Abstract
Background
POR or POI poses a significant challenge to fertility treatment with different ovarian stimulation strategies. Intra-ovarian injection of platelet-rich plasma (PRP) has been hypothesised to improve ovarian reserve and pregnancies in POI or POR. However, its effectiveness on pregnancy, embryology and ovarian reserve outcomes need to be established. Therefore, we systematically searched databases based on PRISMA guidelines that reported on the effects of intra-ovarian autologous PRP injections in sub-fertile women with POI and POR. The following outcome effects were analysed by random model and included in the meta-analysis in pre- and post-PRP injection groups of POI & POR: (a) pregnancy rates, rate of oocyte & embryo formation (b) ovarian reserve markers (Antral follicular count, Anti-Mullerian Hormone, Follicle Stimulating Hormone). A separate analysis of pregnancies, AFC and AMH was done in POI and POR groups and in age groups < 35 years and > 35 years. A total of 12 studies were included. The estimated overall effects size of the log odds ratio (log OR = 2.03; 95% CI = 0.13 to 3.92; P = 0.04; I2 = 0.42) favoured post-PRP with a moderate level of evidence. There are no significant differences in POI/POR and those with < 35 years or > 35 years.
The pooled standard difference of means favoured the post-PRP injection group significantly with regards to rates of embryo formation (1.39; 95% CI = 0.56 to 2.21; P = 0.02; I2 = 46%.), Oocyte (0.84; 95% CI = -1.3 to 3.0; P = 0.24; I 2 93%), Antral follicle count (1.78; 95% CI = 0.73 to 2.84; P = 0.01. I2 = 97%) with a low level of evidence and Anti-Mullerian Hormone (1.11; 95% CI = 0.16 to 2.05; P = 0.03; I2 = 96%) with low level of evidence.
Conclusion
Our study shows that intraovarian PRP injection was associated with no significant increase in the rates of pregnancy, in the rates of pregnancy, oocyte, embryo formation, Anti-Mullerian Hormone and antral follicle count. Live birth rates were not calculated. There was no statistical difference between POR/POI and those with < 35 years or > 35 years. Further randomized studies are warranted to confirm our findings.
Similar content being viewed by others
Introduction
Poor ovarian response (POR), Premature ovarian insufficiency (POI), and low ovarian reserve women pose clinical challenges to fertility treatment. They are (a) women with decreased ovarian response to controlled ovarian stimulation with conventional gonadotrophin dose [1] and (b) premature ovarian insufficiency (POI) [2]. Although they differ in their etiopathogenesis, they all pose similar clinical challenges to fertility treatment i.e., poor fertility outcomes.
Bologna criteria and POSEIDON criteria are the two methods of categorization of patients with POR. Bologna criteria are based on 1. Age 2. Ovarian reserve status. At least two of the following three features must be present. Advanced maternal age (≥ 40 years) or any other risk factor for poor ovarian response (POR); A previous POR (≤ 3 oocytes with a conventional stimulation); An abnormal or low ovarian reserve is AFC < 5–7 follicles or Anti Mullerian hormone (AMH) < 0.5–1.1 ng/ml) [3]. Both POI and low ovarian reserve result in POR.
The POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) criteria brought a change in the terminology from POR to low prognosis. This helps to stratify low-prognosis patients to undergo ovarian stimulation for IVF to address POR[4].
The age-related natural decline in ovarian reserve in women over 40 years is a well-known reason for the POR. On the other hand, in some women, the qualitative and quantitative reduction in oocytes occurring in the younger age group will result in POI and POR. The European Society of Reproductive Medicine (ESHRE) guidelines define POI as the presence of oligomenorrhea-amenorrhea for at least 4 months and serum follicle-stimulating hormone (FSH) levels of ≥ 25 IU/ml measured at least twice with a 4-week interval, with an onset before the age of 40 years [5]. The prevalence of POI has doubled in the last few decades [6, 7]. The available treatment options for POI or POR are either to maximize the ovarian response from the available limited follicular pool or oocyte donation.
Various stimulation strategies have been attempted to maximize the ovarian response in these subsets of patients, without much improvement [8, 9]. The option of oocyte donation results in better outcomes, but this may not be acceptable to infertile couples who desire to have their genetic child [10]. Recently plasma platelets (PRP) have been used in the regeneration of ovarian function by injecting concentrated autologous PRP into the ovaries [11]. It is hypothesized to improve pregnancies and other ovarian reserve markers in women with POI [12].
