Abstract
Background
Patients with primary brain tumors are reported to have an elevated level of distress prevalence, due to the functional sequelae and the unfavorable prognosis, but the estimated prevalence of this disorder varies among studies. The Distress Thermometer (DT) is widely used distress screening tools to identify patients suffering from elevated psychosocial distress. The objective of this meta-analysis is to get a summarized estimate of distress prevalence in adult primary brain tumor patients screened by the DT instrument to identify distress in brain tumor patients.
Method
We searched studies published in PubMed, PsycINFO, and Cochrane library through August 2017 and checked related reviews and meta-analyses for eligible studies. Studies were eligible if they were published in the peer-reviewed literature and evaluated distress level by Distress Thermometer. The prevalence of distress symptoms in patients with the intracranial tumor was estimated by study-level characteristics using stratified meta-analysis. The prevalence of distress level or symptoms during the follow-up examination at different time points was detected by secondary analysis of the longitudinal studies included.
Results
Twelve studies including a total of 2145 brain tumor patients were included in this analysis. Eight used a cross-sectional design and four were longitudinal. The pooled prevalence of distress was 38.2% (95% confidence interval (CI) 28.7%–47.7%) for the overall sample. The pooled prevalence of distress DT ≥4 was 41.1% (642/1686, 95% CI 28.6%–53.5%) and the pooled prevalence of distress by DT ≥6 was 29.7% (137/459, 95% CI 19.5%–39.9%). The distress symptom did not decrease in follow-up studies (Relative Increase Ratio:1.02, 95% CI, (0.78, 1.35)). A huge heterogeneity in different studies was detected, and different screening scales were not compared.
Conclusion
The high prevalence of distress becomes an enormous challenge for primary brain tumor patients. Routine screening and evaluation of distress in brain tumor patients may assist medical workers to develop proper interventions, which may lead to better quality of life and oncology management.
Similar content being viewed by others
Background
Distress is the emotional or mental discomfort under the circumstance of stressful life events [1,2,3]. Patients with distress suffer from a constellation of emotional and physical problems such as depression, insomnia, fatigue, pain, constipation and loss of concentration [2]. Brain tumor patients are reported to have an elevated level of distress prevalence, due to the severe functional sequelae and the unfavorable prognosis [4,5,6]. The high emotional distress experience results in significant emotional burden and greatly affected how patients cope with their diseases and their ability to follow treatment recommendations [7, 8]. These complications reduce health-related quality of life (HRQoL) and have a significant negative impact on prognosis as well as survival in brain tumor patients [5, 7]. A valid and practicable screening instrument for the diagnosis of distress in patients with intracranial tumor should be developed and studied. Different screening standards have been developed for the psychosocial diagnosis and support of cancer patients.
The National Comprehensive Cancer Network distress thermometer (NCCN-DT), a validated distress screening tool, has been widely used for the evaluation of psychiatric distress in cancer patients [2, 7], to improve the identification, management, and treatment of psychological distress [7]. The DT instrument uses a 0–10 scale to assess distress level from no distress to extreme distress [7]. A problem list is also included for patients to find the possible problems and concerns [5, 7]. Cancer patients are encouraged to use DT as part of their routine appointment preparation which makes them easier to talk to their doctors about the emotional effects caused by the diagnosis, symptoms, and treatment of cancer [9]. The Distress Thermometer has been employed in many studies and found to work well. Usually, patients scoring ≥4 are considered to have moderate distress symptoms which need intervention [2, 3, 9]. Also, some researchers recommended applying DT ≥ 6 for screening extreme distress in brain tumor patients [10, 11].
However, the estimated prevalence of this disorder varies among studies in primary neuro-oncological patients [5, 9, 11,12,13]. Different research design, sample size, research years and patients samples with different education level, marriage state, tumor grade and position contribute to the heterogeneity [5, 13, 14]. The purpose of the study is to obtain a reliable pooled distress prevalence in brain tumor patients measured by the DT and discuss the proper identification and treatment of this comorbidity of primary brain tumor.
