Abstract
Background
Retroperitoneal ectopic pregnancy (REP) is an extremely rare type of ectopic pregnancy, with a total of less than 32 cases reported in the English literature. Early diagnosis of REP is very difficult and all treatments entail a high risk of life-threatening complications.
Case presentation
A 29-year-old nulliparous woman presented a history of 50-day amenorrhea and 7-day upper abdominal pain without vaginal spotting. The serum beta-human chorionic gonadotropin (β-hCG) value was 65,004 m-international units per milliliter (mIU/mL), but no intrauterine gestational sac was found via transvaginal sonography (TVS). Then transabdominal ultrasonography (TAS) and abdominal contrast-enhanced computer tomography (CT) identified a retroperitoneal ectopic pregnancy (REP) tightly adjacent to the inferior vena cava and the abdominal aorta. After consultation from a multidisciplinary team, systemic methotrexate (MTX, intramuscular 20 mg daily for 5 consecutive days) combined with ultrasound-guided local potassium chloride solution injection into the gestational sac was scheduled firstly for the patient. However, serum β-hCG continued to increase and the patient experienced worsening abdominal pain. Laparotomy was performed jointly by a gynecologist and a vascular surgeon. During the operation, the gestational sac with fetal bud measuring about 4.5 × 4.0x3.0 cm, tightly adherent to the surface of inferior vena cava and the left side of abdominal aorta, was carefully dissociated out from the surrounding tissues and removed en bloc. Histopathology examination confirmed the diagnosis of REP. The patient recovered uneventfully and her serum β-hCG returned to normal range on the 23th postoperative day.
Conclusions
Considering the possibility of REP and combined radiological examinations, such as ultrasonography and CT, are crucial for the early diagnosis of this rare condition. A multidisciplinary team is necessary to treat REP.
Similar content being viewed by others
Background
Ectopic pregnancy is a major cause of maternal mortality and morbidity encountered in the first trimester [1]. Nearly all ectopic pregnancies (95%) are implanted in the fallopian tube, whereas only merely 1% of ectopic pregnancies are implanted in the abdominal cavity [2]. Retroperitoneal ectopic pregnancy (REP), in which the gestational sac is implanted in the retroperitoneal cavity of the pelvis and abdomen, refers to an extremely rare type of abdominal ectopic pregnancy [3]. Once a retroperitoneal gestational sac ruptures, it can cause a catastrophic hemorrhage, especially for those located close to large blood vessels [3,4,5]. Here we report a case of REP implanted on the surface of the inferior vena cava, as well as the abdominal aorta, which was successfully treated in a multidisciplinary team. In order to provide reference for clinical practice in the diagnosis and treatment of REP, we also conducted a review on all of the reported cases in English literature.
Case presentation
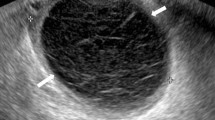
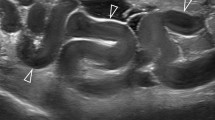
A 29-year-old pregnant woman, gravida 1, para 0, one previous artificial abortion, with regular menstrual cycle, was admitted via the emergency department on December 27 2021 with a history of 50-day amenorrhea and 7-day moderate to intermittent upper abdominal pain. She had no injury history or history of previous pelvic inflammatory diseases or gynecological surgery. Her vital signs were within normal range. General physical examination revealed nothing remarkable. Gynecological examination found no vaginal spotting, and the uterine cervix was smooth without tenderness upon palpation and movement; the uterine body was soft and enlarged equivalent to the size of 50-day-gestation; the right adnexa was slightly thickened without tenderness; and the left adnexa was unremarkable. The serum beta-human chorionic gonadotropin (β-hCG) value was 65,004 m-international units per milliliter (mIU/mL) on admission. Color transvaginal ultrasonography (TVS) of the pelvis demonstrated no intrauterine gestational sac but thicken endometrium of 1.7 centimeter (cm) (Fig. 1a), a right adnexal well-bounded, medially echoic mass approximately 2.3 × 2.0 × 2.0 cm in size with signs of blood supply; no fluid collection in the pouch of Douglas. Because the results of TVS were not parallel with the clinical characteristics