimens sized over 30 mm underwent sampling based on the European guidelines [33, 34].
The World Health Organization criteria were used to determine the histology [35] and nodal status (TNM classification VII ed. AJCC/UICC, 2009) [36]. AFIP and Elston Ellis’s recommendations were considered while assessing the grading in ductal carcinoma in situ and invasive carcinoma, respectively [37, 38]. Peritumoral lymphocytic infiltration, PVI, multifocality/multicentricity, and nodal status were determined as described in previous studies [39, 40].
Estrogen Receptor (ER), Progesterone Receptor (PR), Mib1/Ki-67, and HER2/Neu expression were evaluated by immunohistochemistry. We defined positive ER or PR where positivity was ≥1% in any nuclear staining. In addition, HER2/Neu was defined as overexpressed when staining 3+ or 2+ with FISH amplification and negative if the value was 0, 1+ or 2+ without FISH amplification. Through the combination of ER, PR, HER2/Neu, and Mib1/Ki-67, all invasive breast cancers were classified in the following molecular subtypes as previously described: luminal A, luminal B, luminal HER2, HER2-enriched, and basal-like [39].
Immunohistochemistry and molecular biology analyses
In this study, the following analyses were performed in both the case-cohort and the case-control subject selection. We analyzed the presence and the quantity of mRNA and the relative protein synthesis in the selected breast cancer tumor samples. In addition (solely for subjects selected for the case-control study), the Therascreen PITX2 RGQ PCR was used to test the PITX2 methylation.
Real-time PCR (RT-PCR) to quantify mRNA of TFEB, CARM1, and SIRT1
To perform real-time quantitative PCR, the primers were prepared for TFEB, CARM1, and SIRT1 (Supplemental Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping protein (Supplemental Table 1). The mRNA was extracted from formalin-fixed paraffin-embedded tumor tissue samples by manually micro-dissecting the tumor area that had been histologically marked by a pathologist and using an RNeasy kit (Qiagen®, Venlo, Netherlands) that was used following the manufacturer’s instructions. RNA quantity and purity were measured using the Qubit 2.0 spectrophotometer (Invitrogen®, Carlsbad, CA), and RNA integrity was quantified using the RIN (RNA integrity number) assessed by Agilent 2100 Bioanalyzer (Agilent Technologies®, Santa Clara, CA). From this m-RNA, employing retrotranscription (SuperScript®, III REV transcript; Life Technologies), cDNA was obtained and quantified. Quantitative real-time PCR analysis was performed in triplicate by three independent experiments, using the LightCycler® 480 (Roche®) and LC SYBR Green I Master (Roche®), according to the manufacturers’ instructions. Quantitative data were collected as cycle threshold (CT) values considering the means of the triplicate runs. Finally, quantitative ΔCT expression values (2(−ΔCT)) were calculated.
Immunohistochemical analysis (IHC)
Along with the RT-PCR analysis, TFEB, CARM1, and SIRT1 protein expressions for all breast cancer cases in our samples were investigated by immunohistochemistry. Beclin-1 was investigated by immunohistochemistry. Cases were evaluated with respect to both staining percentage and intensity.
Tissue Micro Array (TMA)
The preparation and analysis of the TMA were carried out as previously described [41,42,43,44,45,46]. Having identified formalin-fixed, paraffin-embedded tumor tissue corresponding with our sample of patients, the hematoxylin-eosin-colored sections were analyzed. Then the tissue core samplings for the TMA were performed, taking care to include neoplastic tissue (two core biopsies per primary tumor). The receiver blocks were assembled. From these, 4-μm cross-sections were obtained, which were stained with hematoxylin-eosin. At a later stage, additional 4-μm cross-sections were obtained to prepare slides for immunohistochemical staining and subsequent analysis.
Immunohistochemical staining was performed according to standard protocol and manufacturer instructions. For antigen retrieval, the slides were heated, after deparaffinization, for 20 min at 98 °C in Target Retrieval Solution (low pH; Dako K8005, Glostrup, DK) with PT-link (Dako), and endogenous peroxidase activity was blocked with H2O2 for 10 min at environmental temperature. Slides were rinsed in PBS and then incubated with the following primary antibodies for 1 h at room temperature: TFEB (OriGene Technologies Inc., diluted 1:100, Rockville, MD, USA); CARM1 (OriGene Technologies Inc., diluted 1:100, Rockville, MD, USA); SIRT1 (OriGene Technologies Inc., diluted 1:200, Rockville, MD, USA); and Beclin-1 (Abcam plc., diluted 1:100, Cambridge, UK). A Dako REAL™ EnVision™ Dako Rabbit/Mouse (Dako, K5007, Glostrup, DK) was used as a second antibody. HRP activity was identified utilizing Dako REAL™ DAB+Chromogen (Dako, K5007, Glostrup, DK) as the substrate for 3 min in accordance with the manufacturer’s instructions. Tissue sections were then counterstained with hematoxylin. Negative controls included sections incubated with non-immune rabbit serum instead of the primary antibody. The immunohistochemical staining was evaluated independently by two pathologists in terms of H-score (the product of the actual percentage of positive-stained cells and intensity score, evaluated as strong 3, moderate 2, and weak 1, giving a possible range of 0–300).
PITX2 DNA methylation assay
The PITX2 promoter methylation was evaluated for DNA extracted from formalin-fixed paraffin-embedded sections (QIAmp DNA mini kit-Qiagen). Specifically, DNA was extracted from each primary tumor tissue sample kept at room temperature until the extraction time. Therascreen® PITX2 RGQ PCR kit is a methylation-specific PCR (MSP) based on real-time PCR, intended for the quantitative assessment of percent methylation ratio (PMR) in the promoter 2 (P2) of the PITX2 gene and it was validated in primary formalin-fixed paraffin-embedded breast cancer tissue [23, 47, 48]. PMR was calculated according to manufacturer instructions.
