Abstract
Background
Immune checkpoint inhibitors (ICI) are an integral part of bladder cancer therapy, however, the relevance of ICI treatment for mixed and pure squamous cell carcinoma of the bladder remains poorly studied. Therefore, we analysed the expression of programmed death-ligand 1 (PD-L1) in urothelial carcinomas with squamous differentiation (UC/SCC) and pure squamous cell carcinoma (SCC) of the bladder and studied a UC/SCC patient with ICI therapy.
Methods
Tissue microarrays of 45 UC/SCC and 63 SCC samples were immunohistochemically stained with four anti-PD-L1 antibodies (28–8, 22C3, SP142 and SP263). PD-L1 expression was determined for tumour cells (TP-Score), immune cells (IC-Score) and combined (CPS, combined positive score). In addition, we present clinical and histological data of an UC/SCC patient with nivolumab therapy.
Results
Overall, positive PD-L1 staining ranged between 4.8 and 61.9% for IC and 0 and 51.2% for TC depending on the used antibody. There were no significant differences between UC/SCC and SCC. According to current FDA guidelines for example for first line therapy of urothelial cancer with pembrolizumab (CPS ≥ 10), a subset of SCC patients up to 20% would be eligible. Finally, our UC/SCC index patient revealed excellent therapy response regarding his lung metastasis.
Conclusions
Our data reveal a PD-L1 expression in squamous differentiated carcinomas comparable with current data shown for urothelial tumours. In accordance with the encouraging clinical data of the index patient we suggest ICI treatment also for mixed and pure SCC of the urinary bladder.
Similar content being viewed by others
Background
The immune system plays an important role in disease protection and cell clearing by orchestrating T-cell mediated immune responses [1]. Several immune checkpoints ensure correct cell recognition. Under normal conditions programmed cell death-1 (PD-1)-receptor is expressed on the surface of activated T-cells and its ligand programmed cell death ligand-1 (PD-L1) on the surface of dendritic cells and macrophages. PD1/PD-L1 interaction induces the activation of Src homology region 2 domain-containing phosphatases modulating the T-cell antigen receptor (TCR) signalling and mediating immune tolerance to self-antigens [2, 3]. However, cancer cells can misuse these checkpoints by overexpressing PD-L1 in tumour cells protecting themselves from cytotoxic T-cell immune detection and elimination [4]. Recently, immunotherapy targeting the PD1/PD-L1 axis has emerged as promising field in anti-cancer therapy for various tumour entities (including non-small lung cancer, renal cell cancer, or head and neck squamous cell cancer) [5]. By blocking theses immune checkpoint proteins, cancer cells’ resistance to immune response can be overcome and effective T-cell response against cancer cells can be restored [4, 6]. Meanwhile ICI treatment is an integral part of first line (in platinum ineligible patients) and second line clinical management of patients with urothelial carcinoma: Five different checkpoint inhibitors, i.e. pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab, have been assessed in clinical trials of advanced bladder cancer during the last years [7] and can be used for second line treatment, but only pembrolizumab and atezolizumab are currently approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for first line therapy in urothelial cancer [8, 9]. In this setting treatment with ICIs depends on complementary PD-L1 assessment based on different PD-L1 antibodies and immunohistochemical assays creating a wealth of different scoring algorithms and evaluation criteria. Recent studies revealed substantial inter-assay heterogeneity of PD-L1 expression in different tumour entities including bladder cancer with also some degree of inter-observer diversity as well [10,11,12].
