Abstract
Background
A Danish cancer pathway has been implemented for patients with serious non-specific symptoms and signs of cancer (NSSC-CPP). The initiative is one of several to improve the long diagnostic interval and the poor survival of Danish cancer patients. However, little is known about the patients investigated under this pathway. We aim to describe the characteristics of patients referred from general practice to the NSSC-CPP and to estimate the cancer probability and distribution in this population.
Methods
A cross-sectional study was performed, including all patients referred to the NSSC-CPP at the hospitals in Aarhus or Silkeborg in the Central Denmark Region between March 2012 and March 2013. Data were based on a questionnaire completed by the patient’s general practitioner (GP) combined with nationwide registers. Cancer probability was the percentage of new cancers per investigated patient. Associations between patient characteristics and cancer diagnosis were estimated with prevalence rate ratios (PRRs) from a generalised linear model.
Results
The mean age of all 1278 included patients was 65.9 years, and 47.5 % were men. In total, 16.2 % of all patients had a cancer diagnosis after six months; the most common types were lung cancer (17.9 %), colorectal cancer (12.6 %), hematopoietic tissue cancer (10.1 %) and pancreatic cancer (9.2 %). All patients in combination had more than 80 different symptoms and 51 different clinical findings at referral. Most symptoms were non-specific and vague; weight loss and fatigue were present in more than half of all cases. The three most common clinical findings were ‘affected general condition’ (35.8 %), ‘GP’s gut feeling’ (22.5 %) and ‘findings from the abdomen’ (13.0 %). A strong association was found between GP-estimated cancer risk at referral and probability of cancer.
Conclusions
In total, 16.2 % of the patients referred through the NSSC-CPP had cancer. They constituted a heterogeneous group with many different symptoms and clinical findings. The GP’s gut feeling was a common reason for referral which proved to be a strong predictor of cancer. The GP’s overall estimation of the patient’s risk of cancer at referral was associated with the probability of finding cancer.
Similar content being viewed by others
Background
Cancer is the most common cause of death in Denmark and many other countries. One in five of all citizens in the developed world will die from cancer [1]. British and Danish cancer patients experience poorer cancer survival rates than patients from other western countries [2, 3]. Differences in public cancer awareness, health-care seeking behaviour, diagnostic pathways and treatment options have been suggested as important contributing factors [3]. Studies indicate that early diagnosis of cancer is important for improving the prognosis [4, 5]. The health care system must, therefore, provide medical services for prompt cancer diagnosis.
The majority of patients with cancer have a symptomatic presentation of the disease [6]. Symptoms are often diverse and may evolve over time as the cancer develops. In many health systems, general practitioners (GPs) form the first line of health care and provide medical advice to an unselected group of people. At the same time, GPs often act as ‘gatekeepers’ to ensure appropriate and timely flow of patients into the more specialized health services [7]. Thus, general practice plays a central role in diagnosing cancer [8–10]. Furthermore, the use of general practice has been shown to increase significantly several months before a patient is diagnosed with cancer [11]; this indicates an open ‘diagnostic window’.
To reduce the length of the diagnostic interval, several countries have implemented urgent referral cancer pathways [9, 12, 13] for patients with clinical suspicion of cancer [14]. In the UK, such pathway was introduced as the 2-week wait referral (2WW) system [15]. The first Danish Cancer Patient Pathways (CPPs) for diagnosis and treatment of suspected cancer were implemented in 2008; these are specific clinical pathways for several of the most common cancers/cancer sites [14, 16]. Once the GP refers the patient to a CPP, all diagnostic and treatment procedures will be promptly organised in well-defined processes; all relevant clinical investigations and treatments will be planned and booked within a given number of days. The aim of the CPP is to offer patients optimal diagnosis and treatment, which may ultimately improve their prognosis, and to provide better quality of life by reducing the insecurity that tends to accompany unwarranted delays.