Methods
Search strategy and inclusion criteria
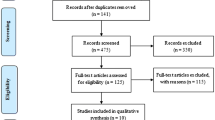
A literature search was performed using PubMed, PsycINFO, and Cochrane library with the following key words: “brain tumor” or “primary brain tumor” or “brain neoplasm” or “meningioma” or “glioblastoma” or “GBM” or “astrocytoma” or “oligodendroglioma” or “oligoastrocytoma” or “high-grade glioma” or “high-grade glioma” or “primary malignant brain tumor” or “intracranial tumors” or “neuro-oncological patients” and “distress” or “distress thermometer” or “psychiatric distress” or “distress symptom” or “emotional distress” or “mental distress”. We also searched reviews and meta-analyses to identify studies that may be missed in the former literature searches. Furthermore, all reference lists of the retrieved articles were obtained and reviewed in full text to search for additional eligible studies. Study authors were contacted to identify additional information if needed. PRISMA guidelines were used for this meta-analysis [15] (Fig. 1).
All studies met the following criteria were eligible for inclusion 1) used an observational or a randomized controlled trial before August 20, 2017; 2) provided distress prevalence in primary brain tumor patients with complication of distress ≥18 years old to ensure they can complete the questionnaire by themselves; 3) evaluated distress level by the National Comprehensive Cancer Network distress thermometer (NCCN-DT); 4) were published in peer-reviewed journals in English language; 5) For longitudinal studies, baseline pre-treatment data were included for the estimate of pooled prevalence of distress symptoms, and data at baseline and after 3 months were analyzed to study prevalence change over time.
Studies were excluded if: data from abstracts without full reports; studies included ≤30 patients; non–English-language studies; case reports. Studies were also excluded if it included tumors with cell origins that differed from that of the brain.
Data extraction and quality assessment of included studies
Two investigators (FL and JH) independently extracted the following information from all eligible studies: study design, year, country or area, patients involved, tumor grade, education levels, DT cut-off, and prevalence. Table 1 summarized the included studies with the demographic and clinical characteristics. Publications potentially reporting data about distress were selected for full-text review and checked for eligibility. Any discrepancies were resolved by consensus, referring back to the original article. Three studies detected distress prevalence in the follow-up period were included and analyzed in our meta-analysis.
Statistical analysis
The statistical heterogeneity among studies was tested by Cochran’s Q statistic, P < 0.10 was considered of significance [16]. The quantity I2 that describes the percentage variation across studies that are attributed to heterogeneity was also assessed. An I2 ≥ 75% indicated significant heterogeneity. We used a random-effects model to calculate all point estimates of analyses and their 95% confidence interval (95% CI) (Fig. 2). Publication bias was evaluated using funnel plots and the Egger test. P < 0.10 was considered to represent statistically significant publication bias. The analysis was performed using Strata software (version 12.1; Stata Corp, College Station, TX). Forest plots were constructed as well. We also used stratified meta-analysis to compare results from different studies separately based on their characteristics (study design, country, sample size, year of the baseline survey, and cutoff score).
Results
The overview of our search process was illustrated in Fig. 1. The initial search strategy identified 426 potentially articles: 354 from PubMed, 57 from Cochrane library, and 15 from PsycINFO. After screening the titles and abstracts according to the selection criteria, we excluded 370 studies. We also identified additional studies by reference scanning and previous meta-analysis or reviews. Overall, 12 eligible studies met the predetermined criteria for inclusion, including eight cross-sectional [4, 5, 9, 10, 13, 17,18,19] and four longitudinal studies [11, 12, 20, 21].