The impact of ICI treatment in patients with rare bladder tumours remains poorly studied. Histologically, bladder cancer comprises a heterogeneous group of tumours including those with squamous differentiation (SD-BLCA), i.e. urothelial cancers with squamous differentiation (UC/SCC) and pure squamous cell carcinoma (SCC). SD-BLCA is characterized by poor outcome and lack of effective (neo) adjuvant therapy [13,14,15]. Pure SCC can be classified into two subgroups, i.e. SCC associated with schistosomiasis whose incidence rate is increased in regions where schistosomiasis is endemic (e.g. in the Middle East), and non-Schistosomiasis associated SCC [16]. Recently PD-L1 expression was studied in Schistosomiasis-related SCC of the bladder highlighting an association between negative PD-L1 expression and clinico-pathological parameters like tumour stage and unfavourable patients’ outcome [17]. In 2018 Udager and colleagues analysed PD-L1 protein expression in 17 pure SCC samples of the urinary bladder demonstrating frequent PD-L1 positivity (65%) [18]. Reis et al. confirmed strong PD-L1 expression in immune and tumour cells in 16 urothelial cancers with squamous differentiation [19], however, a comprehensive study involving the most prominent diagnostic PD-L1 antibodies and corresponding scoring algorithms (immune cell (IC)-score, tumour proportion (TP)-score and combined positivity score (CPS)) in non-Schistosomiasis SCC is still missing. Therefore, we aim to give insights into the therapeutic implications of PD-L1 expression in non-Schistosomiasis associated SD-BLCA by assessing PD-L1 expression using four different PD-L1 antibodies (DAKO 28–8, DAKO 22C3, Ventana SP263, Ventana SP142) in both a retrospective cohort including 45 mixed UC/SCC / 63 pure SCC and in tissue samples derived from a SD-BLCA index patient who showed excellent response of pulmonary metastasis upon nivolumab treatment.
Methods
Patient samples and tissue microarray construction
Formalin-fixed paraffin-embedded (FFPE) samples of primary non-Schistosomiasis-related SCC and mixed UC/SCC (urothelial bladder cancer with substantial squamous components > 50% of tumour area) were collected from collaborating Institutes of Pathology in Germany and the German Study Group of Bladder Cancers (DFBK e.V.). Tissue microarrays (TMA) with a minimum of two cores from different tumour areas of FFPE samples (45 UC/SCC, 63 SCC) were constructed. For the index patient, whole tissue slides were used for analysis. The patient consented the use of his tissue samples stored at the biobank of the Comprehensive Cancer Centre Düsseldorf and the according clinical data (IRB approval: number 4601; April 16th 2014). The retrospective anonymous study was approved by the local ethics committee (RWTH EK 009/12).
Immunohistochemistry
FFPE slides were stained for protein expression of programmed death-ligand 1 (PD-L1) with four different antibodies [28–8 (Agilent/DAKO, California, USA), 22C3 (DAKO), SP263 (Ventana, Tucson, Arizona, USA), SP142 (Ventana)]. Automated pre-treatment was performed at pH 6 for 28–8 / 22C3, and pH 9 for SP142 / SP263. Primary monoclonal antibodies were incubated for 30 min at room temperature and visualized using the appropriate DAB-based detection kits and haematoxylin counterstains (Agilent/DAKO Envision system autostainer plus, Ventana Benchmark Ultra). For lab developed immunohistochemical tests negative controls were run by omitting the primary antibody for both pH conditions compared to positive controls (see Additional files 1 and 2). PD-L1 expression was determined for tumour cells (TP-Score), immune cells (IC-Score) and combined (CPS, combined positivity score) regardless of the staining intensity as follows: TPS/ Cologne Score: 0 = 0 < 1%, 1 = 1 - < 5%, 2 = 5 - < 10%, 3 = 10 - < 25%, 4 = 25 - < 50%, 5 = > 50% [11], IC/Immune cell Score: 0 = < 1%, 1 = 1- < 5%, 2 = 5- < 10%, 3 = > 10% [20], and the combined positivity score (CPS) given by summing the number of PD-L1–stained cells (tumour cells, lymphocytes, macrophages) and dividing the result by the total number of viable tumour cells, multiplied by 100 [21]. Cores with staining artefacts or damage were excluded. Scoring was performed by two independent investigators (RM and NTG). Inter-observer discrepancies regarding the percentage of positivity or scoring were discussed and a consensus was found.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA). Differences were considered statistically significant if the two-sided p-values were equal or below 5% (≤0.05). The non-parametric Mann-Whitney U-test was used in order to compare two groups. In case of more than two groups the non-parametric Dunn’s multiple comparison test was used. Correlation analysis was performed by calculating a non-parametric Spearman’s rank correlation coefficient.