Alarm symptoms of cancer and the related practice guidelines [17] are the primary focus of both the Danish and the British pathways [18, 19]. This approach may result in shorter diagnostic intervals [20] for patients with specific alarm symptoms. However, only approx. 40 % of all cancer patients seem to have benefitted from the implementation of the CPPs based on alarm symptoms as demonstrated by British and Danish studies [21, 22]. This is due to the fact that only half of cancer patients initially present symptoms classified as alarm symptoms by the GP [8, 21], findings from the UK indicate similar figures [20]. As a consequence of these findings, additional CPPs were implemented in Denmark in 2011 for patients with serious non-specific symptoms and signs of cancer (NSSC-CPP) [23]. These provided the Danish GPs with the opportunity to refer patients with serious non-specific symptoms for further diagnostic workup if cancer is suspected although no alarm symptoms (qualifying for specific CPP routes) are present [24]. However, the consequences of this urgent referral modality are not known at present. In particular, more information is needed on i) which patients are referred, ii) which factors constitute the basis of the referral and iii) whether or not the investigated patients have cancer.
This paper aims to describe the characteristics of patients referred from general practice to the Danish NSSC-CPP and to estimate the probability and distribution of cancers in this population.
Methods
We performed a cross-sectional study including all patients aged 18 years or more who were referred to the NSSC-CPP at the hospitals in Aarhus or Silkeborg in the Central Denmark Region between 7 March 2012 and 27 March 2013. All identified patients were followed up for six months for the diagnosis of cancer.
Setting and NSSC-CPP organisation
All Danish residents are entitled to tax-financed public health-care benefits with free access to health care. More than 98 % of Danish citizens are registered with a specific general practice. The GPs act as gatekeepers to the rest of the health-care system, except for emergencies [25]. During one year, 85 % of the Danish population is in contact with general practice.
All patients referred from their GP to the NSSC-CPP underwent a filter function comprising three components: a battery of blood tests, a urine test and diagnostic imaging. The diagnostic imaging consisted of an abdominal ultrasound and a chest X-ray performed at Silkeborg hospital and a CT scan (with contrast) of chest, abdomen and pelvis performed at Aarhus University Hospital. The results of the diagnostic imaging were first assessed by a radiologist, and the GP subsequently interpreted all test results in combination and decided on further diagnostic steps to be taken. Such steps could be either watchful waiting or referral to a diagnostic centre for further investigations. If a specific disease or type of cancer was suspected, further steps could also involve referral to a medical specialist or another cancer-specific CPP (Fig. 1).
A diagnostic centre is a medical unit with comprehensive facilities for diagnostic investigation, including easy access to expertise in a wide range of relevant medical specialties (e.g. oncology, gynaecology, gastroenterological surgery, orthopaedics and radiology). NSSC-CPP patients referred to a diagnostic centre must undergo further investigations on the basis of presented symptoms and clinical findings (e.g. blood tests, diagnostic imaging, endoscopies and biopsies). Based on the findings, the patient is either referred to a CPP for a specific cancer, to a specific hospital department or back to the GP.
The Danish medical services are divided into five regions, and each of these regions must have at least one diagnostic centre. Approx. 15 centres have so far been established in Denmark.
Identification of patients
All patients who underwent the filter function were identified and included. In the Silkeborg catchment area, eligible patients were identified by a digital marker on the battery of blood tests. At the hospital in Aarhus, all patients receiving CT scans as part of the filter function were identified with a particular code.
The unique civil registration number (CRN), which is assigned to all Danish citizens, links the medical records at the personal level across the Danish national registries [26]. Newly identified patients were extracted every two weeks, and we linked these data to the Health Service Registry (HSR) in the Central Denmark Region to identify the GP of each of the included patients.
Some referrals to the NSSC-CPP were made from hospital departments. To ensure inclusion of only relevant patients, we sent a letter to the GPs of the patients who were referred from the hospital to clarify whether the GP had been involved in the referral of this particular patient.
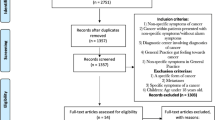
In total, 1899 referrals (1837 unique patients) were identified. We decided to consider two referrals of the same patient as two separate events if six or more months had passed between the referrals.
A total of 167 (8.0 %) referrals were excluded for the following reasons: same patient referred within six months (51 referrals), patient under 18 years (eight referrals), cancer within one year prior to current referral (41 referrals), recurrence of known cancer (15 referrals), questionnaire rejected and returned by the GP for various reasons, e.g. retirement of the referring GP (52 referrals). In total, 1732 referrals were included in the study (Fig. 2).