Main associations of distress with brain tumor
These studies provided a total sample of 2145 brain tumor patients (median sample size = 179 patients, range = 50–798 patients). Four studies were conducted in the United States [4, 5, 9, 17], eight in other countries [10,11,12,13, 18,19,20,21]. These twelve studies were published between 2006 and 2015. Table 1 summarized the study characteristics and corresponding estimated prevalence with 95% CIs.
The pooled prevalence of distress was 38.2% (95% CI 28.7%–47.7%) in the overall sample with random-effects meta-analysis, ranging from 12.3% to 73.6% (Fig. 2). Significant evidence of between-study heterogeneity was observed between studies in the meta-analysis (I2 = 95.5%, P < 0.01). Studies with cut-off scores of ≥4 showed substantial distress 41.1% (642/1686, 95% CI 28.6%–53.5%) and studies with DT cut off score ≥ 6 showed substantial distress 29.7% (137/459, 95% CI 19.5%–39.9%).
The prevalence of distress symptoms by study-level characteristics using stratified meta-analysis was showed in Additional files 1 and 2. To examine consistency across different study designs with potential biases, we stratified data into subgroups on the basis of study design. There was significant difference between cross-sectional vs longitudinal studies (618/1604, 42.1% [95% CI, 29.9% to 54.2%] vs 161/541, 30.5% [95% CI, 15.9% to 45.0%]). A slightly lower prevalence of distress was detected in patients from USA than other countries (383/1161, 35.5% [95% CI, 21.4% to 49.6%] vs 396/984, 40.0% [95% CI, 26.3% to 53.8%]), p < 0.01. Significant differences in prevalence estimates were also noted when studies were stratified by year ≥2010 vs year < 2010 (681/1937, 36.1% [95% CI, 25.1%–47.1%] vs 98/208, 45.0% [95% CI, 29.2%–70.8%]). We then detected the prevalence difference between large sample size (sample ≥ 100) vs small sample size (sample < 100) (645/1841, 35.9% [95% CI, 23.9% to 48.0%] vs 134/304, 43.1% [95% CI, 31.5%–54.1%]). (Additional files 1 and 2). No further analysis was performed for comparison of different position or type of brain tumor. Among all the subgroups we detected, heterogeneity was in part explained by survey country (P < 0.01), sample size (P < 0.01), distress scale (P < 0.01) and study design (P < 0.01).
There were 3 longitudinal studies provided results on the prevalence of distress during further analysis [12, 20, 21]. The prevalence of distress level did not decrease over time (Relative Increase Ratio:1.02, [95% CI, (0.78, 1.35)]) (Table 2).
Publication bias
Publication bias was investigated by funnel plot (Fig. 3) and Egger test. There was no evidence of small studies effect (Egger test P = 0.32).
Discussion
This study provides strong clinical evidence showing primary brain tumor patients have a high level of distress prevalence from 12 observational studies. Based on our findings, patients with intracranial tumor have a higher prevalence of distress compared with a non-clinical population, which ranges between 5% to 27% [22,23,24,25,26]. The high risk of emotional complications and their harms in brain tumor patients become an enormous challenge for disease management. The distress prevalence in patients with intracranial tumor is not higher than that in patients with lung cancer (61.6%) [27] or bone marrow transplant patients (43.0%) [28]. The possible reasons could be the quick disease progression of a malignant brain tumor or early interventions by some of the clinical practitioners [17, 29,30,31]. Fatigue, pain, anxiety, and depression are among the most troubling symptom associated with the prevalence of distress in brain tumor patients which will result in a poorer overall survival and decreased health-related quality of life (HRQoL) [17, 32]. Caregivers also have severe distress experience according to some studies [9, 10]. The mental and physical distress would lead to low quality of life, predicate poor therapeutic effect, and satisfaction with health care [13]. Routine screening and evaluation of distress in brain tumor patients may assist medical workers to develop proper intervention [13, 33, 34], which may improve prognosis [35]. More studies should be planned to identify the risk factors of brain tumor patients and integrate appropriate interventions to improve HRQoL.