PRP injected into ovaries may help to restore proliferation, angiogenesis, and cell migration and reset the programmed cell death of remaining primordial follicles to respond better to ovarian stimulation [13] by releasing growth factors like vascular endothelial growth factors (VEGF) and platelet-derived growth factors AB (PDGF-AB) and TGF-b1[14].
However, this hypothesis is not fully understood. In this review, we would like to evaluate the effects of ovarian PRP injection on pregnancy outcomes and ovarian reserve markers in women with POI who underwent IVF/ICSI so that this intervention can be incorporated into the clinical practice more effectively.
Materials and methods
This study was registered in Prospero (PROSPERO 2021 CRD42021245753).
This review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature search
A systematic literature search of MEDLINE, EMBASE, CINAHL, Google Scholar, Scopus, Web of Science databases, the Cochrane Library, and SciSearch was conducted on studies that reported on the effects of intra-ovarian injections of autologous platelet-rich plasma in sub-fertile women with decreased/low ovarian reserve or premature ovarian insufficiency/failure from the inception of database to March 2022.
To improve our search yield, we adapted the Medical Subjects Headings (MeSH) search strategy to generate two subsets of citations. One subset included the search terms and words related to decreased ovarian reserve (DOR) or low ovarian reserve (LOR) or poor ovarian reserve (POR) or premature ovarian insufficiency (POI) or premature ovarian failure (POF).
The other subset included the search terms and words related to “assisted reproduction techniques (ART) or “in-vitro-fertilization (IVF),” “intracytoplasmic sperm injection (ICSI),” and “Platelet-rich plasma (PRP)”, autologous platelet-rich plasma.
Both subsets were combined and searched again to capture all the relevant articles or citations for our study. The search was restricted to clinical studies in human subjects and published in English language were included in our review.
Data extraction was done by selecting the titles and abstracts, and full manuscripts of the studies that fulfilled our selection criteria were retrieved. They were independently reviewed by two reviewers (SV & PKA) and conflicts regarding inclusion and exclusion of studies were resolved by group consensus and with the third reviewer (NM). We also manually reviewed the bibliographies of retrieved original papers, review papers and relevant studies for additional articles. In this way, missing data from our search criteria were identified and included.
Authors were contacted whenever possible if the full manuscript was not available.
Treatment effect
The log odds ratio was calculated for clinical pregnancy rates and Cohen’s d mean differences were calculated for other outcomes with 95% confidence intervals, estimated overall effect sizes were presented with forest plots. An increase in the outcome values (post-PRP) from the baseline (pre-PRP) of the intervention is graphically displayed to the right of the central line in the meta-analysis favouring intraovarian PRP injection. A p-value of < 0.05 is considered as significant.
Statistical analysis:
-
The characteristics (clinical & methodical) of all included studies were examined.
-
In cases of overlapping data, the studies with the largest number of observations were included.
-
We performed one group (pre-post) treatment summary size effect meta-analysis to look at the estimated overall effect size of intra-ovarian PRP intervention.
-
We performed separate post-PRP analyses between POI and POR for pregnancies, antral follicle count and AMH (key outcomes) to find out any difference between POI and POR.
-
We have done separate post-PRP analyses for those under 35 years and above 35 years to find out whether age impacts the post-PRP reproductive outcomes on pregnancies, antral follicle count and AMH.
-
We used the random effect models for meta-analysis to calculate an overall OR and summary effects size and their 95% confidence intervals (CI) with the forest plots.
-
The missing data on the changes of standard deviations from the included studies were imputed by the Pre/Post correlation value of 0.5 after reasoned argument and doing sensitive analysis.
-
We used IBM SPSS Version 29.0. Armonk, NY: IBM Corp to perform these meta-analyses.
Assessment of heterogeneity
-
The presence of heterogeneity was assessed by the I2 statistic. An I2 > 50% was taken to indicate substantial heterogeneity.
-
The random-effects model was used as the I2 statistic was greater than 50%. Exploration of the causes of heterogeneity was planned using variations in features of the population, exposure, and study quality. Sensitivity analyses were used where possible and appropriate to address the clinical and methodological variations.
Inclusion criteria based on outcomes:
We have included the studies that reported on the fertility outcomes and ovarian reserve markers before (pre) and after (post) intraovarian PRP injection in women with POI or POR.
POI / POR is defined based on the following criteria or in combination.
-
POI: Any of the following
-
o
primary or secondary amenorrhea of > 4 months or oligomenorrhea of > 4 months before the age of 40 years; with FSH elevation > 25 IU/L on 2 assays at > 4 weeks’ intervals, with low estradiol (ESHRE 2016 Guideline).