Data collection
A pilot-tested paper questionnaire was sent to the GP of the identified patient no more than two weeks after inclusion of the patient in the study. This procedure was followed for all included patients. Non-respondents received a reminder after three weeks. In general practices with more than one GP, we asked the GP who was most familiar with the patient to complete the questionnaire. Participating GPs were remunerated for each completed questionnaire (DKK 121 corresponding to approx. EUR 16).
The GPs provided information regarding the patient’s symptoms, known chronic diseases and estimated risk of cancer at referral in addition to clinical findings, abnormal diagnostic test results and level of the GP’s ‘gut feeling’ (understood as clinical intuition) regarding possible serious disease. Furthermore, the date of the first symptom presentation to the GP/practice was reported.
Symptoms were defined as presence or absence of 21 specified symptoms at the time of referral, with the option to add other symptoms that were not listed. As far as possible, all symptoms were classified according to the International Classification of Primary Care, second edition (ICPC-2) [27]. Clinical findings were defined as the GP’s abnormal findings during the clinical examination of the patient. Diagnostic test results were defined as diagnostic tests that were considered abnormal and highly relevant for the overall pathological picture at the time of referral. In accordance with Stolper’s work, we define gut feeling as ‘a physician’s intuitive feeling that something is wrong with the patient, although there are no apparent clinical indications for this, or a physician’s intuitive feeling that the strategy used in relation to the patient is correct, although there is uncertainty about the diagnosis’ [28].
In line with the Aarhus Statement [13], the primary care interval was defined as the time from the patient’s first symptom presentation at the GP/practice until referral to the NSSC-CPP. To ensure accurate data, we used the registered inclusion date as the referral date, i.e. the electronically registered date at which the filter function had been ordered.
Data regarding each patient’s cancer diagnosis were retrieved from the Danish Cancer Registry (DCR) [29–31]. These data were available only for the period until 31 December 2012. Cancer diagnoses made after this date were retrieved from the National Patient Registry (NPR) until six months after the date for inclusion of the last patient. The identification of incident cancers from the NPR has proven to be reliable as 95 % of the cancer diagnoses are displayed after four months and with high validity [32]. The date of diagnosis in the NPR was defined as the first date of the hospital admission at which the cancer diagnosis was confirmed in the DCR. If the patient was diagnosed with ICD-10 codes C760–C800 (i.e. malignant neoplasm’s of ill-defined, other secondary and unspecified sites), we searched and replaced this code with a more cancer-specific diagnostic code if the diagnosis had been made no more than two months after the date at which the cancer incidence had first been registered.
Data collection regarding referral for further examination at the diagnostic centre at the hospital in Aarhus did not start until 1 August 2012. Thus, the data collection for the data shown in Table 4 started nearly five months later than the data collection from the hospital in Silkeborg.
Statistical analyses
We used chi-square (χ2) test and Wilcoxon rank-sum test to identify differences between participating and non-participating GPs, to examine variations in the primary care interval between patients with and without cancer and to calculate the prevalence ratio (PR) in Table 5. The primary care intervals are presented as medians as well as 75 and 90 percentiles.
Cancer probability is presented as the percentage of included patients who were diagnosed with a new cancer within six months after the referral date. Associations between different patient characteristics and subsequent cancer diagnosis were estimated with prevalence rate ratios (PRRs) from a generalised linear model, both unadjusted and adjusted for age and gender, including 95 % confidence intervals (95 % CIs).
The statistical significance level was 0.05 or less. No alterations were made regarding missing data on presence or no presence of cancer. Stata statistical software v. 11 was used for the analyses.
Ethics and approval
The study was approved by the Danish Data Protection Agency (j.no: 2011-41-6118) and the Danish Health and Medicines Authority (j.no: 7-604-04-2/301). This study needed no approval from the Danish National Committee on Health Research Ethics.
Results
Study population
A total of 1278 completed GP questionnaires (73.8 %) were returned and included in the analyses (Fig. 2). Five patients were included twice. No significant differences were found between referrals from participating GPs and non-participating GPs concerning hospital distribution, gender, age or probability of cancer diagnoses (Table 1).
Patient characteristics
The mean age of patients included in the analyses was 65.9 years (sd: 14.7, range: 18–99), and 47.5 % were men. The most frequent chronic diseases at referral were hypertension, chronic lung disease and diabetes (Table 1).