Results
Staining results of four different PD-L1 antibodies in pure SCC and mixed UC/SCC
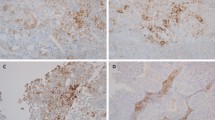
108 squamous differentiated bladder cancers comprising 45 mixed UC/SCC and 63 SCC (for cohort characteristics see Table 1) were immunohistochemically stained with four different anti-PD-L1 antibodies, i.e. the Dako 28–8 and 22C3 and the Ventana SP263 and SP142 (Fig. 1a). PD-L1 antibodies showed variable staining results for both immune (IC) and tumour cells (TPS) in UC/SCC and SCC (Fig. 1b and c). In mixed UC/SCC positive staining was determined for immune cells (IC-score ≥ 1) in 48.8% (28–8; 21/43), 20.5% (22C3; 9/44), 58.1% (SP263; 25/43) and 11.1% (SP142; 5/45) (Fig. 1b). Tumour cells showed PD-L1 expression (TPS ≥1) in 39.5% (28–8; 17/43), 11.3% (22C3; 5/44), 51.2% (SP263; 22/43) and 0% (SP142, 0/45) (Fig. 1c). In pure SCC we observed IC-scores ≥1 in 39.7% (28–8; 25/63), 31.1% (22C3; 19/61), 61.9% (SP263; 39/63) and 4.8% (SP142; 3/63) (Fig. 1b). TPS ≥1 was found in 28.6% (28–8; 18/63), 16.4% (22C3; 10/61), 47.6% (SP263; 30/63) and 0% (SP142, 0/63) (Fig. 1c). Non-parametric Spearman-rank correlation significantly demonstrated a high similarity in PD-L1 staining of SP263 and 28–8 antibodies for IC (r: 0.734, p < 0.001) and TPS (r: 0.773, p < 0.001) (Fig. 1d-e). Including 22C3 assay, inter-assay correlation (p < 0.001) ranged between 0.532 (SP263) and 0.617 (28–8) for IC and 0.409 (SP263) and 0.527 (28–8) for TPS. The SP142 assay showed weakest overlap, i.e. the Spearman correlation coefficient ranged between 0.332 and 0.509 for IC, while for TPS no correlation was accessible (no tumour cell staining). Furthermore, evidence for different PD-L1 expression between mixed UC/SCC and pure SCC was not observed. For detailed scoring results see Table 2.
PD-L1 protein expression in squamous differentiated bladder cancer (SD-BLCA). a Immunohistochemical PD-L1 staining is shown for representative tissue cores illustrating both immune cells (IC) and tumour cells (TC) by applying four different antibodies: DAKO 28–8, DAKO 22C3, Ventana SP263 and Ventana SP142. Squamous tumour components are histologically shown by H&E staining. CD68 staining highlights macrophages. Black scale bar: 100 μM. Please note: Due to tissue loss during the first immunohistochemical staining with the 22C3 antibody deeper tissue sections of the patient’s FFPE material were used which show slight differences in histology. b-c Scatter plot graphs show overall distribution of PD-L1 positive areas of IC and TC for mixed (UC/SCC) and pure squamous cancers (SCC). d-e Spearman correlation analysis demonstrating inter-assay heterogeneity for IC (d) and TC (e)
Therapeutic implications of staining results
According to the current FDA-approved guidelines for first line therapy of bladder cancer with pembrolizumab (CPS ≥10) and atezolizumab (IC-score ≥ 2 / IC ≥ 5%), we determined patients with putative choice of first line ICI therapy, overall ranging between 2 and 20% in SD-BLCA (Fig. 2). For pembrolizumab, a 22C3 CPS cut-off ≥10 indicates putative therapy access in 7% of patients with mixed UC/SCC and in 20% of SCC patients. SP142 completely failed to hold clinical significance. By focusing on the European Medicines Agency (EMA) guidelines according to which strict/mandatory PD-L1 companion diagnostics assay settings are not required by now, up to 47% of UC/SCC and up to 32% of SCC patients would be eligible for first line PD-L1 checkpoint inhibitors (Table 3).
