Abstract
Background
Little is known about the relationship between preoperative body mass index and need for adjuvant radiation therapy (RT) following radical prostatectomy. The goal of this study was to evaluate the utility of body mass index in predicting adverse clinical outcomes which require adjuvant RT among men with organ-confined prostate cancer (PCa).
Methods
We used a prospective cohort of 1,170 low-intermediate PCa risk men who underwent radical prostatectomy and evaluated the effect of body mass index on adverse pathologic features and freedom from biochemical failure (FFbF). Clinical and pathologic variables were compared across the body mass index groups using an analysis of variance model for continuous variables or χ2 for categorical variables. Factors related to adverse pathologic features were examined using logistic regression models. Time to biochemical recurrence was compared across the groups using a log-rank survivorship analysis. Multivariable analysis predicting biochemical recurrence was conducted with a Cox proportional hazards model.
Results
Patients with elevated body mass index (defined as body mass index ≥25 kg/m2) had greater extraprostatic extension (p = 0.004), and positive surgical margins (p = 0.01). Elevated body mass index did not correlate with preoperative risk groupings (p = 0.94). However, when compared with non-obese patients (body mass index <30 kg/m2), obese patients (body mass index ≥30 kg/m2) were much more likely to have higher rate of adverse pathologic features (p = 0.006). In patients with low- and intermediate- risk disease, obesity was strongly associated with rate of pathologic upgrading of tumors (p = 0.01 and p = 0.02), respectively. After controlling for known preoperative risk factors, body mass index was independently associated with ≥2 adverse pathologic features (p = 0.002), an indicator for adjuvant RT as well as FFbF (p = 0.001).
Conclusions
Body mass index of ≥30 kg/m2 is independently associated with adverse pathologic features, which is an indicator for additional RT, particularly in patients with low-intermediate risk disease. Future studies may determine if this select group of patients may be best treated with definitive RT to reduce toxicity from additional RT following radical prostatectomy. We propose including body mass index in clinical decision-making for appropriate treatment recommendation for patients with low-intermediate risk PCa.
Similar content being viewed by others
Background
Obesity, one of the most pressing issues facing the U.S. healthcare system, is a potentially modifiable risk factor for disease progression and poor outcomes for numerous diseases, including prostate cancer (PCa) [1]. Specifically, associations between increased body mass index (BMI) and advanced prostate tumor stage and grade at diagnosis, younger age at diagnosis, and biochemical failure (FFbF, disease recurrence) after treatment have been observed. [2–4] While investigators study the underlying mechanisms that link obesity to poor PCa outcomes [5–8], understanding how BMI may influence treatment recommendations is a critical aspect of ongoing PCa care.
The current guidelines for patients with organ-confined PCa include definitive modalities such as radical prostatectomy (RP) or radiation therapy (RT) [9–12]. RP is a standard surgical management for clinically localized PCa in patients free of surgical contraindications. This procedure confers excellent 10-year long-term disease control of >90 % in patients who are confirmed pathologically to have localized (pT2) disease. Retrospective studies reported that the long-term outcomes of patients with localized and low-risk PCa were equally favorable with RP or external beam radiation therapy [13, 14]. For intermediate and high risk disease, however, monotherapy with either RP or RT did not achieve the excellent long-term outcomes seen in patients with low-risk disease [15, 16]. For pT3 cancer (defined as disease in the extraprostatic extension or seminal vesicle involvement), the risk of 5-year local failure and biochemical progression varies from 20 % to 70 % [17, 18]. Several randomized studies for patients with pT3 (with or without positive margin) or pT2 (with positive surgical margin) disease have been reported, demonstrating that adjuvant RT reduces the risk of local relapse and biochemical progression and disease-specific survival [19–22]. Despite earlier cancer detection with serumPSA screening, approximately 50 % of patients who undergo RP are found to have at least one adverse pathologic feature(APF), including advanced tumor grade/stage and positive margins/lymph nodes, extraprostatic extension and seminal vesicle invasion [23]. These patients may require adjuvant RT.