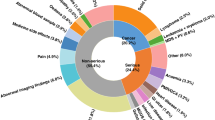
A total of 82 different symptoms and 51 clinical findings were identified from the GP questionnaires (data not shown). The median number of symptoms was 3.0. Non-specific symptoms were the most predominant of all registered symptoms; weight loss and fatigue were both present in more than half of all referrals (Table 2). Symptoms associated with the highest probability of cancer were jaundice (42.9 %), dysphagia (36.7 %), neurological dysfunction (35.3 %) and lump/tumour (26.9 %) (Table 2).
The three most common clinical findings were affected general condition (35.8 %), the GPs’ gut feeling (22.5 %) and abdominal findings (13.0 %). The highest probability of cancer was found for enlarged lymph nodes (27.3 %), neurological findings (26.7 %), the GPs’ gut feeling (24.0 %) and abdominal findings (21.1 %) (Table 2).
Abnormal diagnostic test results were primarily related to blood samples and diagnostic imaging, and no single diagnostic test result was associated with a particularly high probability of cancer.
Cancer and primary care interval
After six months, 16.2 % of all patients had a cancer diagnosis. The most common cancer types were lung cancer (17.9 %), colorectal cancer (12.6 %), hematopoietic tissue cancer (10.1 %) and pancreatic cancer (9.2 %) (Table 3). In comparison, the most common cancer types in Denmark in general for men are prostate cancer, lung cancer, colon cancer and urinary tract cancer, while the most common types for women are breast cancer, lung cancer, colon cancer and malignant melanoma.
The median primary care interval for patients diagnosed with cancer was 15 days; the 75 and 90 percentiles were 72 days and 130 days, respectively. Breast, liver and biliary cancer patients seemed to have shorter than average primary care intervals, while patients with metastases or cancer of the prostate, hematopoietic tissue, oesophagus, stomach or small intestine seemed to have longer primary care intervals than all other patients (Table 3). However, the study population was too small to provide any statistical precision for these estimates.
Men generally had a significantly higher probability of cancer than women when referred (adjusted PRR = 1.32 (95 % CI: 1.03-1.70)) (Table 4).
A more detailed overview of symptoms and clinical findings found to be highly predictive of cancer is presented in Additional file 1.
Cancer probability in different referral groups
Referred patients with five symptoms had a significantly higher probability of having cancer than patients referred with only one symptom (adjusted PRR = 1.68 (95 % CI: 1.06-2.65)) (Table 4). The presence of one or more clinical and/or diagnostic test results implied a significantly higher probability of finding cancer (Table 4).
Patients from Aarhus constituted 44.8 % of the referrals. These patients had a significantly higher probability of cancer than the patients referred to the hospital in Silkeborg (although not in the adjusted analysis) (Table 4).
In total, 59.0 % of the patients from Silkeborg were referred to further examination at the diagnostic centre compared to 18.8 % of the patients from Aarhus. A higher probability of cancer was found among patients who had not been referred to further examination compared to patients who had been referred. However, this difference was only statistically significant in the group of patients from Silkeborg (Silkeborg: adjusted PRR = 1.62 (95 % CI: 1.05-2.50); Aarhus: adjusted PRR = 1.22 (95 % CI: 0.62-2.41)).
The number of chronic diseases and the length of the primary care interval showed no significant associations with the probability of cancer (Table 4).
A strong association was found between the GP’s assessments of estimated cancer risk at referral and the probability of finding cancer (Table 4).
The GPs’ estimations were generally higher than the actual probability of cancer. The probability of cancer was higher if the GP had reported ‘strong’ or ‘very strong’ compared to ‘no’ gut feeling. Furthermore, GP gut feeling showed an association with the four most common clinical findings (weight loss, fatigue, affected general condition and abnormal blood sample) for patients diagnosed with cancer (Prevalence ratio: 1.50 (95 % CI: 0.82-2.75)) (Table 5).
Discussion
Main findings
NSSC-CPP referred patients were a heterogeneous group with over 80 different symptoms, 51 different clinical findings and wide variations in number of symptoms per referral. The most frequent symptoms were non-specific and vague symptoms, which are also very frequent reasons for consultations in general practice [33]. The term ‘non-specific symptom’ is used as opposed to specific alarm symptoms as non-specific symptoms are not necessarily indicative of a specific cancer type, but may suggest several cancers or other diseases. Only a few symptoms were highly predictive of cancer; most of these were rare (<2 % of patients), except for lump/tumour which was present in almost 9 % of the patients. The GP’s estimation of the patient’s risk of cancer at referral showed an expected correlation with the actual probability of cancer. However, it should be noted that the GP’s estimated risk was almost twice the size of the actual probability of cancer.