Therapeutic implications of used PD-L1 antibodies in SD-BLCA according to FDA-approved guidelines for first line therapy in bladder cancer. Scatter plots represent CPS and IC for DAKO 22C3 and Ventana SP142, respectively. Red dotted line: drug-related cut-off values. Below: Percentages of patients with putative choice of first line therapy with pembrolizumab (a) and atezolizumab (b)
Clinical example for immune checkpoint inhibitor treatment in a SD-BLCA patient
A 62-year old male patient was first diagnosed with a high grade (G3) pT1 urothelial carcinoma of the urinary bladder in 2009 and his medical history is shown in Fig. 3a. Subsequently post resection and surveillance biopsy showed no evidence of malignancy but keratinizing squamous metaplasia of the urothelium. He received mitomycin-instillation and following BCG maintenance therapy for 47 months. In 2015 a TUR-B sample displayed moderate to severe squamous epithelial dysplasia, but there was no evidence for invasive carcinoma. 15 months later a subsequent invasive urothelial carcinoma (high grade (G3), min. pT2a, L1, V1) with substantial squamous differentiation without radiological evidence of metastasis was diagnosed. He received 4 cycles gemcitabine/cisplatin chemotherapy. Treatment was switched to second line palliative checkpoint inhibitor therapy with nivolumab due to progressive pulmonary metastasis. CT-staging monitoring is shown before, during and after immune checkpoint inhibitor treatment providing evidence of a partial response, i.e. long-lasting near-complete response of the pulmonary metastasis (Fig. 3b) and initial response (over the first 3 months upon nivolumab treatment) of the local tumour, but thereafter progressive disease (data not shown). Histological documentation and subsequent immunohistochemical PD-L1 staining of tissue samples at two different time points (before and after nivolumab therapy) confirmed squamous differentiation with a proportion of 80 and 30% of the primary tumour lesion, respectively. Biopsies from the pulmonary metastatic site had not been taken. PD-L1 expression was demonstrated for immune cells while it was barely detectable in tumour cells (Fig. 3c). In fact, PD-L1 expression was shown for 28–8 in 30%, for 22C3 in 30% for SP263 in 25% and for SP142 in 7% of IC before nivolumab treatment. In parallel, 1% of tumour cells were stained positively by applying DAKO 28–8 and 22C3 while Ventana SP263 led to 7% PD-L1 staining in tumour cells. Tumour cells were negative using SP142 (Table 4). Interestingly, at the point where ICI therapy has been completed, immune cells showed reduced PD-L1 expression varying between 7 and 10%. PD-L1 staining was not observed in tumour cells (Table 4). Significant differences in PD-L1 expression between urothelial and squamous differentiated tumour areas were not observed.
SD-BLCA index patient treated with nivolumab in a second line therapy. aUpper: History time line of index patient illustrating the diagnostic and therapeutic management over 140 months since first diagnosis. Below: CT images of the index patient show pulmonary metastasis size (white arrow) at different therapy time points. b Immunohistochemical PD-L1 staining of primary tumour lesions of the index patients derived from tissues removed before and after nivolumab treatment is shown. Squamous components are histologically shown by H&E staining and highlighted by K5/6 staining. PD-L1 expression was determined in both tumour cells (TC) and immune cells (IC) by using four different antibodies: DAKO 28–8, DAKO 22C3, Ventana SP263 and Ventana SP142. Black scale bar: 100 μM.HG: high grade; pT: pathological tumour stage; L: invasion into lymphatic vessels; V: invasion into vein; R: the completeness of the operation; pN: pathological degree of spread to regional lymph nodes; cM: clinical metastasis; CR: complete response, PD: progressive disease; Cx: cystectomy; TUR-B: transurethral resection of the bladder; m: months
Discussion
So far, the clinical management of patients with squamous differentiated bladder cancer is limited by the choice of effective (neo) adjuvant therapies [13,14,15]. The five year survival rate is worse varying between 16 and 48% [22, 23]. A ray of hope might be immunotherapy by PD1/PD-L1 checkpoint inhibitors which recently revolutionized the therapeutic landscape of various cancers including urothelial cancer [24]. In urothelial cancers efficacy of different ICIs have been assessed in clinical trials during the last years [7]. For instance, the Keynote-045 trial demonstrated a clinical benefit of pembrolizumab over chemotherapy for efficacy and safety upon treatment of locally advanced/metastatic, platinum-refractory urothelial tumours [25]. Meanwhile two ICI agents, i.e. pembrolizumab and atezolizumab, have been approved by the FDA and EMA for first-line therapy of platinum-ineligible patients with PD-L1 expression as specified by scoring algorithms [8, 9]. Accumulating studies also indicate strong PD-L1 expression in squamous tumors of the urinary bladder [17,18,19], but underlying retrospective cohorts are less suitable to assess the additive value of ICI therapies in SCC disease management: The patient cohort analyzed by Owyong and colleagues comprised mainly Schistosomiasis-associated SCC [17], while the publications by both Reis et al. and Udager et al. lack sufficient SCC sample numbers [18, 19]. Moreover, all studies were characterized by the absence of a clinical setting.