-
o
Bologna criteria of low ovarian reserve
-
o
Anti-Müllerian hormone < 1.1 ng/ml
-
o
Antral follicle count < 5
-
o
-
POR: Any of the following
-
o
≤ 3 oocytes retrieved with a standard gonadotrophin stimulation (based on Age/BMI/ovarian reserve markers)
-
o
POSEIDON criteria 3 or 4 with low prognosis ART outcome
-
o
-
Age above 40 years or any age with any combination of the above
Objectives
Fertility outcomes & ovarian reserve markers:
-
1.
Fertility outcomes are clinical pregnancies or live births either conceived spontaneously or after IVF/ICSI, number of oocytes and embryos after one month of intraovarian PRP administration. Pregnancies reported within one month of intraovarian PRP administration are considered pre-PRP pregnancies.
-
2.
Ovarian reserve markers: AFC and serum AMH, FSH levels after intraovarian PRP administration.
Exclusion criteria:
-
• Studies that have not clearly defined POI or POR.
-
• Studies that did not report Pre and Post PRP values of the outcomes (reported only either pre or post-PRP values of the outcomes).
-
• Studies were excluded if they had no data available for retrieval or overlapping or duplication of the data.
Data extraction and management
The following information and data were extracted.
Trial methods
Prospective non-randomized studies with pre and post-PRP intervention for comparison. None of the studies were randomized controlled trials.
Participants
Data on the participants included infertile couples with POI/POR as defined above conceived either spontaneously or IVF / ICSI.
Intervention
Information on intraovarian injection of PRP with regards to the type of preparation, route of PRP injection, co-intervention and frequency and duration of intervention.
Outcomes
Data on fertility outcomes & ovarian reserve markers (both pre and post-PRP) were reported as means, standard deviations (SD) and the number of subjects in the studies.
Pregnancy outcomes(core reproductive outcome) were represented as dichotomous data (as the number of events occurred) and the remaining outcomes (AMH, AFC, FSH, number of oocytes retrieved, number of embryos formed) were represented as continuous data(means).
Quality of evidence
Assessment of risk of bias
The risk of bias was assessed using a National Institute of Health (NIH) Quality Assessment Tool for Before-After (Pre-Post) Studies (Table 1). The quality rating for each of the studies included was presented as Good, Fair, or Poor by two reviewers (risk of bias table). The studies were assessed for quality based on the NIH risk assessment tool. The studies ranged from being fair to good based on the NIH risk assessment tool (6 good-quality and 6 fair-quality studies). No major side effects were reported after PRP injection in the included studies which is similar to the studies where PRP was used in other faculties of medicine [15, 16].
Assessment of publication integrity
We used the reappraised checklist tool to systematically evaluate the included studies in 11 categories for publication integrity [17]. The integrity rating for each included study was presented as Good, Poor and Unclear in all the categories (Table 2).
The studies were assessed for quality of integrity using the REAPPRAISED checklist tool Nature checklist [17] on 11 categories. The quality of integrity of the studies was assessed as good, poor, and unclear. In the categories of No Plagiarism, 12 studies [10, 12, 23,24,25,26,27,28,29,30,31,32], Productivity 11 studies [10, 12, 24,25,26,27,28,29,30,31,32], Analysis & Methods 11 studies [10, 12, 24,25,26,27,28,29,30,31,32] were assessed as good. In the error reporting category, 8 studies [10, 24,25,26,27,28,29] out of 12 studies were assessed as unclear and 4 studies were assessed as poor [12, 23, 31, 32] and none of the studies were good and more than 3 studies were unclear in the research governance [12, 27, 29, 30], ethics[23, 24, 27, 29, 30], authorship[24,25,26,27, 29], no data duplication categories[10, 12, 23, 30] (Fig. 1).
Summary of findings and assessment of the certainty of the quality of evidence
We have prepared a summary of the findings table using GRADE pro software and Cochrane methods [18] for the outcomes and graded the evidence quality as low, very low, or moderate level of certainty of evidence. The significance of the quality of evidence grading on the outcomes in clinical practice is mentioned in the discussion section under the heading meaning of our findings (Tables 3, 4 and 5).
Results
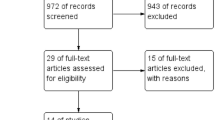
A total of 17 studies were identified and scrutinized in full text, out of which 12 studies were included in the meta-analysis and 5 were excluded for reasons (Fig. 2 PRISMA). Two studies [10, 31] included only POI subjects, eight studies [1, 12, 23, 26,27,28, 30, 32] included POR subjects, and two studies [2, 24] included combined POI and POR subjects.