The overall probability of cancer was 16 %. Cancer was found more often in men than in women, which might be explained by the fact that breast cancer often presents with an alarm symptom [34]. In addition, referred men tended to have a higher probability of cancer than referred women [35, 36].
Affected general condition was the most common clinical finding and the GP’s gut feeling was another important clinical finding, which also showed a high probability of cancer (24.0 %). As seen in Table 4, little influence of gut feeling was less predictive of cancer than no influence, which may be because some patients have clear symptoms where gut feeling has minor importance. Nonetheless, an association was found between the most common findings and gut feeling, as shown in Table 5. These findings indicate that more research is needed to further explore the role of gut feeling in early diagnosis of serious disease. Our study did not allow identification of the specific components of this gut feeling, but it seems to embrace several clinical aspects that in combination increase the patient’s probability of cancer.
The primary care interval for all cancer patients diagnosed in this study was markedly longer than the interval found in previous studies [37, 38]. The long primary care trajectory before referral underlines the complexity of diagnosing these patients, but also stresses the need for quick and easy access to diagnostic investigations [39], including earlier referral by the GP despite non-specific symptoms.
The higher probability of cancer among patients not referred to further examination at a diagnostic centre may be explained by the separation of patients with specific cancer findings through the filter function; these patients are referred to specific CPPs or other pathways and not to the diagnostic centre. This indicates that the filter function prior to the referral to the diagnostic centre is useful. However, some patients who were terminated by the GP without further examination (watchful waiting) may actually have had a cancer or another serious disease. The present study did not gain insight into this issue, and further research in this area is needed.
The lower percentage (18.8 %) of referrals from the hospital in Aarhus to further examination at the diagnostic centre might partly be explained by the use of an initial CT scan, which may be more effective as a diagnostic instrument and thus may reduce the need for referral to further diagnostic workup. However, it could also be false assurance as no difference was found in the proportions of cancer between non-referred patients and patients referred to the diagnostic centre in Aarhus. Furthermore, the NSSC-CPP at the hospital in Silkeborg had been implemented several years before the NSSC-CPP in Aarhus. This difference may also have affected the number of GPs who chose to refer to the diagnostic centre.
Strengths and weaknesses of the study
A major strength of this study is the prospective design, which allowed us to include all patients referred to the NSSC-CPP and not only already diagnosed cancer patients. Although we included patients prospectively, the questionnaires were sent out retrospectively, and this may have introduced recall bias. To minimise recall bias, we posted our questionnaire to the GP no more than two weeks after inclusion of the patient, and the diagnostic workup for many patients had not been finished by the time the GP received the questionnaire. This also minimized possible information bias as the GPs did not know the results of the referral for many of the patients. To further minimize recall bias, we encouraged the GPs to consult their electronic medical records when filling in the questionnaire. Nevertheless, recall bias might be more pronounced for patients referred through a hospital department as the GPs referred the patients to a hospital department before the patients were referred to the NSSC-CPP by the hospital. Further data on this potential recall bias were not available. Lack of complete information in some questionnaires might have introduced information bias, but this is unlikely to have influenced the estimated probability of cancer or the reported clinical findings.
The register data are considered precise and valid as the cancer information in the DCR was registered prospectively. The DCR has an almost complete registration of all Danish cancer data and has been shown to be accurate [29]. We used the NPR to identify cancer patients diagnosed in 2013, and this method of identifying cancer patients has been reported to have an accuracy of 95 % after four months [32]. The introduced misclassification is considered to be non-differential.
The GP response rate is comparable to similar studies using GP questionnaires [34, 37] and must be considered high, which limits potential selection bias. Still, non-responding GPs may have had patients with special characteristics although a non-response analysis revealed no differences between patients of participating GPs and patients of non-participating GPs.