and serum β-hCG level, a full transabdominal ultrasonography (TAS) was applied to extend the scan scope. TAS scan revealed a heterogeneous mass approximately 3.8 × 3.1 × 2.3 cm in size, which consisted of a gestational sac with an 4 mm embryo bud with positive cardiac pulsation (Fig. 1b). The pregnancy mass was tightly adjacent to the inferior vena cava and the abdominal aorta. We furtherly completed an abdominal contrast-enhanced computer tomography (CT), which showed the gestational sac with the embryo in the retroperitoneal space and detailed its tight link with the great vessels alongside (Fig. 1c). Highly suspected of rare REP and lack of experience in the diagnosis and treatment of this disease, a multidisciplinary consultation composed of a gynecologist, a vascular surgeon, a radiologist and an interventional physician was scheduled. For fear of vascular injury and unmanageable intraoperative bleeding potentially associated with excising this mass, the patients decided to administer systemic methotrexate (MTX) combined with local potassium chloride solution injection guided by ultrasonography. Daily 20-miligram(mg) intramuscular MTX for 5 consecutive days was initiated on December 28, 2021. And on the same day, ultrasound-guided paracentesis and local potassium chloride (KCl) injection into the embryo bud was operated successfully (Fig. 1d). On December 30 2021, serum β-hCG elevated to 79,382 mIU/ml, but a repeat TAS showed that though the size of REP mass didn’t change, the fetal heart beat was gone. The patient remained stable with close observation in the hospital. Then the medication therapy was continued. However, on December 31 2021, the patient reported worsening abdominal pain and her serum β-hCG level continued to increase (81,447 mIU/ml). Consequently, the patient agreed to undertake an exploratory laparotomy despite stable vital signs and no drop in hemoglobin level (Hb 118 g/L). This was accomplished through a midline incision about 20 cm in length under general anesthesia. While exploring the pelvic cavity, we found a slightly enlarged and soft uterus with bilateral intact fallopian tubes. The left ovary was completely normal while a corpus luteum about 2.0 × 2.0 cm in size was found in the right ovary without active bleeding. No evidence of lesion and pelvic adhesion was found. No fluid collected in the abdominopelvic cavity. Then an abdominal vascular surgeon joined the operation. Further exploration of the upper abdomen revealed a retroperitoneal mass measuring 4.5 × 4.0 × 3.0 cm, inferior the transverse mesentery and directly attached tightly to the surface of inferior vena cava and the left side of abdominal aorta, with a small amount of local retroperitoneal hemorrhage. The retroperitoneal space was entered. After the surrounding connective tissue was carefully dissociated and the communicating vessels between the mass and the inferior vena cava and abdominal aorta were ligated, the pregnancy mass was removed en bloc. No blood transfusion was required. The small wound surface on the inferior vena cava was sutured meticulously with absorbable suture to ensure sufficient hemostasis. No retroperitoneal drain was placed. The total blood loss was 50 millilitre (ml) and the operation time was 92 min.
The imaging examination before the laparotomy. a Transvaginal ultrasonography (TVS) revealed a thicken endometrium without intrauterine gestational sac. b Transabdominal ultrasonography (TAS) revealed a retroperitoneal pregnancy mass. c Abdominal computer tomography (CT) showed the retroperitoneal gestational sac (red arrow) was tightly adherent to the inferior vena cava (blue arrow) and abdominal aorta (yellow arrow). d Ultrasound-guided paracentesis and local potassium chloride (KCl) injection into the embryo bud
An embryo bud was detected macroscopically inside the resected retroperitoneal mass. Pathological examination confirmed the presence of chorionic villi under an inverted microscope (Olympus, Tokyo, Japan) (Fig. 2).
Serum β-hCG decreased to 21,707 mIU/mL on the first postoperative day and 582 mIU/mL on the 6th postoperative day. The patient recovered smoothly and was discharged on the 6th postoperative day. Her serum β-hCG were strictly monitored in the outpatient setting and returned to normal range on the 23th postoperative day. Changes in the serum β-hCG levels over time are shown in Fig. 3.