Included studies
All the twelve studies included in the meta-analysis were prospective and their details are mentioned in Table No. 3 [10, 12, 23,24,25,26,27,28,29,30,31,32].
Among the 12 included studies, five studies had recruited 20 patients or less, [23, 25, 26, 29, 32] while one study had only five patients [23].
The studies differed concerning the quantity, timing, and frequency of injection of PRP into the ovaries. The volume of PRP injected ranged from 0.2 ml to 4 ml. Six studies have reported changes in at least one fertility outcome and ovarian reserve marker [12, 23, 24, 26, 27, 30]. Three studies reported only changes in at least one ovarian reserve marker [28, 29, 31] while 3 studies have commented on at least one of the fertility outcomes [10, 25, 32].
Excluded studies
Five studies were excluded from the analysis for the reasons explained in Table No. 2 [1, 19,20,21,22]
Ovarian reserve marker
Among the ovarian reserve markers, four studies included all three markers of ovarian reserve, i.e. AMH, AFC, and FSH [11, 13, 25, 32] while four studies included AMH and FSH [23,24,25,26]. The study by Tandulwadkar et al. [26] and Parvanov et al. [30] included AFC and AMH. Stojkosov et al. [29] included only FSH as a marker of ovarian reserve.
Embryology and fertility data
Three studies have included oocytes and embryos [24, 30, 32], while five studies have commented on pregnancies [10, 23,24,25, 32].
Outcomes
-
A)
Comparison of fertility outcomes (both pre and post-PRP):
-
Pregnancies: Among the 12 studies included in the meta-analysis, only 5 studies have reported pregnancies [10, 23,24,25, 32]. A total of 59 pregnancies resulted from 438 patients after (post) PRP in comparison to 6 pregnancies before (pre) PRP. 36 of 59 conceived spontaneously and 23 with ART after PRP administration. 44 pregnancies were with a mean age of < 35 years [10, 28] and 15 pregnancies with a mean age of > 35 years [23, 25, 32]. Pregnancies reported after PRP in the age group above 40 years were less than those below 40 years (4/40 vs 3/30). The estimated overall effects size of random log odds ratio (OR) (log OR = 2.03; 95% CI = 0.13 to 3.92; P= 0.04; I2=0.42) significantly favoured post PRP and is depicted in Figure II. Intra-ovarian PRP did increase pregnancy rates.
The estimated overall effects size of random log odds ratio (OR) (log OR = 2.03; 95% CI = 0.13 to 3.92; P= 0.04; I2=0.42) significantly favoured post PRP and is depicted in Fig. 3. Intra-ovarian PRP did increase pregnancy rates.
The estimated overall effect size of pregnancies after post-PRP in separate analysis between POI and POR did not have any significant difference (log OR -0.52;95%CI: -2.73-1.90; p=0.65) and similarly, the estimated overall effect size of post-PRP in separate analysis among under 35 years and over 35 years of age did not result in any significant differences in pregnancies (log OR -0.26;95%CI:-1.24-0.72;p=0.60).
-
Embryos: Three studies reported the effect of intra-ovarian PRP injection on embryo formation [24, 30, 32]. Cohen’s mean difference in embryo formation significantly favoured injection of PRP (post-PRP). The estimated overall effect size of mean difference (random model) with confidence intervals (CI) is 1.39; 95% CI = 0.56 to 2.21; P = 0.02; I2 = 46% and depicted in Fig. 4.
-
Oocytes: Three studies reported the effect of ovarian PRP injection on oocyte formation [24, 30, 31]. Cohen’s mean difference in oocyte formation favoured injection of PRP (post-PRP). The estimated overall effect size of means difference (random model) with confidence intervals (CI) is 0.84; 95% CI = -1.3 to 3.0; P = 0.24; I2 = 93% and depicted in Fig. 5.
-
No significant increase in pregnancy rates was noted in the present study. Also, the live birth rates were not calculated.
-
-
B)
Comparison of ovarian reserve markers (both pre and post-PRP)
Out of 12 studies,9 studies reported on AMH [10, 12, 23,24,25,26,27,28, 31], while 6 studies reported on AFC [10, 12, 24, 26, 30, 32]. There are 4 studies, which have studied all three ovarian reserve markers, i.e. AMH, AFC and FSH [10, 12, 24, 31], while another four studies have evaluated AMH and FSH [23, 25, 27, 28]. Tandulwadkar et al. [26] and Parvanov et al. [30] studied AFC, AMH and AFC, FSH respectively. Stojkovska et al. [29] studied only FSH.