Several studies have shown increased genitourinary and gastrointestinal toxicity from additional RT after RP [22, 24–26]. In the South West Oncology Group trial, adverse events were more likely to occur in the RP + RT arm compared with the RP arm (23.8 % vs 11.9 %), including urethral strictures (17.8 % vs 9.5 %), total urinary incontinence (6.5 % vs 2.8 %), and rectal complications (3.3 % vs 0 %), respectively [25]. A study on the health-related quality of life (HRQOL) of PCa patients compared short- and long-term effects of adjuvant treatment versus observation after RP [26]. The investigators reported that the addition of RT to RP resulted in more frequent urination, as well as early report of more bowel dysfunction. Another HRQOL in patients treated with multimodality for PCa reported a decline in HRQOL particularly with urinary function, urinary bother and sexual function [24]. Therefore, the ability to preoperatively identify the subset of patients who are at risk of requiring additional RT after RP will be of clinical utility. These patients may benefit from upfront definitive RT to improve quality of life and minimize additional toxicity from a combination of RP followed by RT. To date the most widely utilized predictors of clinical outcomes including PSA, Gleason score (GS) and clinical stage are sub-optimal in predicting adverse pathologic outcomes and adjuvant RT use following RP. Over the last decade, a large body of evidence has emerged associating obesity with incidence of PCa [27–29] as well as adverse outcomes following treatment. Recent studies found increased BMI to be associated with aggressive PCa and FFbF [30–34]. However, no studies have examined the relationship between preoperative BMI and the need for adjuvant RT following RP in patients with adverse pathologic outcomes. We sought to determine whether BMI provides a clinically useful prediction of adverse pathologic outcomes that will guide physicians in recommending RT for select patients with organ-confined PCa. Obesity, in particular, has been related to a number of factors and molecular pathways that may advance cancer progression [35]. We hypothesize that obesity status modifies the relationship between preclinical risk and PCa outcomes among low-intermediate risk patients. The study aims were to utilize a cohort of radical prostatectomy patients to 1) examine the relationship between obesity and adverse pathology, and 2) examine the relationship between obesity and FFBF.
Methods
Patient population
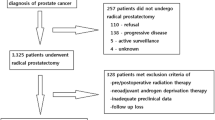
This study utilizes a cohort of 1970 men with PCa treated with RP and bilateral pelvic lymph node dissection at the Hospital of the University of Pennsylvania Health System (UPHS; Philadelphia, PA.) Patients were consented in person and recruited at UPHS to participate in a PCa study, the Study of Clinical Outcomes, Risk and Ethnicity (SCORE) between 1990 and 2012 as previously described [36, 37]. This study was approved by the Institutional Review Board at the University of Pennsylvania.
The SCORE study includes information on patient age, race, height, weight, clinical stage, clinical Gleason on diagnostic biopsy, preoperative PSA levels, surgical pathologic information (tumor grade, stage, surgical margins status, extraprostatic extension, or seminal vesicle involvement, lymph node status). Prospective follow -up was conducted with PSA levels obtained at each visit. For the purpose of this study, patients without height and weight data for BMI calculation were excluded from the analysis (N = 506). Patients without adequate preclinical data including initial PSA (N = 30), or biopsy Gleason (N = 264) at diagnosis were excluded from the analysis. Patients who received androgen deprivation therapy (ADT) or adjuvant RT and/or ADT were included. The remaining 1170 patients were analyzed in this study.
Data collection
The standard protocol for men in the SCORE study was as follows: Patients were evaluated at time of diagnosis by a thorough history and physical examination (including digital rectal examination [DRE]) followed by routine laboratory studies, including serum PSA levels, and GS determined by needle biopsy and reviewed at the UPHS. The patients were examined 1 month postoperatively and then at 3 month intervals for 1 year, every 6 months for 5 years, and then annually. At each follow up visit a complete evaluation, including DRE and serial PSA values, were determined and recorded. Biochemical recurrence (PSA failure) was defined as a single PSA ≥0.2 ng/ml or when two consecutive PSA values of 0.2 ng/ml were obtained after an undetectable value. Time zero (the starting point for follow-up) was defined at the date of surgery for all patients. If PSA was never undetectable postoperatively, then PSA failure was assigned at time zero. Patients with no follow up data were included for the evaluation of differences in preoperative and pathologic characteristics, but not biochemical recurrence.
Data related to patient and clinical characteristics, tumor pathology, and PCa outcomes were collected via medical record abstraction. All patients were staged according to the 1992 American Joint Committee on Cancer staging system [38].
Treatment
Surgical treatment consisted of a radical retropubic prostatectomy and bilateral pelvic lymph node sampling or robotic-assisted laparoscopic prostatectomy. Adverse pathologic features (APF), such as extraprostatic extension (EPE), seminal vesicle invasion (SVI), and surgical margin status (SM), were noted and recorded. At the discretion of the treating physician, patients with APF including EPE, SVI or positive surgical margins were treated with adjuvant RT and/or ADT. ADT consisted of a gonadotropin-releasing hormone agonist (leuprolide acetate or goserelin acetate) with or without antiandrogens (flutamide). The SCORE study is a prospectively maintained database with patients treated from the 1990s until 2012. For this reason the year of prostatectomy was recorded and introduced into our modeling to account for difference in pre-PSA era of diagnosis and improvements in surgical treatment techniques that may impact APFs.
