Abstract
Objectives
To assess the value of intraovarian PRP in women with low ovarian reserve.
Search strategy
Screening of databases from inception to January 2023 using the keywords related to “Platelet-rich plasma” AND “poor ovarian reserve” OR “ovarian failure”.
Selection criteria
Fourteen studies (1632 participants) were included, 10 included women with POR, 1 included women with POI and 3 included both POR and POI women.
Data collection and analysis
Extracted data included study settings, design, sample size, population characteristics, volume, timing and preparation of PRP administration, and outcome parameters.
Main results
AMH level was evaluated in 11 studies (2099 women). The mean difference (MD) was 0.09 with 95% CI of – 0.06, 0.24 (P = 0.25). Antral follicular count level was assessed in 6 studies (1399 women). The MD was 1.73 with 95% CI of 0.81, 2.66 (P < 0.001). The number of oocytes retrieved was evaluated in 7 studies (1413 women). The MD was 1.21 with 95% CI of 0.48, 1.94 (P = 0.001).
Conclusion
This systematic review found a significant improvement of AFC, the number of retrieved oocytes, the number of cleavage embryos and the cancellation rate in women with POR.
Trial registration
Registration number CRD42022365682.
Similar content being viewed by others
Synopsis
Intraovarian PRP injection improved AFC, the number of retrieved oocytes, the number of cleavage embryos and the cancellation rate in women with POR.
Introduction
The ovary is considered as the biological clock that control the aging process in the female [1].
Ovarian aging is defined as gradual decrease in oocyte quality and quantity and eventful ovarian function exhaustion [2].
Two types of ovarian aging are known. Physiological aging is the natural deterioration of ovarian function with age that end in menopause, while pathological aging is the premature diminishment of ovarian function as a result of certain pathogenic factors [3].
Pathological ovarian aging includes premature ovarian insufficiency (POI), diminished ovarian reserve (DOR) and poor ovarian response (POR) for controlled ovarian hyperstimulation (COH) [4].
POR is not uncommonly encountered during COH. Its prevalence is between 5 and 35% of women with subfertility. It is defined as failure of the ovary to respond adequately to standard ovarian induction protocols and production of adequate ova. It is one of the rate limiting steps in success of IVF that is characterized by low or even failure of oocyte retrieval, higher rates of cycle cancelation and the lower probability of pregnancy [5].
Many interventions have been suggested to improve the outcome of COH in POR. These include pretreatment with aromatase inhibitors, human chorionic gonadotropin or androgens [6]; adjuvant treatment with estrogen agonists, luteinizing hormones [7]; starting with the maximum dose of gonadotropin [8]; or the use of alternative protocols as microdose flare up [9], short flare up, agonist stop [10], antagonist (standard or delayed start) [11] or luteal phase support using follicle stimulating hormone [12].
Currently, there is no definitive treatment to reestablish normal ovarian function in women with POI [13].
But there are treatments for associated symptoms, in addition to treatments for reduction of associated risks. These include hormonal therapy, calcium and vitamin D supplementation, regular physical activity, keeping healthy body weight and emotional support [14].
Platelet-rich-plasma (PRP) is prepared from fresh whole blood through its centrifugation. The resultant precipitate is free from both red and white blood cells and rich in cytokines and growth factors as VEGF, TGFβ and PDGF that are released from α-granules of activated platelets [15].
Due to its high regenerative and anti-inflammatory properties, PRP is used in numerous medical fields, including orthopedics and ophthalmology [16].
PRP was first used to improve refractory thin endometrium in IVF [17].
It is currently studied in women with implantation failure, intrauterine synechia and POI. However, the results of its use showed contradictory findings [18].
PRP is a novel technique used in gynecology. The results of its use for improving and restoring ovarian function are conflicting among different studies. There is no sufficient data to support or decline its use. This raises the need for a properly conducted meta-analysis to guide its use in women with inadequate ovarian response.
This systematic review and meta-analysis aimed to assess the effects of intraovarian PRP injection in women with POI and poor ovarian response.
Material and Methods
The study protocol was prepared based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis. The protocol was prospectively registered at PROSPERO with CRD42022365682 number.