-
AFC: Six studies reXported the effect of ovarian PRP injection on AFC [10, 12, 24, 26, 30, 31]. Cohen’s mean difference in AFC favoured the injection of PRP (post-PRP). The estimated overall effect size of means difference (random model) with confidence intervals (CI) is 1.78; 95% CI = 0.73 to 2.84; P = 0.01. I2 = 97% and is depicted in Fig. 6. The AFC may increase by 1.78 after the PRP administration.
-
The estimated overall effect size of mean difference after post-PRP in separate analysis between POI and POR did not have any significant difference(-2.97;95%CI:-2.11–8.04;p = 0.25) similarly, the estimated overall effect size of post-PRP in separate analyses among those under 35 years and over 35 years of age did not result in any significant differences in pregnancies (-2.89;95%CI:-1.46–7.22;p = 0.19).
-
AMH: Nine studies reported on the effect of ovarian PRP injection on AMH [10, 12, 23,24,25,26,27,28, 31]. Cohen’s mean difference in AMH favoured injection of PRP (post PRP). The estimated overall effect size of means difference (random model) with confidence intervals (CI) is 1.11; 95% CI = 0.16 to 2.05; P = 0.03; I2 = 96% and is depicted in Fig. 7. The AMH may increase by 1.11 after administration.
-
The estimated overall effect size of mean difference after post-PRP in separate analysis between POI and POR. Post PRP favoured the POI group with significant difference (-3.08;95%CI:-2.11to-4.05; P = 0.00) however, the number of studies compared AMH are only two studies with high heterogeneity of 85% and wide confidence intervals.
-
The estimated overall effect size of post-PRP in separate analyses among those under 35 years and over 35 years of age did not result in any significant differences in pregnancies (0.76;95%CI:-0.73–2.24; P = 0.32).
-
FSH: Ten studies reported the effect of ovarian PRP injection on FSH [10, 12, 23,24,25, 27,28,29,30,31]. Cohen’s mean difference in FSH favoured injection of PRP (post-PRP). The estimated overall effect size of means difference (random model) with confidence intervals (CI) is -0.62; 95% CI = -1.57 to 0.33; P = 0.17; I2 = 98% and is depicted in Fig. 8. This is a non-significant decrease in FSH after PRP.
-
Discussion
Meaning of our findings
Our study findings suggest that intraovarian PRP administration will increase pregnancy rates and better oocyte and embryo formation. Similarly, there was an improvement in the ovarian reserve markers (AFC, AMH) in POI/POR women.
Pregnancies: The studies reported an increase in pregnancy rates after PRP injection, but many of the pregnancies occurred in younger women (mean age ~ 35 years), and most of them were natural conceptions rather than IVF/ICSI. There was a tendency to favour spontaneous pregnancies in younger women with POI rather than controlled ovarian stimulation after PRP injection. This indicates that maternal age is the most important factor in pregnancy outcomes with or without PRP injection [12]. PRP probably increases pregnancy with the caveat that the quality of evidence is only moderate. The effect of smaller studies needs to be taken with caution and the possibility of high publication bias, with one study having only five subjects [23].
AFC: Our study findings show statistically significant improvement in the AFC. In POI, apoptosis and atresia can occur in all stages of folliculogenesis, i.e. primordial follicle to the antral follicle [33, 34]. The autocrine and paracrine growth factors play a crucial role in all stages of folliculogenesis which can influence embryo quality and implantation potential [35]. We do not know at what stage of folliculogenesis, atresia/apoptosis occurred or what growth factor gene was expressed or mutated at the time of atresia/apoptosis [36]. Henceforth, we can hypothesize that the beneficial effect of PRP to halt the process of apoptosis depends upon the normal gene expression and abnormal gene mutation expression of growth factors at the time of apoptosis/atresia [35, 37]. If normal growth factor gene expressions persist more than abnormal growth factor gene mutations, then PRP might help in the promotion of the development of the remaining antral follicles or may slow down the process of apoptosis, which might quantitatively increase the number of AFC [36]. When the abnormal growth gene mutations are expressed more, then PRP may not necessarily reactivate the already atretic primordial follicle or will lead to low viability and implantation after PRP administration in POI [37]. The quality of evidence about improvement in antral follicle count after PRP is low(uncertain). The mean difference of just 1.78 after PRP has no clinical utilitarian value.