Genomic DNA was extracted from the samples using the QIAmp DNA FFPE Tissue Kit (QIAGEN Inc.). DNA was quantified using a Qubit dsDNA analysis kit for QBIT 2.0 fluorimeter (Thermo Fisher Scientific). The bisulfite conversion of the DNA was performed using the EpiTect Plus DNA Bisulfite Kit (QIAGEN Inc.), and the methylated DNA was then purified using the purification module reagents provided by the same kit and quantified at QBIT 2.0. After bisulfite conversion, the PMR of 3 CpG motifs of the PITX2 gene P2 was quantified by MSP using the Therascreen® PITX2 RGQ PCR kit, this containing a quantitative RT-PCR reaction mix, a primer, probes, and positive and negative controls [47].
The quantitative real-time PCR reaction was performed using the Rotor-Gene Q MDx real-time PCR platform (QIAGEN, Inc.) and evaluated by QIAGEN Rotor-Gene AssayManager® (Version 2.1.0) software with Therascreen PITX2 FFPE (C) analysis plugin for analysis and quality control [49].
During all tissue sample analyses, the operators were blinded to the clinical data of the patients. All primary formalin-fixed paraffin-embedded breast cancer tissue samples were coded to ensure blinding of the operator while conducting the PITX2 DNA methylation assay.
Statistical analysis
Data were analyzed through R (version 3.6.1; R Core Team (2019); R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; URL https://www.R-project.org/) and considering p < 0.05 as significant. The normality of the distribution was tested with the Kolmogorov-Smirnov test. Univariate analysis was performed by the Wilcoxon or t-test in cases of continuous variables, or the Fisher exact test or chi-square test in cases of categorical variables. Univariate and multivariate survival analyses were also performed by Kaplan-Meier curves, Log-rank test, and Cox proportional hazards regression models. OS was considered to be the main outcome. Besides, in the multivariate model, all selected factors and their interactions were accommodated in a single analysis, except when the interaction term was non-significant (in which case we analyzed the non-interaction model). Correlations were tested by Spearman Rho and the relative p-value.
Results
Population description
After the identification of the forty eligible subjects, the sub-cohort of 163 patients was extrapolated. From this first procedure, it was found that four subjects were duplicated between the cases and the sub-cohort. The blocks for the creation of the TMA were then collected. During the creation of the TMA of the 199 selected samples (including 159 that did not relapse within a 12 month follow up and 40 cases that did), only 109 cases that did not relapse within the year and 27 cases that did were able to be successfully included in the TMA. In the final TMA, 50 cases without relapse within 12 months and 13 relapsed cases had to be excluded, as their associated samples were unusable (i.e., they were used in other studies, there was an insufficient quantity of residual neoplastic tissue, or sample was simply not available).
Table 1 shows a description of the included samples. The median age at breast cancer surgery was 55 years (48–65), and the median BMI was 25 kg/m2 (IQR 22–29). In 99.26 and 8.82% of cases, respectively, adjuvant and neoadjuvant chemotherapy was administered. With regards to the surgical treatment, mastectomy was performed in the majority of cases (68.38%), together with complete axillary lymph node dissection (69.12%).
Lymph node macrometastases (neoplastic cell aggregates > 2 μm) and micrometastases (neoplastic cell aggregates of 0.2–2 μm) were discovered in 56.62 and 5.88% of cases, respectively. Considering that before 2015 both macro-and micrometastases were considered an absolute indication for complete axillary lymph node dissection and that this study includes patients operated on between 2002 and 2016, it is likely that the great majority of these cases underwent complete axillary lymphadenectomy. The remaining cases who underwent axillary lymph node dissection included patients who were clinically node-positive before neoadjuvant treatment and converted to clinically node-negative afterward (Table 1), as these particular cases are still the subject of worldwide debate, and there is no unanimous consensus about the best management of their axillary lymph nodes.
In Table 1, further tumor characteristics are described. In 80.88% of cases, the tumor histotype was invasive ductal carcinoma, followed by invasive lobular carcinoma in 11.03% of cases. 55.77% of tumors presented a Mib-1/Ki-67 greater than 20, and 24.26% were found to be histologically multifocal or multicentric.
Table 2 reports breast tumor staging. Most tumors were classified as TNM II and III (respectively 36.03 and 24.26%), and tumor grading was classified as G2 in more than half of patients (52.21%).
TFEB, CARM1, SIRT1, and Beclin-1 expression
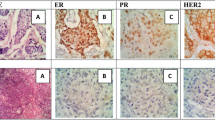
The Median TFEB expression in terms of H-score is 185 (IQR 99–200) with an exclusive nuclear localization (Fig. 1A and B). Median CARM1 expression in terms of H-score is 85 (IQR 25–100) again with a nuclear localization (Fig. 1C and D). The median SIRT1 expression is 190 (IQR 100–200), similarly with a localization in the nucleus (Fig. 1E and F). Finally, The Median Beclin-1 expression in terms of H-score is 90 (IQR 49–162) with an exclusive cytoplasmic localization (Fig. 1G and H).
Immunohistochemical staining. A Image at 20x (and in the box at 40x) of primary breast cancer tissue TFEB immunohistochemical staining in a patient with breast cancer recurrence within 12 months of follow-up. B Image at 20x (and in the box at 40x) of TFEB immunohistochemical staining in a patient’s primary breast cancer tissue without recurrence within 12 months of follow-up. C 20x image (and in the 40x square) of CARM1 immunohistochemical staining in primary breast cancer tissue of a woman with recurrent breast cancer within 12 months of follow-up. D 20x image (and in the 40x frame) of CARM1 immunohistochemical staining in a primary breast cancer specimen of a patient which did not recure within 12 months of follow-up. E Image at 20x (and in the box at 40x) of SIRT1 immunohistochemical staining in a primary breast cancer specimen of a woman with recurrent breast cancer within 12 months of follow-up. F Image at 20x (and in the box at 40x) of SIRT1 immunohistochemical staining in a primary breast cancer specimen of a woman without tumor recurrence within 12 months of follow-up. G Image at 20x (and in the box at 40x) of Beclin-1 immunohistochemical staining in a primary breast cancer specimen of a woman with recurrent breast cancer within 12 months of follow-up. H Image at 20x (and in the box at 40x) of Beclin-1 immunohistochemical staining in a primary breast cancer specimen of a woman without tumor recurrence within 12 months of follow-up
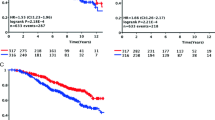
Figure 2A highlights the analysis of survival based on the expression of TFEB. In particular, a higher immunohistochemical expression of TFEB protein correlates with significantly shorter survival (p < 0.05). This difference is also present in the Cox regression analysis with an HR of 3.46 (IC.95 1.27–9.47) (p < 0.05). Furthermore, in the multivariate analysis, this difference is shown to be independent of neoadjuvant chemotherapy (Table 3).