Discussion and conclusions
Abdominal pregnancy is the rarest type of ectopic pregnancies, possessing eight times higher rates of maternal mortality and morbidity than nonabdominal cases [2]. According to the criteria established by Studdiford in 1942 [6], only a very small fraction of the reported cases could be exclusively diagnosed as primary abdominal pregnancy. Reported common sites of primary abdominal pregnancy are the pouch of Douglas, posterior uterine wall, uterine fundus, anterior abdominal wall, omentum, liver, spleen, and diaphragm [7, 8]. However, abdominal pregnancy in the retroperitoneal space is an exceedingly rare occurrence. Due to its rarity, it is impossible to accurately calculate the incidence of REP. Given its propensity to implant along major vessels, REP poses a high risk of fatal rupture and bleeding. To date, however, there is no well-defined consensus or guideline for clinical management. Bizarre implantation locations, non-specific symptoms and varied clinical presentations can make the diagnosis and treatment of REP challenging, sometimes resulting in misdiagnosis. In order to better guide clinical practice, we conducted a search of PubMed database (English language; 1970–2022; search terms: “retroperitoneal ectopic pregnancy” and “retroperitoneal pregnancy”), and supplemented related cases through literature tracking. A total of 31 literatures including 32 REP cases, plus the case presented here, were collected and thoroughly analyzed, focusing on the clinical characteristics, diagnosis, treatment and prognosis (details listed in Table 1 [4, 5, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]).
Pathogenesis
The pathogenesis of primary REP is complex and still unelucidated, but three mechanistic hypotheses have been proposed. It is not surprising that the prevalence of ectopic pregnancy is higher following assisted reproductive technique (ART) procedures than in the general population [38]. Tubal pathology, previous tubal surgery, and previous ectopic pregnancy are the major indications for ART and both have been considered as a major risk factor for the ectopic pregnancy [38]. In this proposed mechanism, embryos are placed in the retroperitoneal space due to iatrogenic uterine perforation, or even less likely, through a fistulous tract formed following salpingectomy. Reviewing all the 33 REP cases, 39.4% (13/33) of the patients had a history of tubal pregnancy, of which 10 cases had 1 time, 1 case had 2 times and another 2 cases had 3 times. 48.5% (16/33) of the patients had a history of tubal surgery, of which 7 cases underwent bilateral salpingectomy and 9 underwent unilateral salpingectomy. 30.3% (10/33) of patients were IVF-ET, and 1 case had undergone intrauterine intro-uterine semination (IUI). However, this mechanism was not likely to explain every case with ART operation because the ET procedure was strictly conducted under sonographic guidance. The iatrogenic placement of the embryos in the retroperitoneal space of the mid or upper abdomen can definitely be excluded considering the length of the transfer catheter and the volume of the ET medium [9,10,11, 14]. Wang et al. [32] speculated that the fallopian tube stumps after resection could be spontaneously reperfused or formed a fistula, creating a possible communication between the uterine and the retroperitoneal cavity. However, in the case reported by Anh et al. [35], both fallopian tube stumps were visible and intact, and detached from the broad ligaments, excluding this explanation. It is also worth mentioning that 16 cases (48.5%) conceived naturally without tubal pathology or resection.
Ferland et al. [9] proposed a second yet not very convincing hypothesis that the embryo implants on the posterior peritoneal surface and reaches a retroperitoneal space by subsequent trophoblastic invasion through the peritoneum. However, there is no direct evidence to confirm this hypothesis.
The third hypothesis suggests that the fertilized ovum may reach the retroperitoneal space via lymphatic system, similar to the metastasis of gynecological cancer, as lymphatic tissue has been found with ectopic masses during postoperative pathological examination [4, 18, 22, 25, 28]. Lymphatic spread may also explain the frequent localization of REPs at the pelvic sidewalls or along the great vessels, corresponding to the known lymphatic drainage from the uterus. This possibility appeared to be the most plausible mechanism in our case for two reasons. First, there was no history of pelvic surgery or tubal pathology before this spontaneous pregnancy, and no abnormal channels were found between the uterus or fallopian tubes and the retroperitoneal cavity. Second, the gestational sac implanted on the inferior vena cava with intact peritoneum overlying it. In addition, the high proportion of cases associated with IVF may be explained by a deposit of a fertilized ovum deep in the endometrium facilitating a subsequent migration into lymph vessels. However, this intralymphatic migration hypothesis is not absolutely persuasive because only a few cases of REP have been reported to be surrounded by lymphatic tissue during pathological examination. The exact pathogenesis of REP is still worthy of further research.