AMH: The AMH is primarily secreted by the granulosa cells of the developing follicles. POI results in the atresia of the follicles and a decrease in the AMH. As mentioned above, PRP may halt the process of atresia but may not reactivate the atretic follicles thus resulting in some improvement in AMH levels [38]. Although the improvement was noted, it may not be clinically significant for good fertility outcomes [38]. The quality of evidence about improvement in AMH after PRP is low (uncertain). The mean difference of improvement of just 1.11 of AMH does not have any clinical utilitarian value.
The estimated overall effect size may favour POI and those under 35 years of age without any statistical significance after PRP concerning pregnancies, AFC and AMH without any clinical utility.
Comparison with other studies
PRP injection favoured more oocyte and embryo formation. The study by Sfakianoudis et al. [24] and Farimani et al. [32] showed an increase in the number of oocytes and embryos, while the study by Parvanov et al. [30] did not show any statistical increase in the number of oocytes and embryos. The age of patients studied ranged from 35–38 years in the study by Sfakianoudis et al. [24] and Farimani et al. [32]. The mean age of the patients in the study by Sfakianoudis et al. [24] and Farimani et al. [32] may be less than that of the mean age of patients in the study by Parvanov et al. [30], as the study by Parvanov et al. [30] did not mention the mean age. The most probable reason for the increase in the number of oocytes and embryos is that PRP may help to activate existing preantral and/or early antral follicles [24], and thus they respond better to fertility treatment.
Strengths and limitations
This study was undertaken to evaluate the effects of intraovarian PRP administration in POI/POR women on pregnancies and ovarian reserve markers. There are no randomized control trials, and all the studies included in our meta-analysis and review were observational studies, which led to a high risk of bias. Also, the number of studies which have reported on pregnancies was only five with one study with only 5 subjects.
Implications for clinical practice
Based on our findings, intraovarian PRP administration may have a role in improving pregnancies and ovarian reserve markers in POI/POR women, albeit the younger women conceived spontaneously in comparison to older women with or without ART.
Implications for future research
Given that there is scope to improve the pregnancy outcomes in POI/PORs, there is no standardized preparation method and PRP injection volume. There is a huge need for large multi-centred randomized trials to standardize the PRP preparation, volume & frequency of administration, duration of repeat PRP injections, and follow-up of patients to evaluate the improvement in fertility outcomes, till that time this procedure is deemed experimental.
Conclusions
Intraovarian PRP injections in POI or POR or low ovarian reserve women did not show significant improvement in the pregnancies (moderate level of evidence), AFC (low level of evidence), AMH(low level of evidence), and number of oocytes and embryos. Live birth rates were not calculated. There is no statistical difference between POI/POR and < 35 years and > 35 years. Large multicentric randomized control trials are required, especially concerning the type of PRP preparation, amount of PRP injected, number and frequency of PRP injections required and duration of follow-up to evaluate the effect of PRP injection, for clinical utility before it is incorporated in the management of POI/POR patients.
Availability of data and materials
Data extracted from published papers.
Abbreviations
- AFC:
-
Antral follicle count
- AMH:
-
Anti-Mullerian hormone
- ART:
-
Assisted reproductive technology
- DOR:
-
Decreased ovarian reserve
- ESHRE:
-
European Society of Human Reproduction and Embryology
- FSH:
-
Follicle stimulating hormone
- IVF:
-
In vitro fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- NIH:
-
National Institute of health
- PDGF:
-
Platelet-derived growth factor
- POI:
-
Premature ovarian insufficiency
- POR:
-
Poor ovarian response
- POSEIDON:
-
Patient Oriented Strategies Encompassing Individualized Oocyte Number
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRP:
-
Platelet-rich plasma
- TGF:
-
Transforming growth factor
- VEGF:
-
Vascular endothelial growth factor
References