Kaplan-Meier survival curves and p-values refers to Log-rank tests. A Curve based on TFEB expression (high expression consists in an H-score greater than the distribution median [> 185] and low expression in an H-score lower or equal to the distribution median) (p < 0.05). B Analysis based on CARM1 expression (i.e., high with an H-score higher than the median distribution [> 85] or low, i.e., with an H-score lower than or equal to the distribution median) (p = 0.156). C Analysis of survival based on SIRT1 expression (i.e., high with an H-score higher than the median distribution [190] or low, i.e., with an H-score lower than or equal to the distribution median) (p = 0.322). D Analysis of survival based on the expression of Beclin-1 (i.e., high with an H-score higher than the median distribution [> 90] or low, i.e., with an H-score lower than or equal to the distribution median) (p < 0.05). E Analysis of survival based on the expression of SIRT1 with a different cut-off (i.e., high with an H-score higher than the first quartile (*) of the distribution [> 100] or low, i.e., with an H-score lower than or equal to the first quartile of the distribution) (p < 0.05). F Subgroup analysis of survival (luminal A, luminal B, and luminal Her sub-types) based on the expression of SIRT1 (i.e., high with an H-score higher than the first quartile (*) of the distribution [132] or low, i.e., with an H-score lower than or equal to the first quartile of the distribution) (p = 0.055). Panel G: Subgroup analysis of survival (Her enriched and Basal-like sub-types) based on the expression of SIRT1 (i.e., high with an H-score higher than the first quartile (*) of the distribution [132] or low, i.e., with an H-score lower than or equal to the first quartile of the distribution) (p = 0.294)
Figure 2B shows the association between a high expression of CARM1 and shorter survival, although this correlation is not statistically significant. Meanwhile, Fig. 2C shows that an H-score of SIRT1 higher than the distribution median is not associated with significant differences in terms of survival.
Figure 2D shows that a higher immunohistochemical expression of Beclin-1 correlates with significantly shorter survival (p < 0.05). This difference is also present in the Cox regression analysis with an HR of 5.36 (IC.95 1.96–14.68) (p < 0.05). Moreover, in the multivariate analysis, this difference is shown to be independent of neoadjuvant chemotherapy (Table 3).
Correlation among immunohistochemical expression of TFEB, CARM1, SIRT1, and Beclin-1
In Supplemental Table 2 and Supplemental Fig. 1, it is possible to observe the correlation among the four analyzed proteins’ expressions. In particular, the immunohistochemical protein expression of SIRT1 correlates directly proportional and significantly with the expression of TFEB, CARM1, and Beclin-1. In addition, the immunohistochemical expression of TFEB also significantly correlates with that of CARM1 and Beclin-1. Finally, the immunohistochemical expression of CARM1 also significantly correlates with that of Beclin-1.
The graph in Supplemental Fig. 1 also shows that a SIRT1 expression within the first quartile of the H-score distribution corresponds to a low expression of CARM1 and TFEB. We consequently decided to evaluate whether survival in this particular subgroup was actually longer. Accordingly, Fig. 2E shows better survival (p < 0.05) in the case of SIRT1 expression under the first quartile of the H-score distribution.
Tumor molecular subtypes and TFEB, CARM1, SIRT1, and Beclin-1 expression
Supplemental Table 3 demonstrates no significant modification to either the CARM1 or SIRT1 result when considering the different tumor molecular subtypes separately. Although not significant, it is remarkable that the H-score of SIRT1 is lower in the basal-like and in the HER2-enriched subtypes than the luminal A, luminal B, and luminal HER2 ones.
Therefore, in Fig. 2F, the Kaplan-Meier survival analysis based on SIRT1 expression was redesigned to include only the luminal A, luminal B, and luminal HER2 molecular subtypes. Under this analysis, no more adverse events were recorded where we observed low SIRT1 expression (p = 0.055).
Furthermore, Fig. 2G shows that, also in the basal-like and in the HER2-enriched subtypes, separately, a low SIRT1 expression is associated with a better prognosis, although not in a statistically significant way.
We also redesigned the correlations between the three molecules in Supplemental Table 2, considering the tumor molecular subtypes. In particular, considering only the basal-like subtype, the correlation between CARM1 and SIRT1 is maintained while the TFEB and Beclin-1 correlations with SIRT1 and CARM1 lose their statistical significance. Finally, considering only the luminal variants (luminal A, luminal B, and luminal HER2), the directly proportional and significant correlations of SIRT1 with TFEB and CARM1 are confirmed as well as the correlation between TFEB and Beclin-1 (Supplemental Table 2).
TFEB, SIRT1, and CRAM1 expression in RT-PCR
The mRNA quantification was only possible in samples with high H-score and low paraffin storage duration. In particular, for CARM1, which was the least expressed protein in immunohistochemistry, no mRNA was detected in the analyzed samples. As regards TFEB (rho = 0.50) and SIRT1 (rho = 0.15), a directly proportional correlation between immunohistochemical score (assessed as H-score) and mRNA expression (assessed as a delta-CT expression) was found, although these correlations are not statistically significant.