Risk classification
Preoperatively patients were stratified into low, intermediate and high risk groups according to the recent National Comprehensive Cancer Network (NCCN) guidelines [39]. Patients who had T1 to T2a tumors, and a Gleason score < 7, and a PSA level < 10 ng/mL were classified as low risk (N = 777); patients who had T2b to T2c tumors, and/or a Gleason score of 7, and/or a PSA level between 10 ng/mL and 20 ng/mL were classified as intermediate risk (N = 270); and patients who had > T3 tumors, or a Gleason score between 8 and 10, or a PSA level > 20 ng/mL were classified as high risk (N = 117) [38]. Following RP patients were further stratified by the number of APFs into low, intermediate and high RPrisk groups. Patients with no APFs were in the low RPrisk (N = 818); patients with only 1 APF were in the intermediate RPrisk (N = 177); and patients with >/=2 APFs were in the high RPrisk group (N = 175).
Statistical analysis
BMI
For the purpose of this study BMI (weight in kilograms divided by height in meters squared) was categorized as follows; normal weight (<25 kg/m2), overweight (≥25 kg/m2 to <30 kg/m2), obese (≥30 kg/m2). BMI was examined as a continuous and a categorical variable in which case BMI was stratified into non-obese (<30 kg/m2) or obese (≥30 kg/m2).
Other patient/clinical variables
Age, PSA, year of surgery, and biopsy Gleason score were examined as continuous variables. Clinical T-stage (T1c, T2a, T2b, and T2c) and race (white, African-American/black, and other) were examined as categorical variables.
Clinical and pathologic variables were compared across the BMI groups using an analysis of variance model for continuous variables or χ2 for categorical variables. Factors associated with the presence of APF were examined using logistic regression models. The predictability of BMI was evaluated using more stringent criteria of ≥2 APF in order to rigorously select for the patients that are most likely to be offered additional RT. For Cox proportional hazards models predicting FFbF, patients who experienced biochemical recurrence or PSA failure, lost to follow up, deceased were censored. Treatment outcomes often correlate with biochemical control rates thus, a PSA rise to 0.2 ng/ml was used to define biochemical disease recurrence, and time to biochemical recurrence was used as a surrogate for biochemical disease-free survival. Time to biochemical recurrence was compared across the groups using a log-rank survivorship analysis. For both univariate and multivariate analyses, BMI, Race, clinical stage, and clinical Gleason score were evaluated as categorical variables as follows: BMI categorical used BMI <25 kg/m2 as reference category; Race used White as reference category; “Other” race was dropped from model due to small numbers. Clinical stage categorical (T1; T2a, T2b; >T2c), used T1c as reference category; clinical Gleason score categorical (6, 7, ≥8) used Gleason 6 as reference category. The analyses were conducted using STATA statistical software version 13.0 (STATA Corporation). A p-value <0.05 was considered statistically significant.
Results
The patient clinical and pathologic characteristics are listed in Table 1. Preoperative factors such as age, PSA at diagnosis, biopsy Gleason, clinical T-stage and year of RP were similar between BMI categories except for race, where African American/Black race was associated with elevated BMI (p < 0.001). There was a statistically significant difference between postoperative pathologic features and BMI. Specifically, extraprostatic extension, p = 0.001; positive surgical margins, p = 0.01; and higher pathologic Gleason (p = 0.001).
As shown in Table 2, BMI was not associated with preoperative risk groupings (p = 0.94). However, obesity (BMI ≥30 kg/m2) directly correlated with increased risk of APFs (; p = 0.006). The effect of BMI and outcomes by pre-operative risk grouping was evaluated as per the National Comprehensive Cancer Network classification. Obesity (BMI ≥30 kg/m2) was strongly associated with a higher rate of pathologic Gleason score upgrading of tumors, particularly for low risk (Fig. 1a-b, 30 % vs. 38 %; p = 0.01) and intermediate risk patients (Fig. 1a-b, 29 % vs 47 %; p = 0.02).
After controlling for known preoperative risk factors, BMI ≥30 kg/m2 was still predictive for the risk of having ≥2 APF (OR, 2.58; 95 % CI 1.30 to 5.09; p = 0.006) using BMI as a categorical variable with BMI <25 kg/m2 as the reference category (Table 3). Although African American/Black race is associated with elevated BMI, race was not associated with adverse pathologic outcomes in the logistic regression models.