Eligibility criteria, information sources, search strategy
Two authors (AM, WSR) independently searched Medline, Embase, Web of Science, Scopus, the Cochrane Central Register of Controlled Trials electronic databases from inception to January 2023 using the keywords “Platelet-rich plasma” OR “PRP” OR “Autologous platelet-rich plasma”) AND “premature ovarian failure” OR “decreased ovarian reserve” OR “premature menopause” OR “premature ovarian insufficiency” and their MeSH terms (Supplementary Table S1). Direct contact with authors via email was done to provide any clarifications or additional data.
Study selection
All published and unpublished studies without language limitations (whether published in English or other languages) that involved intraovarian PRP injection in women with inadequate ovarian response or ovarian insufficiency were searched for. This systematic review included all prospective and retrospective studies, whether quasiexperimental, case control or comparative pilot ones, that involved the intraovarian PRP injection in women with POI and / or POR. Subgroup analysis for quasi-experimental, retrospective and case control studies were done. Both transvaginal and laparoscopic injection routes were also included. Non-human,invitro (cell culture) studies, case reports and studies with non-clearly reported outcomes or non-clear methodology (and cannot be clarified by author correspondence) were excluded from the analysis.
Data extraction
Two authors (AM and AO) independently assessed the titles and abstracts of all search results, then assessed the full articles of the related trials. Any disagreement between the 2 authors for inclusion or data extraction was discussed with other coauthors. Extracted data included study settings, design, participants’ characteristics and number, PRP preparation method, intervention time and technique, outcome parameters, trial registration and funding details. Contacting the authors to clarify any unclear data via email was done.
Outcome parameters included serum AMH, basal FSH, basal E2, antral follicular count, spontaneous pregnancy rate, number of oocytes retrieved, number of cleavage and good quality embryos, fertilization, cancelation, clinical pregnancy, chemical pregnancy and live birth rates.
Assessment of risk of bias
The Newcastle–Ottawa scale (NOS) [19] quality assessment of Non-randomized studies was done. The NOS star system uses 3 main assessments: the selection of the exposed and non-exposed groups; the comparability of the groups (before and after assessment or cases and control); and the ascertainment of both exposure and outcome (proper follow up). Absent and unclear data were requested through authors contact.
The GRADE system was used to assess the quality of evidence [19]. GRADE included risk of bias in the included studies, inconsistency, indirectness, imprecision, and publication bias. Serious concerns in each item decrease the evidence by 1 level while very serious ones decrease the evidence by 2 levels.
The levels were classified as high, moderate, low or very low according to the presence of strong, moderate, low or very low evidence that the true effect is close to the effect estimate, respectively.
Data synthesis
The mean difference with the corresponding 95% CI was calculated for continuous data. No meta-analysis was done for dichotomous data as a result of marked heterogeneity of the outcome parameters. The effect size was obtained using the random effect model through the Mantel-Hansel method.
The I2 statistic and Cochran’s Q test were used to assess the heterogeneity of the included studies. A P-value of < 0.05 in the Q-test or I2 > 40% is considered as significant. The Review Manager (RevMan) version 5.4.1 (The Nordic Cochrane Centre, Cochrane Collaboration, 2020, Copenhagen, Denmark) was used for all statistical analysis.
Results
Study selection
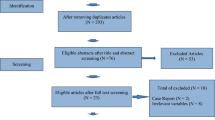
Our search yielded 1885 studies through databases (505 from PubMed, 113 from Embase, 624 from Scopus, 84 from Web of Science, and 559 from clinical trials), 972 of them were screened after removal of duplicates, 29 screened for full text, 14 studies were included in quantitative and qualitative synthesis (Fig. 1).
Study characteristics
Table 1 summarized the main characteristics of the included studies.
Fourteen studies (1632 participants) were included in our analysis, 10 studies included women with POR [21, 23,24,25,26,27,28, 30,31,32], 1 study included women with POI [22] and 3 studies included both POR and POI women [20, 29, 33]. Among the included studies, 9 were Quasi-experimental [20,21,22,23, 26, 28,29,30, 32], 3 were retrospective [24, 27, 33] and 2 were case control studies [25, 31].
