Abstract
Background
Although randomized controlled trials (RCTs) have shown that calcitonin gene-related peptide (CGRP)-targeted monoclonal antibodies (CGRP mAbs) are an efficacious and safe therapeutic modality for migraine prevention, their clinical benefits have not been well validated in Japanese patients in the real-world setting. The present study aimed to evaluate the real-world efficacy and safety of galcanezumab, fremanezumab, and erenumab in Japanese patients with migraine.
Methods
This observational retrospective cohort study was conducted at two headache centers in Japan. Patients with migraine who had experienced treatment failure with at least one traditional oral migraine preventive agent were treated with a CGRP mAb de novo. The primary efficacy endpoints were the changes from baseline in monthly migraine days (MMDs) and Headache Impact Test-6 (HIT-6) score after 3 dosing intervals (V3). We explored whether demographic and clinical characteristics predicted therapeutic outcomes at V3.
Results
Sixty-eight patients who completed three doses of a CGRP mAb (85.3% female [58/68], mean age: 46.2 ± 13.1 years) were included in the analysis. There were 19 patients with chronic migraine. The baseline MMDs were 13.4 ± 6.0. After 3 doses, the MMDs significantly decreased to 7.4 ± 5.5 (p < 0.0001), and the 50% response rate was 50.0%. HIT-6 score was significantly reduced from 66.7 ± 5.4 to 56.2 ± 8.7 after 3 doses (P = 0.0001). There was a positive correlation between the changes in MMDs and HIT-6 score from baseline after 2 doses (p = 0.0189). Those who achieved a ≥ 50% therapeutic response after the first and second doses were significantly more likely to do so at V3 (crude odds ratio: 3.474 [95% CI: 1.037 to 10.4], p = 0.0467). The most frequent adverse event was constipation (7.4%). None of the adverse events were serious, and there was no need for treatment discontinuation.
Conclusions
This real-world study demonstrated that CGRP mAbs conferred Japanese patients with efficacious and safe migraine prevention, and an initial positive therapeutic response was predictive of subsequent favorable outcomes. Concomitant measurement of MMDs and HIT-6 score was useful in evaluating the efficacy of CGRP mAbs in migraine prevention.
Similar content being viewed by others
Background
Migraine is a chronic disorder affecting more than one billion people worldwide [1]. This headache disorder is characterized by recurrent headache attacks of moderate to severe intensity, which interfere with daily activity. The interictal symptoms of migraine include allodynia, hypersensitivity, photophobia, phonophobia, osmophobia, visual/vestibular disturbances, and motion sickness [2]. The Eurolight project revealed that interictal symptoms, reported in 26.0% of patients with episodic migraine (EM), caused loss of productivity [3]. Hence, migraine causes considerable long-term disability in the sufferer’s daily and social life [3,4,5]. The Global Burden of Disease 2019 study showed that migraine is second among the world's causes of disability in terms of years lived with disability [6]. From a therapeutic viewpoint, effective and well-tolerated preventive therapy is key to enhancing the quality of life of migraineurs, especially those affected by high-frequency episodic migraine (HFEM) and chronic migraine (CM). CGRP plays a crucial role in migraine pathogenesis [7,8,9]. This neuropeptide is expressed in the trigeminal afferents innervating the dura, an important disease site associated with migraine [10, 11]. Monoclonal antibodies targeting either CGRP (galcanezumab, fremanezumab, and eptinezumab) or its receptor (erenumab) have been developed for migraine therapy. All of them have been efficacious in migraine prophylaxis, with favorable safety profiles in global RCTs [12,13,14,15]. Subsequent studies reported efficacy and safety for these monoclonal antibodies in migraineurs who had been unsuccessfully treated with preexisting preventive drugs, thus expanding the utility of the novel therapeutic agents [16,17,18,19]. However, it is important to note that migraine patients of Asian ethnicity were underrepresented in these studies. All four CGRP-targeted monoclonal antibodies are now approved for migraine prophylaxis. The strict inclusion and exclusion criteria imposed by the clinical trials limit the generalizability of the obtained results to patients seen in real-world situations.
RCTs have also demonstrated the efficacy and safety of galcanezumab [20], fremanezumab [21], and erenumab [22] for migraine prophylaxis in Japanese patients. Consequently, galcanezumab was first approved for migraine prevention in January 2021, followed by fremanezumab and erenumab in June 2021, in Japan. Although several Japanese single-center real-world studies have been published [23,24,25,26], there is still a paucity of real-world data on the efficacy and safety of CGRP mAbs in Japanese patients with migraine.
The purpose of the present study was to evaluate the effectiveness and safety of CGRP mAbs in real-world migraine therapy at two Japanese headache centers.