Using the Kaplan-Meier survival analysis method, the impact of BMI on freedom from (FFbF) was evaluated. The mean and median follow up time was 42 and 24 months, respectively (range 1–245 months). During this time period, 171 patients (15 %) experienced biochemical recurrence. Higher BMI was significantly associated with worse 7-year FFbF as follows; BMI-normal: 87 % vs. BMI-Overweight: 76 % vs. BMI-Obese: 61 %; log-rank p = 0.0004; Fig. 2. Upon stratifying by risk groupings BMI ≥30 kg/m2 had a significant impact on 7-year FFbF among patients with low risk (90 % vs. 76 %; log-rank p = 0.004), and a trend towards worse outcomes for intermediate risk disease (67 % vs. 40 %; log-rank p = 0.08), as shown in Fig. 3.
Kaplan-Meier curves for FFbF outcomes stratified by body mass index in men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012. Abbreviations: FFbF- Freedom From biochemical Failure, NCCN- National Comprehensive Cancer Network, BMI- Body mass index, BMI- Normal: <25kg/m2; BMI - Overweight: ≥25 to <30 kg/m2; BMI- Obese: ≥30kg/m2
Kaplan-Meier curves for FFbF outcomes by BMI stratified by NCCN risk groups in men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012. Abbreviations: FFbF- Freedom From biochemical Failure, NCCN- National Comprehensive Cancer Network, BMI- Body mass index, Non-Obese: <30kg/m2; Obese: ≥30kg/m2 . NCCN risk groupings: Panel A) Low risk; B) Intermediate risk; C) High risk
Using a multivariate Cox proportional hazard modeling, the significant predictors of risk for FFbF following RP were determined, Table 4. After adjusting for the other preclinical factors BMI ≥30 kg/m2 (HR, 2.56; 95 % CI 1.24 to 5.29; p = 0.01) remained a predictor of biochemical recurrence.
Discussion
In this current study, further evidence was provided to suggest that BMI is a strong predictor of APF and biochemical recurrence following RP as monotherapy, particularly in patients with low- and intermediate-risk PCa. Specific groups of PCa patients with localized disease at presentation may be at increased risk for disease progression and related PCa-specific mortality from pre-treatment patient phenotype (e.g., obesity.) or post-treatment adverse pathologic features (e.g., positive surgical margins, seminal vesicle invasion, extracapsular extension). Multimodal treatment techniques have been employed to increase recurrence-free survival among localized high risk patients [40], and may be useful to treat a subset of patients with lower risk disease characteristics but elevated phenotypic risk factors. Although low- and intermediate risk patients often are treated with monotherapy, obese men are a patient population with unique disease features and medical needs that may require a more aggressive treatment approach including adjuvant RT.
Obesity and PCa outcomes
Previous studies on the association between BMI the risk of developing PCa have provided mixed results. Obesity has been shown to increase the risk of poor PCa outcomes in several studies [2, 41–43]. Recent studies have analyzed the relation between BMI and PCa risk stratified by clinical stage and Gleason score at diagnosis. These studies consistently showed that elevated BMI positively correlated with increased risk of higher Gleason grade or higher stage disease and negatively correlated with low Gleason grade and stage of disease [27, 28, 44]. Previous results suggest that obesity is associated with higher grade tumors, increased risk of positive surgical margins, higher FFbF rates, and risk for PCa-specific mortality [1, 2, 33, 45–47]. In multivariate analyses, obesity also has been associated with significant tumor upgrading and upstaging among pre-operative low-risk patients, which increases risk for FFbF among this patient population [48–52].
However, not all studies support a relationship between poor PCa outcomes and obesity [53–55]. Often, studies differ by the number of obese men, sample population demographics and study methodology, making it difficult to compare across studies. Further complicating the relationship between obesity and PCa are diagnostic and treatment obstacles associated with obesity that make it more likely that cancer will progress and that treatments will fail on the obese patient due to technical difficulty rather than biological processes [41, 56–58]. Obese men are less likely than non-obese men to have abnormal PSA results and undergo biopsy, potentially effecting timely diagnosis. At the time of biopsy, larger prostate glands may make it more difficult to detect and accurately stage cancer [41, 59, 60]. It is also not clear if the relationship between obesity and treatment failure is due to aggressive disease biology or to technical limitations. Potency and continence rates after treatment are similar among weight groups, so technically inferior operations do not account fully for differences in treatment failure [1]. Pelvic surgery in general is more technically challenging in obese patients. Obesity has been associated with 30 % higher odds of capsular incision, a surrogate for poor technical operation. However, some patients receiving poor technique do well after surgery and others that experienced apparently fine surgical technique still experience FFbF [61].