Clinical characteristics
The age of 33 REP patients was 19–38 years old, with an average of 30.6y. Amenorrhea, abdominal pain and vaginal bleeding are the most common symptoms of REP. Compared with the lumen of the fallopian tube, the space of the retroperitoneal cavity is much larger and more complex, and so the ectopic gestational sac can grow bigger. The duration of amenorrhea was 35–161 days with an average of 56.8d. Due to the good embryonic development, the blood β-hCG before treatment was 267.3–99,286 IU/L with an average of as high as 31,673.4 IU/L. At the same time, over half of the patients (22/33, 66.7%) demonstrated embryo and/or fetal heartbeat on preoperative ultrasound. The size of ectopic pregnancy mass without rupture can even reach 10 cm. Meire et al. [13] reported a case of a retroperitoneal anencephalic fetus terminated at 23 weeks’ gestation. Among the 33 cases, only abdominal pain accounts for 57.6% (19/33), and only vaginal bleeding accounts for 9.1% (3/33). 12.1% (4/33) of them presented both abdominal pain and vaginal bleeding, and another 18.2% (6/33) were asymptomatic. The degree of pain is usually related to whether the pregnancy mass ruptures. And significantly, the region of pain does not fully reflect the implantation site of pregnancy. Only one case, reported by Wang et al. [32], complained of pain in the left lumbar back which might be caused by ectopic gestational sac growth resulting in stimulation of the nerve of the left psoas major muscle.
Theoretically, embryo implantation site should be randomly distributed in the retroperitoneal space. However, in fact, most of the reported REPs located along the great vessels. Ouyang et al. [3] suggested that, according to the implantation site, REP can be simply divided into two types: pelvic REP and abdominal REP. The former refers to the REP in the pelvic segment below the common iliac vessels, accounting for 27.3% (9/33); the latter refers to the REP around the abdominal aorta, the inferior vena cava, and the common iliac artery, accounting for 72.7% (24/33). Given its intimacy with great vessels, REPs pose a significant risk of life-threatening hemorrhage. Among them, 15.2% (5/33) had hemorrhagic shock at the time of presentation, and 15.2% (5/33) had blood transfusion during the operation.
Diagnosis and differential diagnosis
Due to the nonspecific clinical manifestations and complex pregnancy sites, the diagnosis of REP can be easily overlooked. In general, clinicians tend to focus the diagnosis on tubal pregnancy, without considering the possibility of REP. TVS examination was firstly undertaken in 63.3% (21/33) of the patients, and except 3 cases of rare heterotopic pregnancy after IVF-ET, the others showed thicken endometrium but no sign of intrauterine pregnancy. For those pelvic REPs, such as obturator fossa pregnancy, or uterosacral ligament pregnancy, TVS can easily misdiagnose it as an adnexal ectopic pregnancy. And those REPs in the mid or upper abdomen may be out of reach for TVS, which potentially increases the risk of misdiagnosis. Fortunately, the development of full abdominal ultrasonography, CT scan and magnetic resonance imaging (MRI) provide a strong support for early diagnosis of rare REP [29, 30, 35]. TAS is the most commonly used examination method (66.7%, 22/33), followed by CT (33.3%, 11/33) and MRI (18.2%, 6/33). Ultrasonography is superior to CT and MRI in determining the presence of yolk sac, embryo or fetal heartbeat, whereas the value of CT and MRI lies more in locating the pregnancy site and delineating the relationship between the gestational sac and the surrounding tissues. However, in some emergent situations, the patients (15.2%, 5/33) needed undertaking laparotomy or laparoscopy directly for life saving, and the diagnosis was made through surgical findings or postoperative pathology. Only 24.2% (8/33) were diagnosed with REP at the initial visit. 12 cases were misdiagnosed as an adnexal ectopic pregnancy and underwent laparoscopy, laparotomy or MTX treatment; 5 cases were misdiagnosed as simple failing intrauterine pregnancy and received medical abortion or curettage; 2 cases were misdiagnosed as cornual pregnancy and underwent laparoscopy; one case was misdiagnosed as intraabdominal pregnancy and underwent laparoscopic abdominal mass resection; and one was misdiagnosed as choriocarcinoma and treated by MTX chemotherapy. Therefore, misdiagnosis rate is quite high among REP cases. Several remarkable points need keeping in mind in the process of diagnosis. Firstly, we should closely monitor β-hCG levels and provide ultrasound