PITX2 methylation (case-control study)
The test failed in 9 samples (35%) (5 cases and 4 controls) of the total 26 tested samples. PMR was found to be > 12 in the 37.50% of cases (3/8) and in 55.56% of controls (5/9) (p = 0.637).
Supplemental Table 4 shows the characteristics of the patients in which the test was successful (8 cases and 9 controls), and no statistically significant differences were found. Although not statistically significant, the age of patients in cases with recurrence within the first year of follow-up was greater than in controls, as well as the number of mastectomies, which, however, could be somehow linked to the increased age of patients.
Supplemental Table 4 also shows the tumor characteristics, where again no statistically significant differences were observed - except for a higher prevalence of basal-like tumor subtypes in the case of early recurrences. Supplemental Table 5 shows the tumor stages, and, here again, there were no statistically significant differences between the two considered groups.
In Supplemental Table 6, the differences in the immunohistochemical expression of TFEB, CARM1, and SIRT1 are reported, as well as the PMR value of the PITX2 methylation. Compared with controls, TFEB, SIRT1, and CARM1 expression levels were significantly higher where relapse occurred within 12 months (p < 0.05), while no statistically significant differences were observed as regards the PITX2 PMR.
For this same group of patients, considering the correlations between PITX2 methylation and immunohistochemical expression of TFEB, CARM1, and SIRT1, PITX2 methylation does not correlate with the immunohistochemical expression of the rest of the TFEB pathway.
Discussion
From this analysis of the role of the TFEB pathway in breast cancer chemoresistance, we reveal evidence that the increased expressions of TFEB and Beclin-1 are associated with a shorter survival period in patients suffering from invasive breast cancer who undergo chemotherapy. Our data also demonstrate a significant and directly proportional correlation of SIRT1 expression with TFEB and CARM1 expression, so that a very low expression of SIRT1 (lower than the first quartile of the H-score distribution) shows an associated low expression of TFEB and CARM1, and consequently with a longer duration of survival. Furthermore, in the basal-like and HER2-enriched breast cancer subtypes, SIRT1 seems to have a lower H-score than in the luminal molecular subtypes. Meanwhile, in the basal-like and HER2-enriched breast cancer subtypes TFEB and Beclin-1 seem to have a higher H-score than in the luminal molecular subtypes. As far as PITX2 methylation analysis is concerned, this was feasible only in 65% of the selected cases (of the case-control study). Furthermore, the methylation of PITX2 did not demonstrate any significant differences between the cases and the controls and did not indicate any correlation with the expression of the TFEB pathway analyzed by immunohistochemistry.
The ability to predict the response to anti-blastic therapies in breast carcinoma is of fundamental importance due to the significant prevalence of this disease in females of all ages. Furthermore, despite the effectiveness of screening with improved early diagnosis and the progressive advance of systemic therapies, no reduction has been observed in the prevalence of metastatic breast cancer at the time of diagnosis [1, 5, 50]. In fact, its prevalence does not depend for the most part on the average size of tumors, which today are frequently smaller than in the past, but depends above all on the intrinsic immunophenotypical characteristics of the malignancy. Specifically, tiny tumors with aggressive behavior are far more likely to develop distant metastases than large tumors with limited proliferation [51].
With regard to tumors with aggressive biological behavior, nowadays, chemotherapy remains the most effective tool to reduce the risk of distant metastases. Prognostic factors indicative of unfavorable outcomes, but also predictive of risk reduction in patients undergoing chemotherapy, are tumor size, loco-regional lymph node involvement, tumor histotype, hormone receptor expression, HER2/neu overexpression, Mib-1/Ki-67 proliferation index, tumor grade, perivascular or perineural invasion, and the young age of the patient [5, 32, 34, 52,53,54]. However, there is still no clarity about the factors which predict tumor chemoresistance. If these factors were available, we would be better equipped to create or design novel drugs to be added to chemotherapy regimens to improve their efficiency.
As for other malignancies, autophagy seems to have a fundamental function in breast cancer resistance to chemotherapy as well as hormonal therapy [7, 9]. In the current literature, it has already been demonstrated that TFEB, SIRT1, CARM1, Beclin-1, and PITX2 play a role of some sort in the autophagy process. However, data explicitly related to breast cancer is extremely limited, especially regarding TFEB. Regarding SIRT1, there are reports which describe a correlation between its increased expression and a poor prognosis in breast cancer [19, 20]. Our study likewise notes an association between SIRT1 expression levels above the first quartile and a diminished survival, and our data confirm a likely association of reduced expression of SIRT1 with the basal-like molecular subtype [55]. Since the correlation between SIRT1 and TFEB disappears when we look exclusively at the basal-like breast cancer subtype, our data actually suggests a different role of SIRT1 in the luminal molecular subtypes compared to that in the basal-like ones, although in both cases, a low expression of SIRT1 is predictive of a better prognosis.
Both TFEB and CARM1 have been seen to play an important role in the onset and regulation of autophagy [15,16,17]. However, at the moment, only one article demonstrates a correlation between an increased TFEB expression in early breast cancer and a poorer prognosis [56]. Our results confirm that an increased immunohistochemical TFEB expression correlates with a less favorable prognosis in women affected by breast cancer and treated with chemotherapy. Furthermore, our study population extends beyond early breast cancers to encompass many locally advanced tumors, which more commonly represent an indication for chemotherapy. Regarding CARM1, its increased expression corresponds with a less favorable prognosis in breast cancer [6, 57, 58] and with HER2-positive tumors [6, 57]. Our study similarly correlates a higher expression level of CARM1 with a poorer prognosis but did not observe differences concerning the expression of CARM1 among the different molecular subtypes. Again, this may be explained by a potential selection bias, as our population includes, on average, more advanced stages than previous studies.