Methods
Study subjects
This is a retrospective, real-world study conducted at the Department of Neurology, Tokyo Dental College Ichikawa General Hospital and Saitama International Headache Center, Saitama Neuropsychiatric Institute, Saitama, Japan. It was approved by the Tokyo Dental College Ichikawa General Hospital Ethics Committee (Authorization number: I 23–02) and the Saitama Neuropsychiatric Institute Ethics Committee (SN I 23–002). We used opt-out procedures to obtain consent in the present study. The need for informed consent was waived by the Tokyo Dental College Ichikawa General Hospital Ethics Committee and the Saitama Neuropsychiatric Institute Ethics Committee, in accordance with national regulations (Ethical Guidelines for Medical and Biological Research Involving Human Subjects). All methods were carried out in accordance with relevant guidelines and regulations.
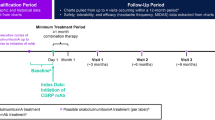
We included migraine patients diagnosed by board-accredited neurologists according to the diagnostic criteria of the International Classification of Headache Disorders 3rd edition (ICHD-3) and treated de novo with one of the CGRP mAbs (galcanezumab, fremanezumab, and erenumab). All participants underwent a cranial MRI or CT scan to exclude secondary headache disorders. Blood tests were conducted as necessary. Before using a CGRP mAb, all the participants were required to have a history of treatment failure with at least one preexisting migraine prophylactic drug or traditional oral migraine preventive (TOMP) (lomerizine, propranolol, valproate, and amitriptyline) due to insufficient effectiveness, side effects, and/or poor tolerability. All patients had to have suffered from migraine attacks on at least 4 days per month on average during the last 3 months prior to their initial CGRP mAb treatment. Among the patients enrolled in this study, only those who completed 3 cycles of antibody administration and clinical assessment were eligible for data analysis (Fig. 1). Migraine cases complicated with medication-overuse headache (MOH) diagnosed in accordance with the ICHD-3 criteria were included. We excluded cases in which the baseline headache status was unclear.
Migraine treatment with CGRP mAbs
CGRP mAb therapy was conducted as follows. For galcanezumab, a loading dose of 240 mg was administered subcutaneously for the first month, and 120 mg was administered monthly thereafter. For fremanezumab, all the analyzed patients received monthly subcutaneous administration at a dose of 225 mg. Erenumab was subcutaneously administered at a dose of 70 mg every 4 weeks. Concomitant use of TOMPs was allowed. No patients were treated with botulinum neurotoxin, which is not approved for chronic migraine in Japan. Triptans, acetaminophen, and NSAIDs were used for acute treatment as needed.
Clinical evaluations and outcomes
Prior to the commencement of CGRP mAb treatment, we collected information about demographic characteristics, comorbidities, headache characteristics, baseline migraine days, accompanying symptoms, and acute headache medication use. The history of acute and preventive migraine therapy was reviewed. We instructed patients to fill out the headache diary every day to capture their headache status (duration, severity, and presence of accompanying symptoms). Patients were asked to complete the Headache Impact Test-6 (HIT-6), Generalized Anxiety Disorder-7 (GAD-7) and Patient Health Questionnaire-9 (PHQ-9).
In the present study, a migraine day was defined as any of the following:
-
➢ A calendar day (00:00 to 23:59) on which there were at least 2 consecutive hours of headache meeting the criteria for migraine with or without aura
-
➢ A calendar day (00:00 to 23:59) on which there were at least 2 consecutive hours of headache meeting the criteria for probable migraine, a migraine subtype where only one migraine criterion is missing.
-
➢ A calendar day (00:00 to 23:59) with headache of any duration that was treated with triptans.
We also verified the migrainous nature of each headache attack recorded in the headache diary by directly asking the patient. The first primary efficacy endpoint was the change from baseline in monthly migraine days (MMDs) during the third dosing interval. The number of MMDs was calculated per 28 days. The other primary efficacy endpoint was the change from baseline in the HIT-6 score during the third dosing interval. Moreover, 50%, 75% and 100% responder rates (RRs), defined as ≥ 50%, ≥ 75% and 100% reductions in migraine days, respectively, were calculated during the first three CGRP mAb dosing intervals. At every visit after the initiation of CGRP mAb treatment, patients were asked to report any adverse events. Among adverse events associated with CGRP mAbs, injection site reactions are known to be common [12,13,14,15]. Hence, only skin changes (erythema, swelling, eruption, etc.) lasting beyond the day of administration were regarded as adverse events. We considered a ≥ 20 mmHg increase in systolic blood pressure a significant blood pressure elevation.