examination timely. If there is a high β-hCG levels but no intrauterine pregnancy or no evidence of ordinary ectopic pregnancy, the possibility of REP should be considered and immediately investigated further with additional diagnostic procedures, especially for those with history of tubal surgery and IVF. Secondly, when there is a highly suspected of rare ectopic pregnancy, combined auxiliary examinations should be applied to exactly locate pregnancy site. Besides ultrasound, CT or MRI examination would be instrumental for diagnosis. Thirdly, when laparoscopy or laparotomy is taken in case of highly suspected ectopic pregnancy, but no obvious pregnancy mass is found, unusual locations such as the retroperitoneum should be carefully examined. If possible, intraoperative real-time ultrasound guidance may assist in finding the pregnancy site. Last but least, when the patient is hemodynamically unstable and imaging is unavailable, laparotomy only revealed retroperitoneal hematoma but no evidence of hemorrhagic spot, evacuation of retroperitoneal hematoma for histopathology may be helpful for diagnosis.
Treatment
Due to the high preoperative misdiagnosis rate, 63.4% (21/33) of REP patients have undergone two or more treatments (medication or surgery treatment), of which 6 cases experienced three treatments. Considering the invasive and vascularized nature of the villi tissue and its intimacy with surrounding organs and vasculature, the opinion of a multidisciplinary team is very important and necessary for selecting a suitable treatment program. Surgery is the mainstay in REP management, including laparoscopy and laparotomy. For women with stable haemodynamics, laparoscopic surgery is generally preferred over laparotomic surgery with advantage of shorter operative time and reduced blood loss. However, because REPs are often located alongside retroperitoneal great vessels, laparoscopic resection would be a great challenge. Otherwise, the choice of surgical approach is also related to the experience of the surgeon. Ferland et al. [9] had an attempt of robot-assisted laparoscopic removal of the REP mass implanted deeply in the right obturator fossa and obtained a good prognosis. Before attempting laparoscopic management, radiological examinations such as MRI, color Doppler ultrasonography may be necessary to elucidate the vascular supply of the pregnancy mass and exclude the infiltration of large retroperitoneal vascular, especially in more advanced gestations [18, 36]. Any gynecologist attempting such a procedure should be well-trained, have a thorough knowledge of the retroperitoneal anatomy, and be ready to convert to laparotomy in case of intraoperative complications or uncontrollable bleeding. Close cooperation with an abdominal surgeon and/or an interventional radiologist may prove invaluable to safely carry out these procedures. During the operation, complete resection of REP lesion is the first choice but not always the best, especially when the trophoblastic tissue invades surrounding organs or tissues. Singh Y et al. [39] suggested that the placenta should be preserved locally to avoid bleeding and organ damage caused by stripping, but the disadvantage was that the risk of postoperative infection, secondary bleeding and even trophoblastic disease increased.
Medical management might be a choice for a proportion of patients. Among the 6 cases of systemic treatment with MTX, 3 cases (including our case) chose such medical treatment after diagnosis of REP for fear of vascular injury and massive intraoperative hemorrhage [19, 37], whereas 2 cases were given intramuscular MTX due to misdiagnosis of adnexal ectopic pregnancy and choriocarcinoma, respectively [14, 21], and the other one was given after surgical resection of REP lesion [20]. In our case, ultrasound-guided local injection of potassium chloride solution into gestation sac was combined with systemic MTX in order to reduce embryonic activity. Zhang et al. [29] reported one patient treated with MTX and selective arterial embolization therapy. Unfortunately, all of the 6 cases were finally treated with retroperitoneal pregnancy resection due to treatment failure. Several factors may be responsible for the failure of systemic methotrexate treatment for REP, such as higher blood β-hCG levels, more advanced gestations, and presence of ectopic viable embryo. Remarkably, Huang et al. [30] reported 2 cases of REP who were successfully by CT-guided paracentesis and local MTX injection in the gestational sac. Although surgery is avoided, this method was time consuming for normalization of hCG levels.