In the presented study, we now provide evidence for suitable ICI treatment of squamous bladder cancer by analysing both PD-L1 staining of a larger retrospective cohort of 108 SD-BLCA samples and of a SD-BLCA index patient whose pulmonary metastasis showed complete response upon nivolumab treatment. In concordance with the current FDA/EMA guidelines of urothelial cancers calling for PD-L1 positivity to protect from side effects [8, 9, 26], we revealed frequent PD-L1 expression in squamous bladder tumours up to 62% for immune and up to 52% for tumour cells. These findings, based on four different antibodies (DAKO 28–8, DAKO 22C3, Ventana SP263 and Ventana SP142), confirmed the data of the recent publications [17,18,19] and are comparable with studies of urothelial cancer [10]. So far, ICI treatment is an integral part of the therapy of squamous cancers in other organs like the lung and head and neck: The Checkmate-017 study revealed an improved overall survival (OS) and a favourable safety profile for nivolumab compared to docetaxel in patients with pre-treated squamous NSCLC [27]. The Keynote-407 study showed clinical significance of combined pembrolizumab treatment with chemotherapy in patients with metastatic squamous NSCLC [28]. In HNSCC the Keynote-012 study demonstrated clinically significant activity in patients with pre-treated tumors for pembrolizumab irrespective of human papillomavirus (HPV) status [29].
However, different PD-L1 antibodies, associated immunohistochemical assays and scoring algorithms, still challenge a robust selection of patients who will benefit from ICI treatment. In line with previous studies in urothelial cancer [10, 12], we also confirmed a substantial inter-assay heterogeneity of PD-L1 expression in squamous bladder cancer. Scheel et al. reported, that the four PD-L1 assays do not show comparable staining patterns in NSCLC [11]. The Blueprint PD-L1 Immunohistochemistry (IHC) Assay Comparison Project also studied the performance of the four PD-L1 IHC assays (22C3, 28–8, SP142, and SP263) in NSCLC, and found -very similar to our results- an analytical comparability of 22C3, 28–8, and SP263 whereas the SP142 assay showed lowest levels of correlation [30]. Hirsch and colleagues concluded that despite similar analytical performance of PD-L1 expression, interchanging assays and cut-offs would lead to “misclassification” of PD-L1 status for a substantial amount of patients. As a consequence, different patient numbers would be eligible for first line therapy with PD-L1 checkpoint inhibitors but still without clear evidence which staining results and cut-off levels really predict therapy response. For urothelial bladder cancer, the clinical consequences with substantial amounts of discordant classifications due to inter-assay and especially inter-algorithm variability, i.e. nearly 50% discordances between eligibility for first line treatment with atezolizumab or pembrolizumab, have been reported previously [10]. In clinical trials, for instance, the objective response rate (ORR) of urothelial cancer patients with nivolumab treatment did not significantly differ between PD-L1 positive (> 1%) and PD-L1 negative tumors (< 1%) (Checkmate-032 study) [31]. Beyond that the reliability of PD-L1 assays to predict ICI response is reduced by various aspects such as non-immunity dependent upregulation of PD-L1 expression (e.g. via PTEN) [32] or intratumoral heterogeneity and dynamic alteration by treatment and cancer progression [24]. In turn, higher ORR have been shown to be associated with increased PD-L1 expression also in urothelial cancer [33]. In this Keynote phase 2 study the subgroup of bladder cancer patients with PD-L1 expression above a cut-off ≥10% showed highest ORR upon pembrolizumab treatment. PD-L1 expression, as revealed in our cohort of squamous bladder cancers, may thereof be suitable to facilitate patient selection for ICI therapies.
This notion is supported by the here presented index patient with a squamous bladder cancer demonstrating partial therapy success upon ICI treatment. Prior to nivolumab treatment, the primary tumour exhibited a substantial percentage of squamous differentiation (80%) with strong PD-L1 expression. Upon ICI treatment the primary tumour showed clinically only short response but thereafter local progress was observed. Interestingly, after nivolumab therapy completion the progressive tumour lesion (< 50% squamous component) was characterized by reduced PD-L1 positivity. Of clinical significance, the pulmonary metastasis showed long-lasting response without any evidence of harmful side effects.