Stojkovska S, Dimitrov G, Stamenkovska N, Hadzi-Lega M, Petanovski Z (2019) Live birth rates in poor responders’ group after previous treatment with autologous platelet-rich plasma and low dose ovarian stimulation compared with poor responders used only low dose ovarian stimulation before in vitro fertilization. Open access Macedonian journal of medical sciences 7(19):3184
Eshre Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, Vermeulen N (2016) ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 31(5):926–937
Ferraretti A, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L, ESHRE working group on Poor Ovarian Response Definition. (2011) ESHRE consensus on the definition of ‘poor response’to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum Reprod 26(7):1616–1624
Esteves SC, Alviggi C, Humaidan P, Fischer R, Andersen CY, Conforti A, Ubaldi FM (2019) The POSEIDON criteria and its measure of success through the eyes of clinicians and embryologists. Frontiers in Endocrinology 10:814
European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber, L., Davies, M., Anderson, R., Bartlett, J., Braat, D., Cartwright, B., Cifkova, R., de Muinck Keizer-Schrama, S., Hogervorst, E., Janse, F., Liao, L., Vlaisavljevic, V., Zillikens, C., & Vermeulen, N (2016) ESHRE Guideline: management of women with premature ovarian insufficiency. Human reproduction (Oxford, England) 31(5):926–937. https://doi.org/10.1093/humrep/dew027
Coulam CB, Adamson SC, Annegers JF (1986) Incidence of premature ovarian failure. Obstet Gynecol 67(4):604–606
Lagergren K, Hammar M, Nedstrand E, Bladh M, Sydsjö G (2018) The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Womens Health 18:1–4
Ovarian Stimulation, T. E. G. G. O., Bosch, E., Broer, S., Griesinger, G., Grynberg, M., Humaidan, P., Kolibianakis, E., Kunicki, M., La Marca, A., Lainas, G., Le Clef, N., Massin, N., Mastenbroek, S., Polyzos, N., Sunkara, S. K., Timeva, T., Töyli, M., Urbancsek, J., Vermeulen, N., & Broekmans, F. (2020). ESHRE guideline: ovarian stimulation for IVF/ICSI†. Human reproduction open, 2020(2), hoaa009. https://doi.org/10.1093/hropen/hoaa009
Kamble L, Gudi A, Shah A, Homburg R (2011) Poor responders to controlled ovarian hyperstimulation for in vitro fertilisation (IVF). Hum Fertil (Camb) 14(4):230–245. https://doi.org/10.3109/14647273.2011.608241
Cakiroglu, Y., Saltik, A., Yuceturk, A., Karaosmanoglu, O., Kopuk, S. Y., Scott, R. T., Tiras, B., & Seli, E. (2020). Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging, 12(11), 10211–10222. https://doi.org/10.18632/aging.103403
Divya P (2021) Platelet-rich plasma in female infertility: A comprehensive review of current literature. Fertility Science and Research 8(1):30
Melo P, Navarro C, Jones C, Coward K, Coleman L (2020) The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet 37(4):855–863. https://doi.org/10.1007/s10815-020-01710-z
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321. https://doi.org/10.1038/nature07039
Dhurat R, Sukesh M (2014) Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J Cutan Aesthet Surg 7(4):189–197. https://doi.org/10.4103/0974-2077.150734
Maisel-Campbell AL, Ismail A, Reynolds KA, Poon E et al (2020) A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch Dermatol Res 312:301–315
Le ADK, Enweze L, DeBaun MR, Dragoo JL (2018) Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med 11(4):624–634
Grey A, Bolland MJ, Avenell A, Klein AA, Gunsalus CK (2020) Check for publication integrity before misconduct. Nature 577(7789):167–169
Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH (2023) Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane. Available from http://www.training.cochrane.org/handbook
Pantos, K., Nitsos, N., Kokkali, G., Vaxevanoglou, T., Markomichali, C., Pantou, A., ... & Sfakianoudis, K. (2016, July). Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. In Abstracts, ESHRE 32nd Annual Meeting (pp. 3–6)
Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, Syrkos S, Pappas A, Koutsilieris M, Sfakianoudis K (2019) A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant 28(9–10):1333–1340. https://doi.org/10.1177/0963689719859539
Hsu CC, Hsu L, Hsu I, Chiu YJ, Dorjee S (2020) Live Birth in Woman With Premature Ovarian Insufficiency Receiving Ovarian Administration of Platelet-Rich Plasma (PRP) in Combination With Gonadotropin: A Case Report. Front Endocrinol 11:50. https://doi.org/10.3389/fendo.2020.00050
Anagani M, Agrawal P, Reddy B, Mishra P (2021). Role of autologous bone marrow derived stem cells and platelet rich plasma for endometrial regeneration and repair and ovarian rejuvenation. Int J Reprod Contracept Obstet Gynecol 10:597. https://doi.org/10.18203/2320-1770.ijrcog20210311