Several studies have previously assessed the prognostic value of Beclin-1 in breast cancer, showing conflicting results. Dong and coworkers found cancers positively expressing Beclin-1 having a favorable prognosis [59]. However, they limited the analysis to ER-positive and HER2-negative cancers, and only a part of these cases was eligible to be treated with chemotherapy [59]. Won and coworkers found no correlations between Beclin-1 expression and survival [60], as well Kim and coworkers found no correlation between Beclin-1 expression and survival in triple-negative breast cancers [61]. However, the last two studies had an average follow-up below 65 months, and it is known that the majority of recurrences can occur within the first 72 months of follow-up, also considering low-risk breast cancer [53, 60, 61]. Furthermore, Liu and coworkers found an increased expression of Beclin-1 in the tamoxifen-resistant breast cancer cell line [22]. In a cohort of high-risk patients treated with chemotherapy, we found that high Beclin-1 immunohistochemical expression was associated with an unfavorable prognosis.
In this study, we also evaluate the correlations among the three considered proteins. In particular, a directly proportional and statistically significant correlation is found among SIRT1, CARM1, TFEB, and Beclin-1. Previous studies demonstrate that TFEB and CARM1 act as effectors of the autophagy process, and their significant correlation in our results confirms this [15]. Besides, SIRT1, CARM1, and TFEB positively correlated with Beclin-1, a known marker of the autophagy process [21]. Even by selecting only the luminal molecular subtypes, it emerges that the rho coefficient of the correlation between TFEB and CARM1 approaches significance (Supplemental Table 2). On the other hand, this correlation is lost in the case of the basal-like subtype, so that in the breast cancer luminal subtypes, we can deduce that both TFEB and CARM1 act in the same pathway, while this may not be true in the case of the basal-like molecular subtype [15]. The correlation between SIRT1 and CARM1 can be explained by the fact that CARM1, through the methylation of HuR, stabilizes the SIRT1 mRNA and hence promotes its production [62, 63]. Finally, while in the basal-like subtype, both TFEB and CARM1 maintain their prognostic value, the correlation of TFEB with SIRT1 and CARM1 expression loses statistical significance. A possible explanation is that TFEB and CRAM1 may act in two different ways, which at the moment cannot be completely discerned from the data present in the current study.
Considering the case-control study, the prognostic value of TFEB and its pathway in chemo-treated patients is confirmed and probably associated with its function in the autophagy mechanism. In fact, PITX2 also seems to play a role in the autophagy process. Indeed, PITX2 regulates DIRAS3 in lung cancer [64], and the re-expression of DIRAS3 promotes autophagy in breast cancer, thus enhancing the inhibitory effect of paclitaxel on breast tumor cells [65]. Although PITX2 and TFEB both have a crucial function in the autophagy process, their role seems to be different. Our data, in fact, show a significant difference regarding the TFEB pathway but no difference as regards the methylation of PITX2 between cases and controls. Furthermore, our data show no correlation between the methylation of PITX2 and the protein expression of the TFEB pathway, which would suggest the independence of these two prognostic markers.
Currently, adjuvant chemotherapeutic regimens are mostly based on the administration of anthracyclines and taxanes and, for this reason, our study focuses on the evaluation of PITX2, which is a molecule that can specifically influence anthracycline resistance [23,24,25,26,27, 66]. Considering that all patients of our case-control study were treated with polychemotherapy, including anthracyclines, it should be emphasized that avoiding the side effects of anthracyclines when there is evidence of their ineffectiveness could be a valuable step in improving the quality of care in this group of patients.
This study’s major limitations are its retrospective design and the limitations linked to perform an analysis on mRNA and DNA of formalin-fixed paraffin-embedded tissues that have been historically collected and stored for many years. At the same time, it is a crucial advantage to have an extended follow-up that allows a broader perspective on prognosis. Despite its limits, this study shows some significant data of great interest. In fact, an increased TFEB expression in terms of H-score appears to have a marked ability to identify patients with shorter durations of survival. In addition, it should be pointed out among the advantages of this study that the cases were treated uniformly by the same team according to the most up-to-date guidelines.
Conclusions
Our preliminary data demonstrate a potential prognostic value of TFEB, SIRT1, and Beclin-1, likely for their role within autophagy regulation in patients affected by breast cancer and treated with anti-blastic therapy. As chemotherapy resistance still represents one of the breast specialist’s major concerns, our encouraging data show that the potential exists to discover new treatments to overcome intrinsic or acquired drug resistance in breast carcinoma and, consequently, improve the prognosis of our patients.
Availability of data and materials
The data that support the findings of this study are available, but restrictions apply to the availability of these data, which was used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Internal Review Board.
Abbreviations
- BMI:
-
body mass index
- CARM1:
-
co-activator-associated arginine methyltransferase 1
- CI.95:
-
95% confidence interval
- CT:
-
cycle threshold
- ER:
-
estrogen receptor
- FFPE:
-
formalin-fixed, paraffin-embedded
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- IHC:
-
Immunohistochemical analysis
- IQR:
-
interquartile range
- MSP:
-
methylation specific PCR
- P2:
-
promotor 2
- PITX2:
-
Pituitary homeobox 2
- PMR:
-
percent methylation ratio
- PR:
-
progesteron receptor
- PVI:
-
peri-vascular invasion
- RT-PCR:
-
Real time PCR
- SIRT1:
-
sirtuin-1
- TFEB:
-
Transcription factor EB
- TMA:
-
Tissue Micro Array
References
Bertozzi S, Londero AP, Cedolini C, Uzzau A, Seriau L, Bernardi S, et al. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. Springerplus. 2015;4(1):688. https://doi.org/10.1186/s40064-015-1449-x.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210.
Bertozzi S, Londero AP, Bernardi S, Cedolini C. Applicability of the Notthingham prognostic index for predicting the survival of triple-negative invasive breast cancer in a single Italian center. Eur J Gynaecol Oncol. 2019;40:787–90.
Cedolini C, Bertozzi S, Londero AP, Yanova M, Seriau L, Bernardi S, et al. Clinicopathological characteristics and outcome of high grade breast cancer: our 9 years’ experience. Eur J Gynaecol Oncol. 2017;38:708–14.