All the studies were conducted at a single center except Navali et al. [26]; Pacu et al. [27] that were conducted in 2 centers. Three studies were conducted in Iran [20, 24, 26], 3 in Turkey [22, 23, 33], 2 in USA [21, 30] and 1 study was conducted in each of the following countries, Greece [29], India [32], Macedonia [31], Romania [27], Ukraine [28] and Venezuela [25].
PRP volume injected was 0.2 ml in one study [25], 1 ml in 1 study [30], 2—4 ml in 9 studies, and 4–8 ml in 4 studies [21,22,23, 32]. The timing of PRP injection was random in all amenorrheic women and those with POI. In women without amenorrhea, PRP injection was done in day 1 -10 in 2 studies [22, 23], day 3–5 in 3 studies [21, 27, 29], day 7, 8 or 9 in one study [25], day 10 in one study [20], at time of follicular rupture in 1 study [24], at time of ovum pickup in 1 study [26] and not determined in 4 studies [28, 30,31,32]. The route in all studies was ultrasound guided transvaginal injection except in those with non-accessible ovarian who underwent laparoscopic injection.
Risk of bias of included studies
Newcastle–Ottawa Scale was used to evaluate quality of the included studies (Table 2) and GRADE quality of evidence was separately done for each individual outcome criteria (Table 3).
Synthesis of results
Anti-Mullerian hormone (AMH) level was evaluated in 11 studies with 2099 POR women. The mean difference (MD) was 0.09 with 95% CI of – 0.06, 0.24 (P = 0.25). Subgroup analysis according to type of the involved studies revealed that AMH was reported in 7 Quasi-experimental studies (1744 women) with MD of 0.10 and 95% CI of [0.04, 0.16] (P < 0.001), 2 retrospective studies (232 women) with MD of 0.02 and 95% CI of [-0.15, 0.18] (P = 0.84) and 2 case control studies (123 women) with MD of 0.09 and 95% CI of [-0.80, 0.98] (P = 0.85) (Fig. 2).
Basal Follicle stimulating hormone (FSH) level was assessed in 9 studies with 1880 POR women. The mean difference (MD) was 1.56 with 95% CI of – 1.53, 4.64 (P = 0.32). Subgroup analysis according to type of the involved studies revealed that FSH was reported in 6 Quasi-experimental studies (1708 women) with MD of 3.39 and 95% CI of [-0.72, 7.49] (P = 0.11), 1 retrospective study (40 women) with MD of -0.22 and 95% CI of [-2.49, 2.05] (P = 0.85) and 2 case control studies (132 women) with MD of -3.02 and 95% CI of [-8.86, 2.82] (P = 0.31) (Fig. 3).
Basal serum estradiol (E2) level was assessed in 4 studies with 598 POR women. The mean difference (MD) was -9.88 with 95% CI of – 26.18, 6.41(P = 0.23). Subgroup analysis according to type of the involved studies revealed that basal E2 was reported in 3 Quasi-experimental studies (558 women) with MD of -11.46 and 95% CI of [-29.76, 6.85] (P = 0.22), and 1 case control study (40 women) with MD of -1.48 and 95% CI of [-20.07, 17.11] (P = 0.88) (Fig. 4).
Antral follicular count (AFC) level was assessed in 6 studies with 1399 POR women. The mean difference (MD) was 1.73 with 95% CI of 0.81, 2.66 (P < 0.001). Subgroup analysis according to type of the involved studies revealed that AFC was reported in 4 Quasi-experimental studies (1276 women) with MD of 1.73 and 95% CI of [1.03, 2.43] (P < 0.001), 1 retrospective study (40 women) with MD of 0.40 and 95% CI of [-0.38, 1.18] (P = 0.31) and 1 case control study (83 women) with MD of 3.24 and 95% CI of [3.14, 3.34] (P < 0.001) (Fig. 5).
The number of oocytes retrieved was evaluated in 7 studies with 1413 POR women. The mean difference (MD) was 1.21 with 95% CI of 0.48, 1.94 (P = 0.001). Subgroup analysis according to type of the involved studies revealed that the number of oocytes retrieved was reported in 3 Quasi-experimental studies (1150 women) with MD of 1.50 and 95% CI of [1.16, 1.83] (P < 0.001), 2 retrospective studies (140 women) with MD of 0.87 and 95% CI of [0.48, 1.25] (P < 0.001)and 2 case control studies (123 women) with MD of 0.62 and 95% CI of [-4.13, 5.37] (P = 0.8) (Fig. 6).