MTX can also be used in combination with surgery. Ansong et al. [40] suggested that compared with operation alone, operation combined with MTX (i.m. 50 mg/m2) for abdominal pregnancy could significantly reduce bleeding and shorten the hospitalization time. Therefore, two cases underwent local MTX injection in gestational sac implantation site during the operation, for purpose of killing trophoblast cells, decreasing β-hCG, and reducing relevant complications [32, 36].
There were several limitations existing in our study. Because of the rarity of REP, the number of cases was small. Though reviewed all the included cases in detail, we still can’t figure out a definitive consensus or guideline for the management of REP. Through the case reported here, we emphasize the cooperation of a multidisciplinary team in clinical practice, and a treatment consensus is best devised via input from gynecologists, vascular surgeons, radiologists, interventional physicians, pathologists, and the patient. Besides, only English literature published in PubMed database was included in our study. Many cases reported in other languages or databases must have been missed.
In conclusion, REP is exceedingly rare and its pathogenesis is still unelucidated currently. Due to the non-specific clinical manifestations and complex pregnancy site, REP requires a high index of suspicion to reach a timely diagnosis and management. Abdominal ultrasound, CT and MRI are extremely important in the diagnosis and localization of REP. Although successful conservative treatment has been reported, surgery is still the mainstay in REP management. Given the propensity of REPs to implant alongside great vessels, a multidisciplinary approach and adequate preparation are essential to make a suitable surgical plan to alleviate life-threatening complications.
Availability of data and materials
All data analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- hCG:
-
Human chorionic gonadotropin
- REP:
-
Retroperitoneal ectopic pregnancy
- TVS:
-
Transvaginal ultrasonography
- TAS:
-
Transabdominal ultrasonography
- CT:
-
Computer tomography
- MRI:
-
Magnetic resonance imaging
- IVF-ET:
-
In vitro fertilization – embryo transfer
- MTX:
-
Methotrexate
References
Marion L, Meeks G. Ectopic Pregnancy: History, Incidence, Epidemiology, and Risk Factors. Clin Obstet Gynecol. 2012;55(2):376–86.
Poole A, Haas D, Magann EF. Early abdominal ectopic pregnancies: a systematic review of the literature. Gynecol Obstet Invest. 2012;74(4):249–60.
OuYang Z, Wei S, Wu J, Wan Z, Zhang M, Zhong B. Retroperitoneal ectopic pregnancy: A literature review of reported cases. Eur J Obstet Gynecol Reprod Biol. 2021;259:113–8.
Hall J, Harris M, Levy R, Walrond E. Retroperitoneal ectopic pregnancy. J Obstet Gynaecol Br Commonw. 1973;80:92–4.
Sotus P. Retroperitoneal ectopic pregnancy a case report. JAMA. 1977;238(13):1363–4.
Studdiford W. Primary peritoneal pregnancy. Am J Obstet Gynecol. 1942;44(3):487–91.
Shen L, Fu J, Huang W, Zhu H, Wang Q, Yang S, Wu T. Interventions for non-tubal ectopic pregnancy. Cochrane Database Syst Rev. 2014;7:CD011174.
Eisner SM, Ebert AD, David M. Rare Ectopic Pregnancies - A Literature Review for the Period 2007–2019 on Locations Outside the Uterus and Fallopian Tubes. Geburtshilfe Frauenheilkd. 2020;80(7):686–701.
Ferland R, Chadwick D, O’Brien J, Granai C. An ectopic pregnancy in the upper retroperitoneum following in vitro fertilization and embryo transfer. Obstet Gynecol. 1991;78:544–6.