Cedolini C, Bertozzi S, Londero AP, Bernardi S, Seriau L, Concina S, et al. Type of breast cancer diagnosis, screening, and survival. Clin Breast Cancer. 2014;14(4):235–40. https://doi.org/10.1016/j.clbc.2014.02.004.
Davis MB, Liu X, Wang S, Reeves J, Khramtsov A, Huo D, et al. Expression and sub-cellular localization of an epigenetic regulator, co-activator arginine methyltransferase 1 (CARM1), is associated with specific breast cancer subtypes and ethnicity. Mol Cancer. 2013;12(1):40. https://doi.org/10.1186/1476-4598-12-40.
Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Rev Anticancer Ther. 2011;11(8):1283–94. https://doi.org/10.1586/era.11.111.
Hurvitz SA, Hu Y, O’Brien N, Finn RS. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev. 2013;39(3):219–29. https://doi.org/10.1016/j.ctrv.2012.04.008.
Vicier C, Dieci MV, Arnedos M, Delaloge S, Viens P, Andre F. Clinical development of mTOR inhibitors in breast cancer. Breast Cancer Res. 2014;16(1):203. https://doi.org/10.1186/bcr3618.
Manna S, Holz MK. Tamoxifen action in ER-negative breast Cancer. Sign Transduct Insights. 2016;5:1–7. https://doi.org/10.4137/STI.S29901.
Kim YR, Park MS, Eum KH, Kim J, Lee JW, Bae T, et al. Transcriptome analysis indicates TFEB1 and YEATS4 as regulatory transcription factors for drug resistance of ovarian cancer. Oncotarget. 2015;6(31):31030–8. https://doi.org/10.18632/oncotarget.5208.
Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129(13):2475–81. https://doi.org/10.1242/jcs.146365.
Kauffman EC, Ricketts CJ, Rais-Bahrami S, Yang Y, Merino MJ, Bottaro DP, et al. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat Rev Urol. 2014;11(8):465–75. https://doi.org/10.1038/nrurol.2014.162.
Martina JA, Diab HI, Li H, Puertollano R. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci. 2014;71(13):2483–97. https://doi.org/10.1007/s00018-014-1565-8.
Shin HJR, Kim H, Oh S, Lee JG, Kee M, Ko HJ, et al. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534(7608):553–7. https://doi.org/10.1038/nature18014.
Lapierre LR, Kumsta C, Sandri M, Ballabio A, Hansen M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015;11(6):867–80. https://doi.org/10.1080/15548627.2015.1034410.
Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9(2):123–8. https://doi.org/10.1038/nrc2562.
Qiu G, Li X, Che X, Wei C, He S, Lu J, et al. SIRT1 is a regulator of autophagy: implications in gastric cancer progression and treatment. FEBS Lett. 2015;589(16):2034–42. https://doi.org/10.1016/j.febslet.2015.05.042.
Lee H, Kim KR, Noh SJ, Park HS, Kwon KS, Park BH, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42(2):204–13. https://doi.org/10.1016/j.humpath.2010.05.023.
Wu M, Wei W, Xiao X, Guo J, Xie X, Li L, et al. Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer. Med Oncol. 2012;29(5):3240–9. https://doi.org/10.1007/s12032-012-0260-6.
Mustafa MF, Saliluddin SM, Fakurazi S, Tizen Laim NMS, Md Pauzi SH, Nik Yahya NH, et al. Expression of autophagy and Mitophagy markers in breast Cancer tissues. Front Oncol. 2021;11:612009. https://doi.org/10.3389/fonc.2021.612009.
Liu J, Yue W, Chen H. The correlation between autophagy and tamoxifen resistance in breast cancer. Int J Clin Exp Pathol. 2019;12(6):2066–74.
Schmitt M, Wilhelm OG, Noske A, Schricker G, Napieralski R, Vetter M, et al. Clinical Validation of PITX2 DNA Methylation to Predict Outcome in High-Risk Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy. Breast Care (Basel). 2018;13:425–433.
Aubele M, Schmitt M, Napieralski R, Paepke S, Ettl J, Absmaier M, et al. The predictive value of PITX2 DNA methylation for high-risk breast Cancer therapy: current guidelines, medical needs, and challenges. Dis Markers. 2017;2017:4934608–14. https://doi.org/10.1155/2017/4934608.
Hartmann O, Spyratos F, Harbeck N, Dietrich D, Fassbender A, Schmitt M, et al. DNA methylation markers predict outcome in node-positive, estrogen receptor-positive breast cancer with adjuvant anthracycline-based chemotherapy. Clin Cancer Res. 2009;15(1):315–23. https://doi.org/10.1158/1078-0432.CCR-08-0166.
Nimmrich I, Sieuwerts AM, Meijer-van Gelder ME, Schwope I, Bolt-de Vries J, Harbeck N, et al. DNA hypermethylation of PITX2 is a marker of poor prognosis in untreated lymph node-negative hormone receptor-positive breast cancer patients. Breast Cancer Res Treat. 2008;111(3):429–37. https://doi.org/10.1007/s10549-007-9800-8.
Harbeck N, Nimmrich I, Hartmann A, Ross JS, Cufer T, Grützmann R, et al. Multicenter study using paraffin-embedded tumor tissue testing PITX2 DNA methylation as a marker for outcome prediction in tamoxifen-treated, node-negative breast cancer patients. J Clin Oncol. 2008;26(31):5036–42. https://doi.org/10.1200/JCO.2007.14.1697.
Langholz B. Case–Cohort Study. In Encyclopedia of Biostatistics. Chichester: John Wiley & Sons, Ltd; 2005.
Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomark Prev. 2010;19(2):492–502. https://doi.org/10.1158/1055-9965.EPI-09-0532.
Myckatyn T, Wagner I, Mehrara B, Crosby M, Park J, Qaqish B, et al. Cancer recurrence after fat transfer (CRAFT)- a multicenter case-cohort study. Plast Reconstr Surg. 2017;139(1):11–8. https://doi.org/10.1097/PRS.0000000000002838.
Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics. 2004;60(4):1015–24. https://doi.org/10.1111/j.0006-341X.2004.00257.x.
Arnone P, Zurrida S, Viale G, Dellapasqua S, Montagna E, Arnaboldi P, et al. The TNM classification of breast cancer: need for change. Updat Surg. 2010;62(2):75–81. https://doi.org/10.1007/s13304-010-0014-y.
Perry N, Puthaar E, European Commission Directorate-General Health & Consumer Protection. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities; 2006.
Cedolini C, Bertozzi S, Londero AP, Seriau L, Andretta M, Agakiza D, et al. Impact of the presence and quantity of ductal carcinoma in situ component on the outcome of invasive breast cancer. Int J Clin Exp Pathol. 2015;8(10):13304–13.
Lakhani SR, International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of the Breast. In: Number fourth in World Health Organization Classification of Tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2012.
Compton CC. AJCC Cancer staging atlas a companion to the seventh editions of the AJCC Cancer staging manual and handbook. New York, NY: Springer; 2012.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. the value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x.
Tavassoli FA, Eusebi V. Tumors of the Mammary Gland. In: Number fasc. 10 in AFIP Atlas of Tumor Pathology. 4rth Series ed. Washington, D.C: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2009.
Bernardi S, Bertozzi S, Londero AP, Angione V, Petri R, Giacomuzzi F. Prevalence and risk factors of intraoperative identification failure of sentinel lymph nodes in patients affected by breast cancer. Nucl Med Commun. 2013;34(7):664–73. https://doi.org/10.1097/MNM.0b013e328361cd84.
Cedolini C, Bertozzi S, Seriau L, Londero AP, Concina S, Cattin F, et al. Eight-year experience with the intraoperative frozen section examination of sentinel lymph node biopsy for breast cancer in a north-Italian university center. Int J Clin Exp Pathol. 2014;7(1):364–71.
Calcagno A, Grassi T, Mariuzzi L, Marzinotto S, Londero AP, Orsaria M, et al. Expression patterns of Aurora a and B kinases, Ki-67 and the estrogen and progesterone receptors determined using an endometriosis tissue microarray model. Hum Reprod. 2011;26(10):2731–41. https://doi.org/10.1093/humrep/der264.
Londero AP, Calcagno A, Grassi T, Marzinotto S, Orsaria M, Beltrami CA, et al. Survivin, MMP-2, MT1-MMP, and TIMP-2: their impact on survival, implantation, and proliferation of endometriotic tissues. Virchows Arch. 2012;461(5):589–99. https://doi.org/10.1007/s00428-012-1301-4.
Londero AP, Bernardi S, Bertozzi S, Angione V, Gentile G, Dri C, et al. Synchronous and metachronous breast malignancies: a cross-sectional retrospective study and review of the literature. Biomed Res Int. 2014;2014:250727–10. https://doi.org/10.1155/2014/250727.
Grassi T, Calcagno A, Marzinotto S, Londero AP, Orsaria M, Canciani GN, et al. Mismatch repair system in endometriotic tissue and eutopic endometrium of unaffected women. Int J Clin Exp Pathol. 2015;8(2):1867–77.
Orsaria M, Londero AP, Marzinotto S, Di Loreto C, Marchesoni D, Mariuzzi L. Placental type alkaline phosphatase tissue expression in ovarian serous carcinoma. Cancer Biomark. 2016;17(4):479–86. https://doi.org/10.3233/CBM-160665.
Londero AP, Orsaria M, Marzinotto S, Grassi T, Fruscalzo A, Calcagno A, et al. Placental aging and oxidation damage in a tissue micro-array model: an immunohistochemistry study. Histochem Cell Biol. 2016;146(2):191–204. https://doi.org/10.1007/s00418-016-1435-6.
Maier S, Nimmrich I, Koenig T, Eppenberger-Castori S, Bohlmann I, Paradiso A, et al. DNA-methylation of the homeodomain transcription factor PITX2 reliably predicts risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients–technical and clinical validation in a multi-Centre setting in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J Cancer. 2007;43(11):1679–86. https://doi.org/10.1016/j.ejca.2007.04.025.
Hernández HG, Tse MY, Pang SC, Arboleda H, Forero DA. Optimizing methodologies for PCR-based DNA methylation analysis. BioTechniques. 2013;55(4):181–97. https://doi.org/10.2144/000114087.
Perkins J, Napieralski R, Schricker G, Piednoir E, Manner O, Bona A, et al. therascreenPITX2 RGQ PCR assay for the assessment of PITX2 DNA-methylation status to investigate the role of the transcription factor PITX2 and the regulation of the Wnt/ß-catenin pathway in pathophysiological processes. Protoc Exch. 2018:1–38.
Driul L, Bernardi S, Bertozzi S, Schiavon M, Londero AP, Petri R. New surgical trends in breast cancer treatment: conservative interventions and oncoplastic breast surgery. Minerva Ginecol. 2013;65(3):289–96.
Bertozzi S, Londero AP, Petri R, Bernardi S. Isolated axillary nodal swelling and cancer of unknown primary. Eur J Gynaecol Oncol. 2015;36(2):131–7.
Bertozzi S, Cedolini C, Londero AP, Baita B, Giacomuzzi F, Capobianco D, et al. Sentinel lymph node biopsy in patients affected by breast ductal carcinoma in situ with and without microinvasion: Retrospective observational study. Medicine (Baltimore). 2019;98:e13831.
Bernardi S, Bertozzi S, Londero AP, Giacomuzzi F, Angione V, Dri C, et al. Nine years of experience with the sentinel lymph node biopsy in a single Italian center: a retrospective analysis of 1,050 cases. World J Surg. 2012;36(4):714–22. https://doi.org/10.1007/s00268-011-1420-0.
Bernardi S, Bertozzi S, Londero AP, Gentile G, Angione V, Petri R. Influence of surgical margins on the outcome of breast cancer patients: a retrospective analysis. World J Surg. 2014;38(9):2279–87. https://doi.org/10.1007/s00268-014-2596-x.