The number of cleavage embryos was evaluated in 4 studies with 625 POR women. The mean difference (MD) was -1.16 with 95% CI of -1.76, -0.57 (P < 0.001) (Fig. 7).
The cancellation rate was evaluated in 3 studies with 234 POR women. The Odds Ratio (OR) was 0.36 with 95% CI of 0.21, 0.63 (P < 0.001) (Fig. 8).
In women with POI, AMH and basal FSH levels were reported in 2 studies with 78 women and revealed a MD of 0.23 and -1.76 with a 95%CI of -0.29, 0.75 and-2.53, -1.0 and P values of 0.39 and < 0.001 respectively.
The rate of spontaneous pregnancy in both women with POR and POI are reported in Table 4.
Table 4 summarized the number of good quality embryos, fertilization rate, clinical pregnancy rate, chemical pregnancy rate, and live birth rate in women with POR and those with POI. No meta-analysis was done for these outcomes as a result of marked heterogeneity and incomplete reporting (data were not completed after several emails to authors).
Discussion
Main findings
In our meta-analysis, we included 14 studies that evaluated the value of intraovarian injection of PRP in women with POR and POI.
Although there was an improvement of baseline hormones (AMH, FSH and E2) after intraovarian injection of PRP, this improvement failed to reach statistical significance (except the improvement of serum AMH analyzed in quasi-experimental studies).
This meta-analysis found a beneficial effect of intraovarian PRP injection on AFC (in quasi-experimental and case control studies), the number of retrieved oocytes (in quasi-experimental and retrospective studies), the number of cleavage embryos and the cancelation rate. These effects had moderate evidence regarding AFC, the number of oocyte retrieved and the number of cleavage embryos and low evidence regarding cancelation rate.
The effects of intraovarian PRP injection on clinical, chemical and live birth rate cannot be properly assessed as most of the included studies reported no data about these outcomes before PRP injection. However, the occurrence of spontaneous pregnancy, clinical pregnancy and live birth in women with POI reflects a significant change in these women.
The effects of PRP are linked to its high regenerative and anti-inflammatory properties. PRP was found to reduce inflammation, postoperative bleeding and infection. It also accelerates wound healing, osteogenesis and soft tissue healing [34].
The effect of PRP on AFC and the number of retrieved oocyte is more obvious than its effect on hormonal assessment. This may be explained by the physical recovery of the ovarian tissue that may precede its functional and hormonal recovery. Longer follow up may detect a functional recovery with improvement of the ovarian reserve hormone markers.
These tissue regenerative effects are linked to the growth factors contained in platelet granules. These growth factors include insulin-like growth factors, transforming growth factor-β, epidermal growth factor, and vascular endothelial growth factor [35].
These growth factors play important roles in cell migration, differentiation, and proliferation besides the activation of angiogenesis [36].
The inverse correlation between the concentration of growth hormone and growth factors with aging is documented in a previous study [37].
In a recent meta-analysis by Maged and colleagues in 2023, intrauterine and subendometrial injection of PRP were proved to improve the IVF cycle outcomes as implantation, clinical pregnancy, live birth rates and endometrial thickness in infertile women with previous implantation failure and those with refractory thin endometrium [19].
In rats with bilateral adnexal torsion, PRP injection was successful in prevention of ischemia and promotion of reperfusion through increase in growth factors, mainly VEGF [38].
Strengths and limitations
This meta-analysis is the first comprehensive one evaluating the effects of intraovarian PRP injection in women with POR and POI. Although intraovarian PRP injection is a recent procedure, this meta-analysis included 14 studies. These represent all the available trials reached by extensive independent searching of all available published and unpublished. A separate analysis was done for POR and another one for women with POI. Adequate subgroup analysis according to different study designs for all the available outcomes was done.
This meta-analysis is not without limitations. None of the included studies was RCT, so it carries a high risk of bias. Most of the studies did not report the clinically significant outcomes such as clinical pregnancy and live birth rates. Even the studies that reported these outcomes failed to compare them either to before intervention nor to controls. There is marked heterogeneity among the included studies regarding the study design, baseline hormonal levels, timing of PRP injection, the time for the outcomes assessment and reporting of outcomes. We used the random effect method for comparison to compensate for this heterogeneity. The data may be limited by the fact that some of the patients included have received concomitant other treatments.