Conclusion
Our data reveal strong PD-L1 expression in squamous differentiated bladder cancers comparable with urothelial cancer whose disease management has been successfully improved by ICI therapy. Considering the encouraging clinical data of our index patient we propose to consider treatment of ICI also for both mixed and pure SCC of the urinary bladder. However, according to the tumour and inter-assay heterogeneity of PD-L1 expression, the utility of given scoring algorithms for robust patients’ therapy selection remains questionable and should be considered in future study designs.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CPS:
-
Combined positive score
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- FFPE:
-
Formalin-fixed paraffin-embedded
- IC-score:
-
Immune cell score
- ICI:
-
Immune checkpoint inhibitors
- ORR:
-
Objective response rate
- PD-L1:
-
Programmed death-ligand 1
- SD-BLCA:
-
Squamous differentiated bladder cancer
- SCC:
-
Squamous cell carcinoma
- TPS:
-
Tumour proportion score
- TMA:
-
Tissue microarray
- UC/SCC:
-
Urothelial carcinoma with squamous differentiation
References
Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. https://doi.org/10.1146/annurev.immunol.25.022106.141623..
Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. https://doi.org/10.1097/COC.0000000000000239.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. https://doi.org/10.1038/nrc3239.
Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. https://doi.org/10.1186/s40425-018-0316-z.
Lee HT, Lee JY, Lim H, et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7:5532. https://doi.org/10.1038/s41598-017-06002-8.
Stenehjem DD, Tran D, Nkrumah MA, et al. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. 2018;11:5973–89. https://doi.org/10.2147/OTT.S135157.
[Internet] FDA US FDA, Keytruda (pembrolizumab) or Tecentriq (atezolizumab): FDA Alerts Health Care Professionals and Investigators. FDA statement- decreased survival in some patients in clinial trials associated with monotherapy, 2018. https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-health-care-professionals-and-oncology-clinical-investigators-about-efficacy-issue. Accessed 16 Aug 2018.
[Internet] EMA EMA restricts use of Keytruda and Tecentriq in bladder cancer: Data show lower survival in some patients with low levels of cancer protein PD-L1, 2018. https://www.ema.europa.eu/en/documents/press-release/ema-restricts-use-keytruda-tecentriq-bladder-cancer_en.pdf. Accessed 1 June 2018.
Eckstein M, Erben P, Kriegmair MC, et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur J Cancer. 2019;106:234–43. https://doi.org/10.1016/j.ejca.2018.11.007.
Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016;29:1165–72. https://doi.org/10.1038/modpathol.2016.117.
Schwamborn K, Ammann JU, Knüchel R, et al. Multicentric analytical comparability study of programmed death-ligand 1 expression on tumor-infiltrating immune cells and tumor cells in urothelial bladder cancer using four clinically developed immunohistochemistry assays. Virchows Arch. 2019; [Epub ahead of print]. https://doi.org/10.1007/s00428-019-02610-z.
Minato A, Noguchi H, Tomisaki I, et al. Clinical significance of squamous differentiation in Urothelial carcinoma of the bladder. Cancer Control. 2018;25:1073274818800269. https://doi.org/10.1177/1073274818800269.
Dotson A, May A, Davaro F, et al. Squamous cell carcinoma of the bladder: poor response to neoadjuvant chemotherapy. Int J Clin Oncol. 2019;24:706–11. https://doi.org/10.1007/s10147-019-01409-x.
Lin X, Deng T, Wu S, et al. The clinicopathological characteristics and prognostic value of squamous differentiation in patients with bladder urothelial carcinoma: a meta-analysis. World J Urol. 2019; [Epub ahead of print]. https://doi.org/10.1007/s00345-019-02771-1.
Abol-Enein H, Kava BR, Carmack AJ. Nonurothelial cancer of the bladder. Urology. 2007;69:93–104.
Owyong M, Lotan Y, Kapur P, et al. Expression and prognostic utility of PD-L1 in patients with squamous cell carcinoma of the bladder. Urol Oncol. 2019;37:478–84. https://doi.org/10.1016/j.urolonc.2019.02.017.
Udager AM, McDaniel AS, Hovelson DH, et al. Frequent PD-L1 protein expression and molecular correlates in urinary bladder squamous cell carcinoma. Eur Urol. 2018;74:529–31. https://doi.org/10.1016/j.eururo.2018.06.019.
Reis H, Serrette R, Posada J, et al. PD-L1 expression in Urothelial carcinoma with predominant or pure variant histology: concordance among 3 commonly used and commercially available antibodies. Am J Surg Pathol. 2019;43:920–7. https://doi.org/10.1097/PAS.0000000000001264.