The study has some limitations. A huge heterogeneity in different studies was detected. After sub-group analysis, we found that different study design, sample size, study country, cut-off point and year published contributed to the heterogeneity. The effect of tumor size and grade on distress remains controversial [13, 36]. Tumor biology has an influence on cognition function and physiological environment in patients [37, 38], and intracranial tumors could invade and affect function area, but they did not alter the Distress Thermometer scoring according to Goebel’s research [13]. However, similar studies using Hospital Anxiety and Depression Scale (HADS) and Beck Depression Inventory (BDI) to assess distress, anxiety and depressive symptoms, have found that patients with meningioma are more likely to develop emotional stress, but other studies did not support this finding [36, 39,40,41,42].
Our findings are based on one single screening method, the Distress Thermometer produced by the National Comprehensive Cancer Network (NCCN) [2]. There are other screening scales used in clinical setting to assess the distress-related symptoms such as depression, anxiety, and fatigue [8, 43,44,45,46,47,48,49,50]. For example, the hospital anxiety and depression scale (HADS) was used to identify distress in some studies by which DT was compared [47]. Simone Goebel et al. found that the ability of DT to screening distress in brain tumor patients was efficient and excellent by comparing different DT scores with HADS [13]. And correlation analysis for the relationship between DT scores and HADS anxiety and depression found that they are closely relevant. Distress levels could reflect emotional problems including anxiety and depression. To date, there seems no consensus to define the best-standardized scale to evaluate distress-related symptoms in clinical settings. In the future studies, different scales should be compared to analyze their accuracy and consistency in the identification of disease.
It would be better if we can monitor the distress prevalence change during a routine follow-up examination [35]. There were limited studies monitoring distress change over time and recording relative indicators during this period [12, 20, 21]. We hope more studies will track distress over time in combination with feedback information to provide better insight into this field and develop appropriate supportive care options. And study design including healthy control group or extracranial tumor patients is recommended.
Conclusions
The high prevalence of distress becomes an enormous challenge for primary brain tumor patients. The role of distress in intracranial tumor patients should be studied and understood to develop proper management and maintain the good Health-related quality of life. More studies to track distress over time are needed to develop appropriate supportive care options for intracranial tumor patients.
Abbreviations
- BDI:
-
Beck Depression Inventory
- DT:
-
The Distress thermometer
- HADS-D:
-
Depression Subscale of Hospital Anxiety and Depression Scale
- HRQoL:
-
Health-related quality of life
- NA:
-
not applicable
References
Ridner SH. Psychological distress: concept analysis. J Adv Nurs. 2004;45(5):536–45.
O'Donnell E. The distress thermometer: a rapid and effective tool for the oncology social worker. Int J Health Care Qual Assur. 2013;26(4):353–9.
Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8(4):487–94.
Keir ST, Farland MM, Lipp ES, Friedman HS. Distress persists in long-term brain tumor survivors with glioblastoma multiforme. J Cancer Surviv. 2008;2(4):269–74.
Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, Friedman HS. Screening for distress in patients with brain cancer using the NCCN's rapid screening measure. Psycho-Oncology. 2008;17(6):621–5.
Huang J, Liu F, Liu Z, Tang H, Wu H, Gong Q, Chen J. Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol. 2017;8:242.
Hoffman BM, Zevon MA, D'Arrigo MC, Cecchini TB. Screening for distress in cancer patients: the NCCN rapid-screening measure. Psycho-Oncology. 2004;13(11):792–9.
Jacobsen PB, Donovan KA, Trask PC, Fleishman SB, Zabora J, Baker F, Holland JC. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494–502.
Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer. 2009;17(7):793–9.
Goebel S, Mehdorn HM. Measurement of psychological distress in patients with intracranial tumours: the NCCN distress thermometer. J Neuro-Oncol. 2011;104(1):357–64.