Despite these limitations that were expected as this line of treatment is recently introduced in the field of infertility, the promising findings of our study encourage the conduction of a well-designed randomized control study with proper selection criteria and low risk of bias to confirm these results.
Comparison with existing literature
Although there are many systematic reviews conducted to assess the benefits of PRP in skin, eye and bone diseases, only a few studies were conducted on infertility. Only one systematic review studied intraovarian PRP in women with POR or ovarian Insufficiency [39].
However, this systematic review included only 4 studies. Most of them did not evaluate pregnancy characteristics as clinical pregnancy, chemical pregnancy, or live birth rates. They failed to conduct a meta-analysis of 4 studies with marked heterogeneity. Also, this review lacks any subgroup analysis.
Our meta-analysis suggests that intraovarian PRP injection could be tried in all women with POI and those with POR in whom other measurements to improve their ovarian response failed. PRP is relatively a safe procedure that improves the ovarian response and function. With progress in preparation of PRP and addition of other stimulatory, growth factors and stem cells, it can provide future hope for fertility in those women suffering from POI.
Conclusions
This systematic review found a non-significant improvement in ovarian hormones (AMH, basal FSH or basal E2) and a significant improvement of AFC, the number of retrieved oocytes, the number of cleavage embryos and the cancellation rate. However the quality of evidence of these findings was not high. A well designed RCT with adequate blinding, with properly selected inclusion criteria considering the level of ovarian reserve markers should be conducted to provide the needed evidence. Also setting an optimum level of different ovarian reserve markers to achieve the maximum benefits from intraovarian PRP injection is recommended.
Availability of data and materials
Data used and/or analised during the study are available from the corresponding author upon reasonable request.
References
Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, Henseb JD, et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech Ageing Dev. 2021;194:111425.
Wu J, Liu Y, Song Y, Wang L, Ai J, Li K. Aging conundrum: a perspective for ovarian aging. Front Endocrinol (Lausanne). 2022;19(13):952471. https://doi.org/10.3389/fendo.2022.952471. PMID:36060963;PMCID:PMC9437485.
Tesarik J, Galán-Lázaro M, Mendoza-Tesarik R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci. 2021;22(3):1371.
Yan F, Zhao Q, Li Y, et al. The role of oxidative stress in ovarian aging: a review. J Ovarian Res. 2022;15:100. https://doi.org/10.1186/s13048-022-01032-x.
Maged AM, Fahmy RM, Rashwan H, et al. Effect of body mass index on the outcome of IVF cycles among patients with poor ovarian response. Int J Gynaecol Obstet. 2019;144:161–6.
Schoolcraft W, Surrey E, Minjarez DA, Stevens JM, Gardner DK. Management of poor responders: can outcomes be improved with novel GnRH antagonist/letrozole protocol? Fertil Steril. 2009;89:151–6.
Barrenetxea G, Agirregoikoa JA, Jimenez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor responder women: a randomized controlled trial on the effect of LH supplementation on in vitro fertilization cycle. Fertil Steril. 2008;89:546–53.
Hofmann GE, Toner JP, Muasher SJ, Jones GS. High dose follicle stimulating hormone (FSH) ovarian stimulation in low responder patient for in vitro fertilization. J In Vitro Fert Embryo Transf. 1989;6:285–9.
Surrey ES, Bower J, Hill DM, Ramsey J, Surrey MW. Clinical and endocrine effects of a micro dose GnRH agonist flare regimen administered to poor responders who are undergoing in vitro fertilization. Fertil Steril. 1998;69:419–24.
Garcia-Valesco JA, Isaza V, Requena A, et al. High dose of gonadotropins combined with stop versus non-stop protocol of GnRH analogue administration in low responders IVF patients: a prospective, randomized controlled trial. Hum Reprod. 2000;15:2292–6.
Maged AM, Nada AM, Abohamila F, Hashem AT, Mostafa WA, Elzayat AR. Delayed start versus conventional GnRH antagonist protocol in poor responders pretreated with estradiol in luteal phase: a randomized controlled trial. Reprod Sci. 2015;22(12):1627–31. https://doi.org/10.1177/1933719115590666. Epub 2015 Jun 4 PMID: 26045549.