Although ’gut feeling’ is a well-known and common phenomenon among GPs [28], this notion may have introduced a problem regarding the construct validity as it is uncertain whether GPs regard ‘gut feeling’ in the same way. Furthermore, ‘gut feeling’ can be difficult to separate from e.g. the GP’s estimation of the patient’s risk of cancer in this study design. The association between gut feeling and the four most common findings indicates that gut feeling is often seen in combination with other findings. Further sub analysis showed that no symptoms, clinical findings or abnormal diagnostic test results were stated in the medical records for only 11 of the patients; none of these patients were registered with a GP gut feeling. Furthermore, the fact that the probability of cancer appeared higher with no gut feeling (compared to little gut feeling) indicates that presence of clear signs of cancer does not generally prompt activation of gut feeling. Our results warrant further studies into the importance of ‘gut feeling’ in early detection of cancer.
Comparison with other studies
Bosch et al. [40] published a paper on referrals from GPs to a quick diagnostic unit (QDU) similar to the one described in this paper, but their aim was different from ours. The study showed that 30 % of the patients referred directly to the QDU had cancer compared to the 16 % found in our study. Data from the UK have shown that 11 % of the patients referred to the ordinary urgent referral pathways were diagnosed with cancer [22]. Apart from the study by Bosch et al. [40], we are unaware of any published studies examining and quantifying GP referrals to NSSC-CPPs and related outcomes.
An earlier study confirmed that action should be taken when the GP suspects serious disease as these patients have a high risk of a new diagnosis of cancer or another serious disease within 2 months [41]. Furthermore, Hamilton has also highlighted the importance of the GP’s suspicion [6]. Our study adds to this evidence within primary care diagnostics.
Jensen et al. [21] documented that only 40 % of the Danish cancer patients were referred to a ‘cancer specific’ CPP. This finding stresses the importance of providing the GPs with diagnostic tools like the NSSC-CPP as well as direct access to diagnostic investigations [39, 42, 43].
Conclusions
This study documents that 16.2 % of all patients referred through the Danish NSSC-CPP because of non-specific serious symptoms had cancer. Patients referred to the NSSC-CPP were a heterogeneous group with many different symptoms and clinical findings. The GP’s gut feeling was a common clinical finding which was a strong predictor of cancer. Likewise, the GP’s assessment of the patient’s risk of cancer at referral was also strongly associated with the actual probability of finding cancer.
References
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7):26–9.
Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–38.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5-a population-based stud. Lancet Oncol. 2014;15(1):23–34.
Torring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. Eur J Cancer. 2013;49(9):2187–98.
Torring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65(6):669–78.
Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. 2009;59(563):441–5. 447; discussion 446.
Starfield B. Is primary care essential? Lancet. 1994;344:1129–33.
Nielsen TN, Hansen RP, Vedsted P. Symptom presentation in cancer patients in general practice. Ugeskr Laeger. 2010;172(41):2827–31.
Hansen RP. Delay in the diagnosis of cancer PhD thesis. Aarhus: Faculty of Health Sciences, University of Aarhus; 2008.
Allgar VL, Neal RD. General practictioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients-Cancer. Eur J Cancer Care (Engl). 2005;14(5):409–16.
Christensen KG, Fenger-Gron M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis-a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12(1):224.
Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101:S5–8.
Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):126210–1267.
The National Board oH. National Cancer Plan II Denmark National Board of Health recommendations for improving cancer healthcare services. Copenhagen: The National Board of Health; 2005.
Department oH. The NHS Cancer Plan. A plan for investment, A plan for reform. London: Department of Health; 2000.
Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians-A national Danish project. Health Policy. 2012;105(1):65–70.
Mulka O. NICE suspected cancer guidelines. Br J Gen Pract. 2005;55(517):580–1.
Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians-A national Danish project. Health Policy. 2012;105(1):65–70.
Jones R, Rubin G, Hungin P. Is the two week rule for cancer referrals working? BMJ. 2001;322(7302):1555–6.
Neal RD, Din NU, Hamilton W, Ukoumunne OC, Carter B, Stapley S, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110(3):584–92.
Jensen H, Torring ML, Olesen F, Overgaard J, Vedsted P. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14(1):636–2407. 14-636.
Meechan D, Gildea C, Hollingworth L, Richards MA, Riley D, Rubin G. Variation in use of the 2-week referral pathway for suspected cancer: A cross-sectional analysis. Br J Gen Pract. 2012;62(602):590–7.
Vedsted P, Olesen F. A differentiated approach to referrals from general practice to support early cancer diagnosis - the Danish three-legged strategy. Br J Cancer. 2015;112(Suppl):S65–9.