Treatment guidelines for low and intermediate risk patients
Treatment outcomes for patients with low- and intermediate risk disease have been inconsistent in part due to tumor heterogeneity and inaccuracies in staging [62, 63]. For this reason, low risk patients have recently been reclassified into very low-risk group (active surveillance eligible) and low-risk, and there are ongoing discussions to re-classify intermediate risk patients into low- and high- intermediate risk groups [64]. Therefore, the ability to preoperatively identify low or intermediate risk patients with elevated BMI who are at highest risk of FFbF after RP as monotherapy will be very useful in guiding upfront treatment recommendations. Perhaps, these patients may be best treated with combination therapy (surgery and RT), or other approaches such as definitive RT with/without hormonal therapy to improve disease control [40].
The current treatment guidelines recommend that patients with ≥1 APF be offered RT adjuvantly, or as part of a salvage regimen upon a detectable rise in PSA above 0.2 ng/ml following RP. Adjuvant RT has been shown in randomized trials to improve PSA-relapse free survival [19, 22, 65], distant metastasis-free survival and overall survival [66], compared to observation. Despite these results, referral patterns for additional RT for these patients remain very low. In fact less than 20 % of qualifying patients in the United States actually receive adjuvant RT [67–70] suggesting that many clinicians are reluctant to deliver RT after RP. In our study, only 2 % of entire cohort had documented treatment with additional RT. However, this result may be an underestimation and should be interpreted with caution, since a good number of patients could undergo RP at UPHS and then RT locally. RT information for these patients may not be accurately captured in our database. The primary reasons for withholding post-RP RT include increased treatment-related toxicity and potentially over treating patients with RT who may not recurred after RP [71]. It is estimated that approximately 50 % of patients with APF will not experience FFbF. Therefore, patients are offered “active surveillance” post-RP, and RT is only recommended at the earliest signs of PSA failure termed “early salvage”. However, whether early salvage RT is equivalent to adjuvant RT is a topic of current investigation [29]. Unlike RT post-RP, the use of ADT for patients in this setting is even much less standardized since physicians often recommend ADT for a number of reasons including attempting to reduce the prostate size prior to surgery or at the earliest signs of PSA failure after surgery.
Currently, the decision to recommend definitive radiation treatment for patients with low-intermediate risk prostate disease is often based on many factors including patient preference, and/or preexisting comorbid conditions that precludes surgery. However, in patients with no contraindications for surgery, decision for RT or RP is largely driven by age, genitourinary toxicity, and the desire to preserve sexual function [71]. The ability to identify patients with low-intermediate disease yet at increased risk for APF as well as FFbF will enable clinicians to better counsel patients with the appropriate treatment option that provides the best disease control with minimal side effects. The existing preclinical factors used to predict APF and biochemical outcomes are suboptimal. In this report elevated BMI was identified as a preclinical factor that is independently associated with adverse pathologic outcomes as well as biochemical recurrence, particularly in patients with low-intermediate disease or ≤ one adverse pathologic feature. Therefore, incorporating BMI into the current predictive models may shows promise in identifying the group of patients with low-intermediate risk disease who are likely to experience biochemical recurrence following RP as monotherapy. These patients could be best treated with definitive RT with or without hormones thus sparing them the added toxicity of requiring additional RT after RP. Further studies are required to develop and validate the predictive performance of BMI using an independent patient cohort.
Study limitations
It is important to emphasize that results from this study cannot be extrapolated to imply that RP is suboptimal for obese men since not all patients with elevated BMI experience adverse pathologic outcomes or biochemical recurrence after surgery. Although elevated BMI was associated with increased positive surgical margins, BMI still was associated with adverse pathologic outcomes after adjusting for margin status. This suggests that poorer surgical outcomes did not account for worse pathologic outcomes in obese men. Therefore, patients with elevated BMI may harbor a biologically more aggressive PCa. Limitations to this study were that important measures of obesity, such as waist-to-hip ratio and percent lean body fat were unavailable. Information on the biologic factors that may contribute to the effect of elevated BMI on disease aggressiveness and treatment outcomes could not be evaluated since blood biosamples were not obtained at the time of surgery. Furthermore, the median follow-up for the study cohort was relatively short.