Selvaraj Y, Malaisamy K (2021) Rejuvenation of ovary and thin endometrium by autologous PRP injection in POR and recurrent implantation failure. Advances in Sexual Medicine 11(01):1
Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G, Koutsilieris M, Pantos K (2020) Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J Clin Med 9(6):1809. https://doi.org/10.3390/jcm9061809
Aflatoonian, A., Lotfi, M., Saeed, L., & Tabibnejad, N. (2021). Effects of Intraovarian Injection of Autologous Platelet-Rich Plasma on Ovarian Rejuvenation in Poor Responders and Women with Primary Ovarian Insufficiency. Reproductive sciences (Thousand Oaks, Calif.), 28(7), 2050–2059. https://doi.org/10.1007/s43032-021-00483-9
Tandulwadkar S, Karthick MS (2020) Combined Use of Autologous Bone Marrow-derived Stem Cells and Platelet-rich Plasma for Ovarian Rejuvenation in Poor Responders. Journal of human reproductive sciences 13(3):184–190. https://doi.org/10.4103/jhrs.JHRS_130_19
Petryk, N., & Petryk, M. (2020). Ovarian Rejuvenation Through Platelet-Rich Autologous Plasma (PRP)-a Chance to Have a Baby Without Donor Eggs, Improving the Life Quality of Women Suffering from Early Menopause Without Synthetic Hormonal Treatment. Reproductive sciences (Thousand Oaks, Calif.), 27(11), 1975–1982. https://doi.org/10.1007/s43032-020-00266-8
Sills ES, Petersen JL, Rickers NS, Wood SH, Li X (2020) Regenerative effect of intraovarian injection of activated autologous platelet rich plasma: serum anti-mullerian hormone levels measured among poor-prognosis in vitro fertilization patients. International Journal of Regenerative Medicine 2020(1):1–5
Stojkovska S, Dimitrov G, Saltirovski S, Pantos K, Hadzi Lega M (2020) Autologous platelet rich plasma intracortical ovarian injection restored ovarian function and foliculogenensis in poor responders after one month: a control pilot study. Medicus 25(1):7–10
Parvanov D, Ganeva R, Vidolova N, Nikolova K, Vasileva M, Stoykov I, Stamenov G (2020) Ovarian autologous platelet-rich plasma (PRP) treatment improves oocyte and embryo quality in women with poor ovarian response. Fertility and Sterility. 114(3):e452–e453
Abdullah TH, Abbas SH, Al-Obaidi MT, Abdulraheem Y (2019) The Efficacy of Platelets Rich Plasma (PRP) for Ovarian Rejuvenation. Indian J Public Health 10(8):729
Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M (2019) A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep 46(2):1611–1616. https://doi.org/10.1007/s11033-019-04609-w
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383(6600):531–535. https://doi.org/10.1038/383531a0
Otsuka F, McTavish KJ, Shimasaki S (2011) Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev 78(1):9–21. https://doi.org/10.1002/mrd.21265
Nagashima, T., Kim, J., Li, Q., Lydon, J. P., DeMayo, F. J., Lyons, K. M., & Matzuk, M. M. (2011). Connective tissue growth factor is required for normal follicle development and ovulation. Molecular endocrinology (Baltimore, Md.), 25(10), 1740–1759. https://doi.org/10.1210/me.2011-1045
Cremonesi F, Bonfanti S, Idda A, Anna LC (2020) Improvement of Embryo Recovery in Holstein Cows Treated by Intra-Ovarian Platelet Rich Plasma before Superovulation. Veterinary sciences 7(1):16. https://doi.org/10.3390/vetsci7010016
Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM (2017) Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online 35(4):343–350. https://doi.org/10.1016/j.rbmo.2017.04.007
Dayal M, Sagar S, Chaurasia A, Singh U (2014) Anti-mullerian hormone: a new marker of ovarian function. J Obstet Gynaecol India 64(2):130–133. https://doi.org/10.1007/s13224-013-0482-3
Acknowledgements
None.
Code availability
PROSPERO Registration number: 2021 CRD42021245753.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception – Dr. Srisailesh Vitthala and Dr. Prashanth K Adiga. Acquisition – Dr. Srisailesh Vitthala and Dr. Prashanth K Adiga. Analysis- Dr. Ravishankar N and Dr. Nicola Marconi. Interpretation –Dr. Nicola Marconi. Drafting – Dr. Srisailesh Vitthala. Final approval—Dr. Prashanth K Adiga.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable as it is a systematic review and meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adiga, P.K., Marconi, N., N, R. et al. Effect of intra-ovarian injection of platelet-rich plasma on the patients with a poor ovarian response (POR) or premature ovarian insufficiency (POI): a systematic review and meta-analysis. Middle East Fertil Soc J 29, 24 (2024). https://doi.org/10.1186/s43043-024-00180-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00180-y