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. https://doi.org/10.1016/S0140-6736(16)00587-0.
Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of Pembrolizumab for treatment of gastric Cancer. Arch Pathol Lab Med. 2019;143:330–7. https://doi.org/10.5858/arpa.2018-0043-OA.
Serretta V, Pomara G, Piazza F, et al. Pure squamous cell carcinoma of the bladder in western countries. Report on 19 consecutive cases. Eur Urol. 2000;37:85–9.
Riadh BS, El Atat R, Sfaxi M, et al. Clinical presentation and outcome of bladder schistosoma-unrelated squamous cell carcinoma: report on 33 consecutive cases. Clin Genitourin Cancer. 2007;5:409–12.
Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. https://doi.org/10.1186/s12943-018-0864-3.
Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of Pembrolizumab versus chemotherapy for previously treated advanced Urothelial Cancer. J Clin Oncol. 2018;36:1579–87. https://doi.org/10.1200/JCO.2017.76.9562.
Gourd E. EMA restricts use of anti-PD-1 drugs for bladder cancer. Lancet Oncol. 2018;19:e341. https://doi.org/10.1016/S1470-2045(18)30433-9.
Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus Docetaxel in previously treated patients with advanced non-small-cell lung Cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924–33. https://doi.org/10.1200/JCO.2017.74.3062.
Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung Cancer. N Engl J Med. 2018;379:2040–51. https://doi.org/10.1056/NEJMoa1810865.
Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of Pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–45. https://doi.org/10.1200/JCO.2016.68.1478.
Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung Cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–22. https://doi.org/10.1016/j.jtho.2016.11.2228.
Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–8. https://doi.org/10.1016/S1470-2045(16)30496-X.
Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. 2015;51:221–8. https://doi.org/10.1016/j.oraloncology.2014.11.014.
Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–92. https://doi.org/10.1016/S1470-2045(17)30616-2.
Acknowledgments
The authors thank all members of the DFBK e.V. for contributing samples to our squamous bladder cancer cohort. We are grateful to the (immune) histochemistry labs at the Institute of Pathology at RWTH Aachen University and the Institute of Pathology at the University of Erlangen.
Funding
Not applicable.
Author information
Authors and Affiliations
Consortia
Contributions
NTG, MR, GN designed and supervised the study. TB, RK, TAV, ME, VW, TE, GN, IE, MA and NTG provided material, collected samples and data. MAC prepared the samples and carried out experiments. RM, NTG, VW and ME analysed the slides. RM, AM and NTG collected and organized data. RM and MR analysed the data. RM, MR and NTG interpreted the data and prepared the figures. RM wrote the manuscript. MR, GN and NTG corrected and edited the manuscript. RK, TAV, ME and VW revised the study and the manuscript, and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As noted in the manuscript, all analyses involving human patient samples and clinical data were performed in accordance with the ethical standards and the local Institutional Review Board (IRB)-approved protocols of the Medical Faculty of RWTH Aachen University (RWTH EK 009/12) and of the Heinrich Heine University Düsseldorf (IRB approval: April 16 2014). The index patient provided written informed consent for the use of both patient material as well as medical data acquired by examination at the university hospital Düsseldorf starting in 2009 and gave verbal consent for publication in accordance with the local ethics committee of the Heinrich Heine University Düsseldorf. Finally the patient passed away before a written consent for publication could be realized.
Consent for publication
Not applicable.
Competing interests
ME: Financial interest and/or other relationship with Astra Zeneca, Janssen, Roche Pharma, MSD, Genomic Health, GN: Financial interest and/or other relationship with BMS, Roche Pharma, MSD; NTG: Financial interest and/or other relationship with Astra Zeneca. All other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Lab developed immunohistochemistry: HE staining and negative controls are shown for pH 6 as well as pH 9 by omitting the primary antibody.
Additional file 2: Figure S2.
Tonsil tissue used as positive control. HE staining and PDL1 immunohistochemistry using different antibody clones: DAKO 28–8, DAKO 22C3, Ventana SP263 and Ventana SP142.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morsch, R., Rose, M., Maurer, A. et al. Therapeutic implications of PD-L1 expression in bladder cancer with squamous differentiation. BMC Cancer 20, 230 (2020). https://doi.org/10.1186/s12885-020-06727-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-06727-2