Conclusions
Elevated BMI is independently associated with APF, particularly in patients with low-intermediate risk disease. BMI may be useful as a predictive tool to augment the performance of the known preclinical factors in predicting adverse pathologic outcome and the use of adjuvant RT post-RP. Therefore, BMI should be considered in clinical decision-making for appropriate treatment recommendation for patients with low-intermediate risk PCa.
Abbreviations
ADT, androgen deprivation therapy; APF, adverse pathologic features; BMI, body mass index; DRE, digital rectal exam; EPE, extraprostatic extension; FFbF, biochemical failure; GS, gleason score; HRQOL, health-related quality of life; NCCN, National comprehensive cancer network; PCa, prostate cancer; PSA, prostate specific antigen; RP, radical prostatectomy; RT, radiation therapy; SCORE, study of clinical outcomes, risk and ethnicity; SM, surgical margin status; SVI, seminal vesicle invasion; UPHS, University of Pennsylvania health system
References
Herman M, Raman J, Dong S, Samadi D, Scherr D. Increasing body mass index negatively impacts outcomes following robotic radical prostatectomy. Journal of the Society of Laparoendoscopic Surgeons. 2007;11:438–42.
Spangler E, Zeigler-Johnson C, Coomes M, Malkowicz S, Wein A, Rebbeck T. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;1939–1945.
King C, Freedland S, Terris M, Kane C, Amling C, Aronson W, Presti JJ. Impact of obesity on the utility of preoperative prostate-specific antigen velocity to predict for relapse after prostatectomy: a report from the SEARCH database. Urology. 2007;69(5):921–6.
Calle E. Obesity and cancer. BMJ. 2007;335(7630):1107–8.
Li A, Elmore R, Pavelka C, Karlan B. Hyperandrogenism, mediated by obesity and receptor polymorphisms, promotes aggressive epithelial ovarian cancer biology. Gynecol Oncol. 2007;107:420–3.
Wu F, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24(2):183–217.
Zeigler-Johnson C, Weber A, Spangler E, Panossian S, Rebbeck TR, Malkowicz SB. Relationship of obesity, androgen receptor genotypes and biochemical failure after radical prostatectomy. Prostate. 2012;72(9):984–90.
Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel R, Ferrante Jr A. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol. 1996;20(3):286–92.
Gerber GS, Thisted RA, Scardino PT, Frohmuller HG, Schroeder FH, Paulson DF, Middleton Jr AW, Rukstalis DB, Smith Jr JA, Schellhammer PF, et al. Results of radical prostatectomy in men with clinically localized prostate cancer. JAMA : the journal of the American Medical Association. 1996;276(8):615–9.
Perez CA, Hanks GE, Leibel SA, Zietman AL, Fuks Z, Lee WR. Localized carcinoma of the prostate (stages T1B, T1C, T2, and T3). Review of management with external beam radiation therapy. Cancer. 1993;72(11):3156–73.
Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, Walz J, Karakiewicz PI, Huland H. Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol. 2006;24(3):273–80.
Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(16):3376–85.
Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, Carlson TP, Klein EA. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58(1):25–33.
van den Ouden D, Davidson PJ, Hop W, Schroder FH. Radical prostatectomy as a monotherapy for locally advanced (stage T3) prostate cancer. J Urol. 1994;151(3):646–51.
Akakura K, Furuya Y, Suzuki H, Komiya A, Ichikawa T, Igarashi T, Tanaka M, Murakami S, Ito H. External beam radiation monotherapy for prostate cancer. International journal of urology : official journal of the Japanese Urological Association. 1999;6(8):408–13.
Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, Maru N, Slawin K, Scardino PT. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174(3):903–7.
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–23.
Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366(9485):572–8.
Thompson I, Tangen C, Tolcher A, Crawford E, Eisenberger M, Moinpour C. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst. 2001;93(3):219–25.
Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, Forman JD, Thompson IM, Crawford ED, Swog. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(16):2225–9.
Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, Willich N, Semjonow A, Souchon R, Stockle M, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30.
Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, Cangiano T, Schroder FH, Scardino PT, Kattan MW. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66(6):1245–50.
Wu AK, Cooperberg MR, Sadetsky N, Carroll PR. Health related quality of life in patients treated with multimodal therapy for prostate cancer. J Urol. 2008;180(6):2415–22. discussion 2422.
Thompson Jr IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–35.
Moinpour CM, Hayden KA, Unger JM, Thompson Jr IM, Redman MW, Canby-Hagino ED, Higgins BA, Sullivan JW, Lemmon D, Breslin S, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26(1):112–20.
Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1977–83.
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–84.