Dmowski W, Rana N, Ding J, Wu W. Retroperitoneal Subpancreatic Ectopic Pregnancy Following In Vitro Fertilization in a Patient with Previous Bilateral Salpingectomy: How Did It Get There? J Assist Reprod Genet. 2002;19(2):90–3.
Reid F, Steel M. An exceptionally rare ectopic pregnancy. BJOG. 2003;110:222–3.
Lee J, Sohn K, Jung H. Retroperitoneal Ectopic Pregnancy. AJR Am J Roentgenol. 2005;184(5):1600–1.
Meire I, van Heusden A, Roukema MS, Niezen RA, Dhont M. A retroperitoneal pregnancy of an anencephalic fetus. J Obstet Gynaecol. 2007;27(5):518–9.
Iwama H, Tsutsumi S, Igarashi H, Takahashi K, Nakahara K, Kurachi H. A case of retroperitoneal ectopic pregnancy following IVF-ET in a patient with previous bilateral salpingectomy. Am J Perinatol. 2008;25(1):33–6.
Chang YL, Ko PC, Yen CF. Retroperitoneal abdominal pregnancy at left paracolic sulcus. J Minim Invasive Gynecol. 2008;15(6):660–1.
Lin J, Liu Q, Ju Y, Guan Q, Wu Y, Zheng N. Primary_obturator_foramen_pregnancy: a case report. Chin Med J (Engl). 2008;121(14):1328–30.
Bae S, Kim C, Kim K, Hwang I, Choi Y, Lee M, Cho B, Kang Y, Park J. Laparoscopic treatment of early retroperitoneal abdominal pregnancy implanted on inferior vena cava. Surg Laparosc Endosc Percutan Tech. 2009;19(4):e156-158.
Persson J, Reynisson P, Masback A, Epstein E, Saldeen P. Histopathology indicates lymphatic spread of a pelvic retroperitoneal ectopic pregnancy removed by robot-assisted laparoscopy with temporary occlusion of the blood supply. Acta Obstet Gynecol Scand. 2010;89(6):835–9.
Okorie CO. Retroperitoneal ectopic pregnancy: is there any place for non-surgical treatment with methotrexate? J Obstet Gynaecol Res. 2010;36(5):1133–6.
Martinez-Varea A, Hidalgo-Mora JJ, Paya V, Morcillo I, Martin E, Pellicer A. Retroperitoneal ectopic pregnancy after intrauterine insemination. Fertil Steril. 2011;95(7):2433-e2431-2433.
Jiang W, Lv S, Sun L, Singer G, Xu C, Lu X. Diagnosis and treatment of retroperitoneal ectopic pregnancy: review of the literature. Gynecol Obstet Invest. 2014;77(4):205–10.
Liang C, Li X, Zhao B, Du Y, Xu S. Demonstration of the route of embryo migration in retroperitoneal ectopic pregnancy using contrast-enhanced computed tomography. J Obstet Gynaecol Res. 2014;40(3):849–52.
Protopapas A, Akrivos N, Athanasiou S, Chatzipapas I, Domali A, Loutradis D. Ultrasound-assisted intraoperative localization and laparoscopic management of a previously missed unruptured retroperitoneal ectopic pregnancy. Gynecol Surg. 2014;11(3):207–11.
Ouassour S, Filali AA, Raiss M, Bezad R, Tazi Z, Alami MH, Bennani J, Dafiri R. Retroperitoneal Ectopic Pregnancy: Diagnosis and Therapeutic Challenges. Case Rep Surg. 2017;2017:9871865.
Yang M, Cidan L, Zhang D. Retroperitoneal ectopic pregnancy: a case report and review of the literature. BMC Pregnancy Childbirth. 2017;17(1):358.
Pak J, Durfee J, Pedro L, Osborne A, Larkins-Pettigrew M. Retroperitoneal Ectopic Pregnancy. Obstet Gynecol. 2018;132(6):1491–3.
Yang Y, Liu Z, Song L, Liu H, Li L, Meng Y. Diagnosis and surgical therapy of the retroperitoneal ectopic pregnancy: A case report. Int J Surg Case Rep. 2018;49:21–4.