Renovanz M, Gutenberg A, Haug M, Strittmatter E, Mazur J, Nadji-Ohl M, Giese A, Hopf N. Postsurgical screening for psychosocial disorders in neurooncological patients. Acta Neurochir. 2013;155(12):2255–61.
Trad W, Koh ES, Daher M, Bailey A, Kastelan M, Legge D, Fleet M, Simpson GK, Hovey E. Screening for psychological distress in adult primary brain tumor patients and caregivers: considerations for cancer care coordination. Front Oncol. 2015;5:203.
Goebel S, Stark AM, Kaup L, von Harscher M, Mehdorn HM. Distress in patients with newly diagnosed brain tumours. Psycho-Oncology. 2011;20(6):623–30.
Su D, Wu XN, Zhang YX, Li HP, Wang WL, Zhang JP, Zhou LS. Depression and social support between China' rural and urban empty-nest elderly. Arch Gerontol Geriatr. 2012;55(3):564–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Randazzo DM, McSherry F, Herndon JE 2nd, Affronti ML, Lipp ES, Flahiff C, Miller E, Woodring S, Freeman M, Healy P, et al. A cross sectional analysis from a single institution's experience of psychosocial distress and health-related quality of life in the primary brain tumor population. J Neuro-Oncol. 2017;134(2):363-69.
Renovanz M, Hechtner M, Janko M, Kohlmann K, Coburger J, Nadji-Ohl M, Konig J, Ringel F, Singer S, Hickmann AK. Factors associated with supportive care needs in glioma patients in the neuro-oncological outpatient setting. J Neuro-Oncol. 2017;133(3):653–62.
Halkett GK, Lobb EA, Rogers MM, Shaw T, Long AP, Wheeler HR, Nowak AK. Predictors of distress and poorer quality of life in high grade glioma patients. Patient Educ Couns. 2015;98(4):525–32.
Rooney AG, McNamara S, Mackinnon M, Fraser M, Rampling R, Carson A, Grant R. Screening for major depressive disorder in adults with cerebral glioma: an initial validation of 3 self-report instruments. Neuro-Oncology. 2013;15(1):122–9.
Rooney AG, McNamara S, Mackinnon M, Fraser M, Rampling R, Carson A, Grant R. The frequency, longitudinal course, clinical associations, and causes of emotional distress during primary treatment of cerebral glioma. Neuro-Oncology. 2013;15(5):635–43.
Benzeval M, Judge K. Income and health: the time dimension. Soc Sci Med. 2001;52(9):1371–90.
Gispert R, Rajmil L, Schiaffino A, Herdman M. Sociodemographic and health-related correlates of psychiatric distress in a general population. Soc Psychiatry Psychiatr Epidemiol. 2003;38(12):677–83.
Kuriyama S, Nakaya N, Ohmori-Matsuda K, Shimazu T, Kikuchi N, Kakizaki M, Sone T, Sato F, Nagai M, Sugawara Y, et al. Factors associated with psychological distress in a community-dwelling Japanese population: the Ohsaki cohort 2006 study. J Epidemiol. 2009;19(6):294–302.
Sparrenberger F, Dos Santos I, Lima Rda C. Epidemiology of psychological distress: a population-based cross-sectional study. Rev Saude Publica. 2003;37(4):434–9.
Mcveigh KH, Galea S, Thorpe LE, Maulsby C, Henning K, Sederer LI. The epidemiology of nonspecific psychological distress in new York City, 2002 and 2003. J Urban Health. 2006;83(3):394–405.
Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55(2):215–24.
Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow transplant patients. Psycho-Oncology. 2006;15(7):604–12.
Liu H, Liu Z, Jiang B, Ding X, Huo L, Wan X, Liu J, Xia Z. Prognostic significance of hyperglycemia in patients with brain tumors: a meta-analysis. Mol Neurobiol. 2016;53(3):1654–60.