Mishra MV, Scher ED, Andrel J, Margules AC, Hegarty SE, Trabulsi EJ, Hyslop T, Den RB, Lallas CD, Gomella LG, et al. Adjuvant versus salvage radiation therapy for prostate cancer patients with adverse pathologic features: comparative analysis of long-term outcomes. Am J Clin Oncol. 2013.
Amling CL, Kane CJ, Riffenburgh RH, Ward JF, Roberts JL, Lance RS, Friedrichs PA, Moul JW. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58(5):723–8.
Mydlo JH, Tieng NL, Volpe MA, Chaiken R, Kral JG. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4(2):101–5.
Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55(2):140–6.
Freedland SJ, Aronson WJ, Kane CJ, Presti Jr JC, Amling CL, Elashoff D, Terris MK. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the shared equal access regional cancer hospital database study group. J Clin Oncol. 2004;22(3):446–53.
Freedland SJ, Terris MK, Presti Jr JC, Amling CL, Kane CJ, Trock B, Aronson WJ, Search Database Study G. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172(2):520–4.
De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546.
Rebbeck TR, Weber AL, Walker AH, Stefflova K, Tran TV, Spangler E, Chang BL, Zeigler-Johnson CM. Context-dependent effects of genome-wide association study genotypes and macroenvironment on time to biochemical (prostate specific antigen) failure after prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2115–23.
Zeigler-Johnson C, Morales KH, Spangler E, Chang BL, Rebbeck TR. Relationship of early-onset baldness to prostate cancer in African-American men. Cancer Epidemiol Biomarkers Prev. 2013;22(4):589–96.
Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145.
Perez BA, Koontz BF. Radiotherapy before and after radical prostatectomy for high-risk and locally advanced prostate cancer. Urol Oncol. 2015;33(5):226–34.
Rodriguez C, Freedland S, Deka A, Jacobs E, McCullough M, Patel A, Thun M, Calle E. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;161(1):63–9.
Freedland S, Banez L, Sun L, Fitzsimons N, Moul J. Obese men have higher-grade and larger tumors: an analysis of the Duke Prostate Center database. Prostate Cancer Prostatic Dis. 2009;12:259–63.
Gross M, Ramirez C, Luthringer D, Nepomuceno E, Vollmer R, Burchette J, Freedland S. Expression of androgen and estrogen related proteins in normal weight and obese prostate cancer patients. Prostate. 2009;69:520–7.
Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Body mass index, weight change, and risk of prostate cancer in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63–9.
Amling C, Riffenburgh R, Sun L, Moul J, Lance R, Kusuda L, Sexton W, Soderdahl D, Donahue T, Foley J, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22(3):439–45.
Freedland S, Terris M, Presti Jr J, Amling C, Kane C, Trock B, Aronson W, Group SDS. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172(2):520–4.
Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM, Shipley WU. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92-02. J Clin Oncol. 2008;26(26):4333–9.
Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Presti Jr JC, Group SDS. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69(3):495–9.
Truong M, Slezak JA, Lin CP, Iremashvili V, Sado M, Razmaria AA, Leverson G, Soloway MS, Eggener SE, Abel EJ, et al. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119(22):3992–4002.
Ploussard G, de la Taille A, Bayoud Y, Durand X, Terry S, Xylinas E, Allory Y, Vacherot F, Abbou CC, Salomon L. The risk of upstaged disease increases with body mass index in low-risk prostate cancer patients eligible for active surveillance. Eur Urol. 2012;61(2):356–62.
Kane C, Im R, Amling C, Presti Jr J, Aronson W, Terris M, Freedland S. Outcomes after radical prostatectomy among men who are candidates for active surveillance: Results from the SEARCH database. Urology. 2010;76(3):695–700.
Vora A, Large T, Aronica J, Haynes S, Harbin A, Marchalik D, Nissim H, Lynch J, Bandi G, McGeagh K, et al. Predictors of Gleason score upgrading in a large African-American population. Int Urol Nephrol. 2013;45(5):1257–62.
Buschemeyer III W, Freedland S. Obesity and prostate cancer: epidemiology and clinical implications. European Association of Urology. 2007;52:331–43.
van Roermund J, Hinnen K, Battermann J, Witjes J, Bosch J, Kiemeney L, van Vulpen M. Body mass index is not a prognostic marker for prostate-specific antigen failure and survival in Dutch men treated with brachytherapy. BJU Int. 2010;105(1):42–8.