Kucuk T, Sozen E. Luteal start of exogenous FSH in poor responder women. J Assisst Reprod Genet. 2007;24:635–8.
National Library of Medicine. Premature ovarian failure. 2011. Retrieved January 4, 2012, from https://medlineplus.gov/primaryovarianinsufficiency.html.
National Institute of child health and human development. Primary ovarian insufficiency. 2022. Last Reviewed Date 1/4/2022 from https://www.nichd.nih.gov/health/topics/poi/conditioninfo/treatments.
Baba K, Yamazaki Y, Sone Y, Sugimoto Y, Moriyama K, Sugimoto T, et al. An in vitro long-term study of cryopreserved umbilical cord blood-derived platelet-rich plasma containing growth factors-PDGF-BB, TGF-β, and VEGF. J Craniomaxillofaci Surg. 2019;47(4):668–75.
Makrigiannakis A, Makrygiannakis F, Vrekoussis T. Approaches to improve endometrial receptivity in case of repeated implantation failures. Front cell Dev Biol. 2021;9:613277.
Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286–90.
Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol. 2020;137:103078.
Maged AM, El-Mazny A, Kamal N, Mahmoud SI, Fouad M, El-Nassery N, Kotb A, Ragab WS, Ogila AI, Metwally AA, Fahmy RM, Saad H, Shaeer EM, Salah N, Lasheen Y. The value of platelet rich plasma in women with previous implantation failure. A systematic review and meta-analysis. Journal of assisted reproduction and genetics. 2023. Online ahead of print.
Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Effects of intraovarian injection of autologous platelet-rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Reprod Sci. 2021;28(7):2050–9. https://doi.org/10.1007/s43032-021-00483-9. Epub 2021 Mar 8 PMID: 33683669.
Barad DH, Albertini DF, Molinari E, Gleicher N. Preliminary report of intraovarian injections of autologous platelet-rich plasma (PRP) in extremely poor prognosis patients with only oocyte donation as alternative: a prospective cohort study. Hum Reprod Open. 2022;2022(3):hoac027. https://doi.org/10.1093/hropen/hoac027. PMID: 35795849; PMCID: PMC9247703.
Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Tiras B, Seli E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020;12(11):10211–22. https://doi.org/10.18632/aging.103403. Epub 2020 Jun 5. PMID: 32507764; PMCID: PMC7346073.
Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, Scott RT, Tiras B, Seli E. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging (Albany NY). 2022;14(6):2513–23. https://doi.org/10.18632/aging.203972. Epub 2022 Mar 22. PMID: 35320118; PMCID: PMC9004561.
Farimani M, Nazari A, Mohammadi S, Anvari AR. Evaluation of intra-ovarian platelet-rich plasma administration on oocytes-dependent variables in patients with poor ovarian response: a retrospective study according to the POSEIDON criteria. Reprod Biol Endocrinol. 2021;19(1):137. https://doi.org/10.1186/s12958-021-00826-w. Erratum in: Reprod Biol Endocrinol. 2021 Nov 18;19(1):169. PMID: 34496887; PMCID: PMC8425058.
Melo P, Navarro C, Jones C, Coward K, Coleman L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet. 2020;37(4):855–63. https://doi.org/10.1007/s10815-020-01710-z. Epub 2020 Feb 7. PMID: 32030554; PMCID: PMC7183031.
Navali N, Sadeghi L, Farzadi L, Ghasemzadeh A, Hamdi K, Hakimi P, Niknafs B. Intraovarian injection of autologous platelet-rich plasma improves therapeutic approaches in the patients with poor ovarian response: a before-after study. Int J Fertil Steril. 2022;16(2):90–4. https://doi.org/10.22074/IJFS.2021.533576.1154. Epub 2022 May 8. PMID: 35639652; PMCID: PMC9108296.
Pacu I, Zygouropoulos N, Dimitriu M, Rosu G, Ionescu CA. Use of platelet-rich plasma in the treatment of infertility in poor responders in assisted human reproduction procedures. Exp Ther Med. 2021;22(6):1412. https://doi.org/10.3892/etm.2021.10848. Epub 2021 Oct 7. PMID: 34676005; PMCID: PMC8524761.