Li J, Qu Q, Qu J, Luo WM, Wang SY, He YZ, Luo QS, Xu YX, Wang YF. Association between XRCC1 polymorphisms and glioma risk among Chinese population. Med Oncol. 2014;31(10):186.
Wang H, Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, Li L, Zhao J. Clinical significance of increased cerebellar default-mode network connectivity in resting-state patients with drug-naive somatization disorder. Medicine. 2016;95(28):e4043.
Huang J, Zeng C, Xiao J, Zhao D, Tang H, Wu H, Chen J. Association between depression and brain tumor: a systematic review and meta-analysis. Oncotarget. 2017;8(55):94932-43.
Langbecker D, Ekberg S, Yates P. Don't need help, don't want help, can't get help: how patients with brain tumors account for not using rehabilitation, psychosocial and community services. Patient Educ Couns. 2017;100(9):1744–50.
Yao S, Zhang C, Zhu X, Jing X, McWhinnie CM, Abela JR. Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J Adolesc Health. 2009;45(1):55–62.
Langbecker D, Yates P. Primary brain tumor patients' supportive care needs and multidisciplinary rehabilitation, community and psychosocial support services: awareness, referral and utilization. J Neuro-Oncol. 2016;127(1):91–102.
Arnold SD, Forman LM, Brigidi BD, Carter KE, Schweitzer HA, Quinn HE, Guill AB, Herndon JE 2nd, Raynor RH. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-Oncology. 2008;10(2):171–81.
Pyter LM. The influence of cancer on endocrine, immune, and behavioral stress responses. Physiol Behav. 2016;166:4–13.
Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: neuroinflammatory mechanisms in humans and rodents. Brain Behav Immun. 2015;49:1–17.
Anderson SI, Taylor R, Whittle IR. Mood disorders in patients after treatment for primary intracranial tumours. Br J Neurosurg. 1999;13(5):480–5.
Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study. J Neurosurg. 2005;103(5):841–7.
Mainio A, Hakko H, Niemela A, Tuurinkoski T, Koivukangas J, Rasanen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J Neurol Neurosurg Psychiatry. 2003;74(9):1278–82.
Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46–51.
Giovagnoli AR, Tamburini M, Boiardi A. Quality of life in brain tumor patients. J Neuro-Oncol. 1996;30(1):71–80.
Kerr LK, Kerr LD. Screening tools for depression in primary care. The Western journal of medicine. 2001;175(5):349.
Pranckeviciene A, Bunevicius A. Depression screening in patients with brain tumors: a review. CNS oncology. 2015;4(2):71–8.
Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63–70.
Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–87.
Gao W, Bennett MI, Stark D, Murray S, Higginson IJ. Psychological distress in cancer from survivorship to end of life care: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46(11):2036–44.
Acknowledgements
The authors would like to thank the reviewers for their valuable comments and suggestions to improve the quality of the paper.
Funding
This work was supported by National Natural Science Foundation of China (No. 81472693). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Author information
Authors and Affiliations
Contributions
FL, JH, JC, and ZL conceptualized and designed the study; FL led the review process, drafted the initial manuscript, and JH reviewed all articles and extracted data; and JH, LZ, FF, JC and KX analyzed and interpreted the data. All authors made substantial contributions to revising the manuscript. ZL is responsible for the overall content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Meta-analysis of the prevalence of distress symptoms among brain tumor patients stratified by study-level characteristics. (DOC 40 kb)
Additional file 2:
Meta-analysis of the prevalence of distress symptoms among brain tumor patients stratified by study design (A), country (B), sample size (C), year (D) and distress scale cut-off (E). CI, confidence interval. (ZIP 6618 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, F., Huang, J., Zhang, L. et al. Screening for distress in patients with primary brain tumor using distress thermometer: a systematic review and meta-analysis. BMC Cancer 18, 124 (2018). https://doi.org/10.1186/s12885-018-3990-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-3990-9