Leon P, Seisen T, Cussenot O, Drouin SJ, Cattarino S, Comperat E, Renard-Penna R, Mozer P, Bitker MO, Roupret M. Low circulating free and bioavailable testosterone levels as predictors of high-grade tumors in patients undergoing radical prostatectomy for localized prostate cancer. Urol Oncol. 2015;33(9):384. e321-387.
Millender L, Aubin M, Pouliot J, Shinohara K, Roach M. Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiation Oncology Biol Phys. 2004;59(1):6–10.
Merrick G, Butler W, Wallner K, Galbreath R, Anderson R, Kurko B, Lief J. Permanent prostate brachytherapy-induced morbidity in patients with grade II and III obesity. Urology. 2002;60(1):104–8.
Pavelka J, Ben-Shachar I, Fowler J, Ramirez N, Copeland L, Eaton L, Manolitsas T, Cohn D. Morbid obesity and endometrial cancer: surgical, clinical, and pathologic outcomes in surgically managed patients. Gynecol Oncol. 2004;95(3):588–92.
Freedland S, Platz E, Presti Jr J, Aronson W, Amling C, Kane C, Terris M. Obesity, serum prostate specific antigen and prostate size: Implications for prostate cancer detection. J Urol. 2006;175(2):500–4.
Efstathiou J, Bae K, Shipley W, Hanks G, Pilepich M, Sandler H, Smith M. Obesity and mortality in men with locally advanced prostate cancer. Cancer. 2007;110:2691–9.
Freedland S, Grubb K, Yiu S, Nielsen M, Mangold L, Isaacs W, Epstein J, Partin A. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005;174:1798–801.
Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the national comprehensive cancer network prostate cancer risk classification system. Urology. 2012;80(5):1075–9.
Reese AC, Sadetsky N, Carroll PR, Cooperberg MR. Inaccuracies in assignment of clinical stage for localized prostate cancer. Cancer. 2011;117(2):283–9.
Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D'Amico AV, Eastham JA, Enke CA, Farrington T, Higano CS, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(9):1081–7.
Thompson Jr IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–35.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–62.
Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76(5):1169–74.
Hoffman KE, Nguyen PL, Chen MH, Chen RC, Choueiri TK, Hu JC, Kuban DA, D'Amico AV. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trial. J Urol. 2010;2010:11 [Epub ahead of print].
Schreiber D, Rineer J, Yu JB, Oisheski M, Nwokedi E, Schwartz D, Choi K, Rotman M. Analysis of pathologic extent of disease for clinically localized prostate cancer after radical prostatectomy and subsequent use of adjuvant radiation in a national cohort. Cancer. 2010;116(24):5757–66.
Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. J Cancer Educ. 2010;2:1–19.
Showalter TN, Ohri N, Teti KG, Foley KA, Keith SW, Trabulsi EJ, Lallas CD, Dicker AP, Hoffman-Censits J, Pizzi LT, et al. Physician beliefs and practices for adjuvant and salvage radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82(2):e233–238.
Acknowledgements
We thank the Prostate Cancer Foundation, and the Department of Defense for supporting the analysis for this manuscript. We also thank Dr. Adam Dicker, Professor and Chair of the Department of Radiation Oncology at Sidney Kimmel Medical College - Thomas Jefferson University, for his advice and generous support.
Funding
This work was funded in part by PHS grants R01-CA085074, P50-CA105641, P60-MD-006900 (to T.R.R.), and DOD grant PC-121189, and the Prostate Cancer Foundation Young Investigator Award (to K.Y.).
Availability of data and materials
SCORE database is housed at the University of Pennsylvania and is available via collaborative agreement or request through a Materials Transfer agreement between institutions. Currently, database is not available in any publicly accessible repository.
Authors’ contributions
KY developed manuscript concept and study design; analyzed data; drafted manuscript. CMZJ helped draft manuscript; helped with data collection. AJ Revised manuscript. BM Revised manuscript. ES helped with data collection and data management. JYP Revised manuscript. AW Revised manuscript. TRR helped with data collection and interpret results. All authors read and approved final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Patients were consented in person and recruited at UPHS to participate in a PCa study, the Study of Clinical Outcomes, Risk and Ethnicity (SCORE) between 1990 and 2012 as previously described (reference provided for SCORE study participation).
This study was approved by the Institutional Review Board at the University of Pennsylvania.
The committee name is the “Institutional Review Board #8”. The approval number is 361402.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yamoah, K., Zeigler-Johnson, C.M., Jeffers, A. et al. The impact of body mass index on treatment outcomes for patients with low-intermediate risk prostate cancer. BMC Cancer 16, 557 (2016). https://doi.org/10.1186/s12885-016-2572-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2572-y