Petryk N, Petryk M. Ovarian rejuvenation through Platelet-Rich Autologous Plasma (PRP)-a chance to have a baby without donor eggs, improving the life quality of women suffering from early menopause without synthetic hormonal treatment. Reprod Sci. 2020;27(11):1975–82. https://doi.org/10.1007/s43032-020-00266-8. Epub 2020 Jul 22 PMID: 32700285.
Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G, Koutsilieris M, Pantos K. Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med. 2020;9(6):1809. https://doi.org/10.3390/jcm9061809. PMID: 32532000; PMCID: PMC7355907.
Sills JES. L, Rickers PNS, Wood SH, Li X. Regenerative effect of intraovarian injection of activated autologous platelet rich plasma: serum anti-mullerian hormone levels measured among poor-prognosis in vitro fertilization patients. Int J Regener Med. 2020:2613–5914. https://doi.org/10.31487/j.RGM.2020.01.02.
Stojkovska S, Dimitrov G, Stamenkovska N, Hadzi-Lega M, Petanovski Z. Live birth rates in poor responders’ group after previous treatment with autologous platelet-rich plasma and low dose ovarian stimulation compared with poor responders used only low dose ovarian stimulation before in vitro fertilization. Open Access Maced J Med Sci. 2019;7(19):3184–8. https://doi.org/10.3889/oamjms.2019.825. PMID: 31949513; PMCID: PMC6953937.
Tandulwadkar S, Karthick MS. Combined use of autologous bone marrow-derived stem cells and platelet-rich plasma for ovarian rejuvenation in poor responders. J Hum Reprod Sci. 2020;13(3):184–90. https://doi.org/10.4103/jhrs.JHRS_130_19. Epub 2020 Oct 27. PMID: 33311903; PMCID: PMC7727891.
Tülek F, Kahraman A. The effects of intra-ovarian autologous platelet rich plasma injection on IVF outcomes of poor responder women and women with premature ovarian insufficiency. J Turk Ger Gynecol Assoc. 2022;23(1):14–21. https://doi.org/10.4274/jtgga.galenos.2021.2021.0134. Epub 2021 Dec 6. PMID: 34866374; PMCID: PMC8907433.
Na JI, Choi JW, Choi HR, Jeong JB, Park KC, Youn SW, Huh CH. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet rich plasma. Dermatol Surg. 2011;37:463–8. https://doi.org/10.1111/j.1524-4725.2011.01916.x.
Ramaswamy Reddy SH, Reddy R, Babu NC, Ashok GN. Stem-cell therapy and platelet-rich plasma in regenerative medicines: a review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;3:367–74. https://doi.org/10.4103/jomfp.JOMFP_93_18.
Borrione P, Gianfrancesco AD, Pereira MT, Pigozzi F. Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil. 2010;89:854–61. https://doi.org/10.1097/PHM.0b013e3181f1c1c7.
Fanciulli G, Delitala A, Delitala G. Growth hormone, menopause and ageing: no definite evidence for ‘rejuvenation’ with growth hormone. Hum Reprod Update. 2009;15:341–58. https://doi.org/10.1093/humupd/dmp005.
Bakacak M, Bostanci MS, İnanc F, et al. Protective effect of platelet rich plasma on experimental ischemia/reperfusion injury in rat ovary. Gynecol Obstet Invest. 2016;81:225–31. https://doi.org/10.1159/000440617.
Panda SR, Sachan S, Hota S. A systematic review evaluating the efficacy of intra-ovarian infusion of autologous platelet-rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus. 2020;12(12):e12037. https://doi.org/10.7759/cureus.12037. PMID: 33457137; PMCID: PMC7797441.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AMM search, assessment, writing, revision and approval of manuscript. RAM Data analysis, writing, revision and approval of manuscript. NS search, assessment of risk of bias, writing, revision and approval of manuscript. WSR Data extraction, writing, revision and approval of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maged, A.M., Mohsen, R.A., Salah, N. et al. The value of intraovarian autologous platelet rich plasma in women with poor ovarian reserve or ovarian insufficiency: a systematic review and meta-analysis. BMC Pregnancy Childbirth 24, 85 (2024). https://doi.org/10.1186/s12884-024-06251-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06251-2