Velemínský M, Štěpánek O, Koznar P, Michal M, Mainzerová P, Štiková Z. A rare case of ectopic pregnancy - retroperitoneal ectopic pregnancy. Neuroendocrinol Lett. 2018;39(3):156–8.
Zhang M, Qin LL. A case of retroperitoneal para-aortic ectopic pregnancy detected by sonography. J Clin Ultrasound. 2018;46(6):412–4.
Huang X, Zhong R, Tan X, Zeng L, Jiang K, Mei S, Ye Z, Luo X. Conservative management of retroperitoneal ectopic pregnancy by computed tomographic-guided methotrexate injection in the gestational sac: 2 Case Reports and Literature Review. J Minim Invasive Gynecol. 2019;26(6):1187–92.
Lu Q, Zhang Z, Zhang Z. Laparoscopic Management of Retroperitoneal Ectopic Pregnancy. J Minim Invasive Gynecol. 2019;26(3):405–6.
Wang X, Ma D, Zhang Y, Chen Y, Zhang Y, Liu Z, Bi X, Wu X, Fan J. Rare heterotopic pregnancy after frozen embryo transfer: a case report and literature review. BMC Pregnancy Childbirth. 2020;20(1):542.
Le MT, Huynh MH, Cao CH, Hoang YM, Le KC, Dang VQ. Retroperitoneal ectopic pregnancy after in vitro fertilization/embryo transfer in patient with previous bilateral salpingectomy: A case report. Int J Gynaecol Obstet. 2020;150(3):418–9.
Hou Q, Xin L, Jian L, Pan J, Chen L, Song W. Retroperitoneal ectopic pregnancy: A case report and literature review. J Obstet Gynaecol Res. 2021;47(3):1186–90.
Anh ND, Hai NX, Ha NT, Toan NK, Thuong PH, Duc NM. Retroperitoneal ectopic pregnancy after in vitro fertilization: A case report of a patient with bilateral salpingectomy. Radiol Case Rep. 2022;17(3):721–4.
Wen X, Yan X, Zhang Q, Dong P, Zhou L, Wang S. Retroperitoneal Ectopic Pregnancy: A Case Report. J Minim Invasive Gynecol. 2021;28(9):1662–5.
Di Lorenzo G, Romano F, Mirenda G, Cracco F, Buonomo F, Stabile G, Facchin S, Ricci G. “Nerve-sparing” laparoscopic treatment of parametrial ectopic pregnancy. Fertil Steril. 2021;116(4):1197–9.
Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril. 1999;71(2):282–6.
Singh Y, Singh SK, Ganguly M, Singh S, Kumar P. Secondary abdominal pregnancy. Med J Armed Forces India. 2016;72(2):186–8.
Ansong E, Illahi G, Shen L, Wu X. Analyzing the clinical significance of postoperative methotrexate in the management of early abdominal pregnancy: analysis of 10 cases. Ginekol Pol. 2019;90(8):438–43.
Acknowledgements
The authors would like to thank the patient for her permission to present this case report to sensitize practitioners. The authors also thank all the medical staff who participated in the diagnosis and treatment of this patient.
Funding
The present study was supported by grants awarded to Qing Yang by the National Natural Science Foundation of China (No 81872125) which played a role in the design of the study, and Dandan Wang by the 345 Talent Project (Category 30C) of Shengjing Hospital of China Medical University (No 14) which played a role in the collection, analysis and interpretation of data.
Author information
Authors and Affiliations
Contributions
DDW and HNX carried out the retrospective review of the case, the search of related literature and prepared the manuscript draft. QY and DLC conceived of the study and were responsible for revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This paper was approved by the Ethics Committee of the Institutional Review Board (IRB) of Shengjing Hospital of China Medical University. The patient described in this case report provided informed consent.
Consent for publication
Written consent has been obtained from the patient for publication of this case report and any accompanying images.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, H., Cheng, D., Yang, Q. et al. Multidisciplinary treatment of retroperitoneal ectopic pregnancy: a case report and literature review. BMC Pregnancy Childbirth 22, 472 (2022). https://doi.org/10.1186/s12884-022-04799-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-04799-5