Rifaï K, Judes G, Idrissou M, Daures M, Bignon YJ, Penault-Llorca F, et al. Dual SIRT1 expression patterns strongly suggests its bivalent role in human breast cancer. Oncotarget. 2017;8(67):110922–30. https://doi.org/10.18632/oncotarget.23006.
Giatromanolaki A, Sivridis E, Kalamida D, Koukourakis MI. Transcription factor EB expression in early breast Cancer relates to lysosomal/Autophagosomal markers and prognosis. Clin Breast Cancer. 2017;17(3):e119–25. https://doi.org/10.1016/j.clbc.2016.11.006.
Cheng H, Qin Y, Fan H, Su P, Zhang X, Zhang H, et al. Overexpression of CARM1 in breast cancer is correlated with poorly characterized clinicopathologic parameters and molecular subtypes. Diagn Pathol. 2013;8(1):129. https://doi.org/10.1186/1746-1596-8-129.
Habashy HO, Rakha EA, Ellis IO, Powe DG. The oestrogen receptor coactivator CARM1 has an oncogenic effect and is associated with poor prognosis in breast cancer. Breast Cancer Res Treat. 2013;140(2):307–16. https://doi.org/10.1007/s10549-013-2614-y.
Dong M, Wan X, Yuan ZY, Wei L, Fan XJ, Wang T, et al. Low expression of Beclin 1 and elevated expression of HIF-1α refine distant metastasis risk and predict poor prognosis of ER-positive, HER2-negative breast cancer. Med Oncol. 2013;30:355.
Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41(1):107–12. https://doi.org/10.1016/j.humpath.2009.07.006.
Kim S, Jung WH, Koo JS. Differences in autophagy-related activity by molecular subtype in triple-negative breast cancer. Tumour Biol. 2012;33(5):1681–94. https://doi.org/10.1007/s13277-012-0424-1.
Fujiwara T, Mori Y, Chu DL, Koyama Y, Miyata S, Tanaka H, et al. CARM1 regulates proliferation of PC12 cells by methylating HuD. Mol Cell Biol. 2006;26(6):2273–85. https://doi.org/10.1128/MCB.26.6.2273-2285.2006.
Calvanese V, Lara E, Suárez-Alvarez B, Abu Dawud R, Vázquez-Chantada M, Martínez-Chantar ML, et al. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc Natl Acad Sci U S A. 2010;107(31):13736–41. https://doi.org/10.1073/pnas.1001399107.
Paylakhi SH, Fan JB, Mehrabian M, Sadeghizadeh M, Yazdani S, Katanforoush A, et al. Effect of PITX2 knockdown on transcriptome of primary human trabecular meshwork cell cultures. Mol Vis. 2011;17:1209–21.
Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan J, et al. Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer. 2011;11(1):22. https://doi.org/10.1186/1471-2407-11-22.
Absmaier M, Napieralski R, Schuster T, Aubele M, Walch A, Magdolen V, et al. PITX2 DNA-methylation predicts response to anthracycline-based adjuvant chemotherapy in triple-negative breast cancer patients. Int J Oncol. 2018;52(3):755–67. https://doi.org/10.3892/ijo.2018.4241.
Acknowledgements
We are grateful to Eilidh PJ Medley (McIntosh) for her suggestions on the style and composition of our English. The authors would like to thank the whole staff collaborating in article collection, selection, reading and in paper writing and reviewing. This work was conducted as part of the PhD thesis of Dr. Serena Bertozzi.
Consent to publish
Not applicable.
Funding
This study has had no financial support.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design or acquisition of data or to analysis and interpretation of data (SB, APL, LV, MO, MB, SM, BC, UB, CD, CC, LM). Drafting the article or revising it critically for important intellectual content (SB, APL, LV, MO, MB, SM, BC, UB, CD, CC, LM) All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the internal review board of the Department of Medical Area (University of Udine), it was conducted in accordance with the Helsinki Declaration and it followed the dictates of the general authorization to process personal data for scientific research purposes by the Italian Data Protection Authority. The need for an informed consent, in accordance with national legislation, was waived by the IRB listed above because this was a retrospective cohort study.
Competing interests
The authors declare that they have no potential conflicts of interest relevant to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Condensation: TFEB, SIRT1, and Beclin-1 seem to have a potential prognostic significance in patients with chemo-treated breast cancer, likely because of their role in the regulation of autophagy. Besides, no correlation between TFEB and PITX2 methylation was found, likely because they perform two different roles within the autophagy process.
Supplementary Information
Additional file 1
: Supplemental Fig. 1. The plot shows the correlation analysis between the immunohistochemical protein expression evaluated as the H-score of the four proteins analyzed. Correlations evaluated by the Spearman test.
Additional file 2
: Supplemental Table 1. RT-PCR primers of TFEB, CARM1, SIRT1, and GAPDH. Supplemental Table 2. Analysis of the correlation between the immunohistochemical protein expression evaluated in H-score of the four proteins analyzed. Correlations evaluated by the Spearman test. Supplemental Table 3. Analysis of the expression of the considered proteins among the various molecular subtypes where the data is present. The values reported are median and range of interquartiles (IQR) while p refers to the Wilcoxon test. Supplemental Table 4. Description of the population and tumor characteristics divided into the two groups (cases who recurred within 12 months from treatment initiation and controls who did not recur). Supplemental Table 5. Tumor staging divided into the two groups (cases who recurred within 12 months from treatment initiation and controls who did not recur). Supplemental Table 6. PITX2 (PMR) and immunoistochemical expression of TFEB, CARM1, SIRT1, and Beclin-1 divided into the two groups (cases who recurred within 12 months from treatment initiation and controls who did not recur).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bertozzi, S., Londero, A.P., Viola, L. et al. TFEB, SIRT1, CARM1, Beclin-1 expression and PITX2 methylation in breast cancer chemoresistance: a retrospective study. BMC Cancer 21, 1118 (2021). https://doi.org/10.1186/s12885-021-08844-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08844-y