Hamilton W. The CAPER studies: five case–control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101 Suppl 2:S80–6. S80-S86.
Pedersen KM, Andersen JS, Sondergaard J. General practice and primary health care in Denmark. J Am Board Fam Med. 2012;25 Suppl 1:S34–8.
Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7):22–5.
Soler JK, Okkes I, Wood M, Lamberts H. The coming of age of ICPC: celebrating the 21st birthday of the International Classification of Primary Care. Fam Pract. 2008;25(4):312–7.
Stolper E, Van RP, Dinant GJ. The ‘sense of alarm’ (‘gut feeling’) in clinical practice. A survey among European general practitioners on recognition and expression. Eur J Gen Pract. 2010;16(2):72–4.
Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–5.
Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry-history, content, quality and use. Dan Med Bull. 1997;44:535–9.
Danish National Board of Health. The Cancer Registry 2009 in Danish. 2010. p. 1–55.
Larsen MB, Jensen H, Hansen RP, Olesen F, Vedsted P. Identification of patients with incident cancers using administrative registry data. Dan Med J. 2014;61(2):A4777.
Moth G, Vedsted P, Olesen F. Kontakt- og sygdomsmønsteret i almen praksis. KOS 2008. Aarhus: Forskningsenheden for Almen Praksis i Aarhus, Aarhus Universitet; 2010.
Jensen H, Torring ML, Larsen MB, Vedsted P. Existing data sources for clinical epidemiology: Danish Cancer in Primary Care cohort. Clin Epidemiol. 2014;6:237–46.
Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174–82.
Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27(3):187–96.
Hansen RP, Vedsted P, Sokolowski I, Sondergaard J, Olesen F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11(1):284.
Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(3):686–90.
Guldbrandt LM, Fenger-Gron M, Folkersen BH, Rasmussen TR, Vedsted P. Reduced specialist time with direct computed tomography for suspected lung cancer in primary care. Dan Med J. 2013;60(12):A4738.
Bosch X, Escoda O, Nicolas D, Coloma E, Fernandez S, Coca A, et al. Primary care referrals of patients with potentially serious diseases to the emergency department or a quick diagnosis unit: a cross-sectional retrospective study. BMC Fam Pract. 2014;15(1):75.
Hjertholm P, Moth G, Ingeman ML, Vedsted P. Predictive values of GPs’ suspicion of serious disease: a population-based follow-up study. Br J Gen Pract. 2014;64(623):e346–53.
Rubin G, Vedsted P, Emery J. Improving cancer outcomes: Better access to diagnostics in primary care could be critical. 2011. p. 317–8.
Baughan P, Keatings J, O’Neill B. Urgent suspected cancer referrals from general practice: audit of compliance with guidelines and referral outcomes. Br J Gen Pract. 2011;61(592):700–6.
Acknowledgements
We thank the contributing GPs for their time and effort with completing the questionnaire. We also thank the personnel at the hospitals in Aarhus and Silkeborg for providing the data used to include the relevant patients for this study. Data manager Kaare Rud Flarup is also acknowledged for his substantial assistance with the data retrieval from the Danish national registries.
The project was supported by the Committee for Quality Improvement and Continuing Medical Education (KEU) of the Central Denmark Region, the Danish Cancer Society and the Novo Nordisk Foundation. Sponsoring organizations were not involved in any part of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MLI participated in the design of the study, drafted the GP questionnaire, performed the data analysis and drafted the manuscript. PV conceived the study, contributed to the drafting of the GP questionnaire, the data analysis and the interpretation of results as well as the revision of the manuscript. MBC and FB contributed to the design of the study, the GP questionnaire and the revision of the manuscript. STK contributed to the design of the study, the data collection at the hospital in Aarhus and the revision of the manuscript. All authors read and approved the final manuscript.
Additional file
Below is the link to the electronic supplementary material.
Additional file 1:
Symptoms and abnormal clinical findings highly predictive of cancer.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ingeman, M.L., Christensen, M.B., Bro, F. et al. The Danish cancer pathway for patients with serious non-specific symptoms and signs of cancer–a cross-sectional study of patient characteristics and cancer probability. BMC Cancer 15, 421 (2015). https://doi.org/10.1186/s12885-015-1424-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1424-5