Statistical analysis
Statistical analyses were conducted with GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics ver. 29 (Armonk, NY, USA). Numerical data are expressed as mean with standard deviation (SD) or as 95% confidence interval (CI). Frequency data analyses were performed using the chi-square test or Fisher’s exact test. For numerical data, between-group comparisons were performed using analysis of variance (ANOVA) or the Kruskal–Wallis test, in accordance with the results of the D'Agostino-Pearson test for the normality of the data distribution. Multiple comparisons were carried out with Dunnett’s post hoc test or Dunn’s post hoc test. Two-way ANOVA was performed to compare the effects of the CGRP mAbs on the temporal trajectories of MMD and the HIT-6 score. For two-group comparisons, statistical analyses were conducted using Student’s t test or the Mann–Whitney test in accordance with the results of the D'Agostino-Pearson test. The correlation between MMD and HIT-6 score was evaluated as the Spearman rank-order correlation coefficient. The predictive ability of demographic and clinical parameters for 50% RR at visit 3 was analyzed by univariate and multivariate logistic regression models. No missing data were imputed. Statistical significance was set at p < 0.05 (two-tailed).
Results
Study participants
From May 2021 through March 2023, 87 patients with migraine were treated de novo with one of the CGRP mAbs (Fig. 2). Of them, 83 patients completed 3 doses of CGRP mAbs (Fig. 2). We were not able to collect information about the baseline headache status in 15 patients, who were excluded from the study. Thus, 68 patients (30 patients at Tokyo Dental College Ichikawa General Hospital and 38 patients at Saitama Neuropsychiatric Institute) were eligible for data analysis (Fig. 2). The included patients (85.3% female [58/68], mean age: 46.2 ± 13.1 years) were of Japanese ethnicity. CM and medication overuse were found in 27.9% and 14.7% of patients, respectively. The demographic and baseline clinical characteristics of the analyzed participants are shown in Table 1. Galcanezumab, fremanezumab, and erenumab were administered in 31, 24, and 13 patients, respectively. There were no significant differences in demographic parameters among the treatment groups. Among the previously used TOMPs, amitriptyline was used by the most people (33%, Fig. 3). During the CGRP mAb dosing intervals, there was concomitant use of preexisting preventive drugs in 42 cases (61.8%, Table 1).
Effects of CGRP mAbs on MMDs
We examined the effect of overall antibody treatment on the temporal changes in MMDs. The first, second, and third dosing intervals were 31.0 ± 5.3 days, 30.9 ± 4.0 days, and 31.4 ± 5.2 days, respectively. The baseline value of MMDs was 13.4 ± 6.0. After 3 doses, MMDs significantly decreased to 7.4 ± 5.5 (p < 0.0001, Fig. 4A). The 50% RR was 39.4%, 43.3% and 50.0% at V1, V2, and V3, respectively (Fig. 4B, red bars). Significant MMD reductions from baseline to V3 were observed in both CM (9.0 ± 6.0 vs. 19.1 ± 6,1, p = 0.0001, Supplementary Fig. 1A) and EM (6.8 ± 5.2 vs. 11.2 ± 4.2, p < 0.0001, Supplementary Fig. 1B). The RRs for EM and CM are shown in Supplementary Fig. 1C and D, respectively.
Effects of all the CGRP mAbs on MMDs. A Temporal profile of MMDs in patients treated with any CGRP mAb (n = 68). Data are shown as mean ± SD. Statistical analysis was performed using the Kruskal–Wallis test with Dunn’s post hoc test. ***p < 0.001, ****p < 0.0001. B 50%, 75%, and 100% RRs at V1, V2, and V3
Comparison of the therapeutic effects on MMDs among CGRP mAbs
As shown in Fig. 5A–C, galcanezumab and fremanezumab significantly reduced MMDs after 3 doses (galcanezumab: 7.1 ± 5.8 vs. 14.0 ± 5.9, p = 0.0001; fremanezumab: 7.8 ± 5.4 vs. 12.1 ± 5.5, p = 0.0042), whereas there was no significant change in MMDs after 3 doses of erenumab (7.6 ± 5.2 vs. 12.9 ± 7.0, p = 0.1229). We next analyzed the effects of each CGRP mAb on MMDs using two-way ANOVA. There was a significant effect of time (F (3, 257) = 11.74, p < 0.001), but there was no significant effect of CGRP mAb type (F (2, 257) = 0.6174, p = 0.154) or the interaction between them (F (6, 257) = 0.4534, p = 0.8423). There were no significant differences in MMDs among CGRP mAbs at any timepoint (Fig. 5D).
Effects of each CGRP mAb on MMDs. Temporal profiles of MMDs in patients treated with galcanezumab (n = 31, A), fremanezumab (n = 24, B), and erenumab (n = 13, C). Data are shown as mean ± SD. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test for galcanezumab and the Kruskal–Wallis test with Dunn’s post hoc test for fremanezumab and erenumab. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. D Overlay line graph depicting the temporal profiles of each CGRP mAb
Effects of CGRP mAb treatment on HIT-6 score
We investigated the effects of CGRP mAb treatment on the HIT-6 score. Considering CGRP mAb treatment overall, the baseline HIT-6 score was 66.7 ± 5.4, and after 3 doses, we observed a significant reduction to 56.2 ± 8.7 (P = 0.0001, Fig. 6). Significant improvements in the HIT-6 score at V3 were observed in both EM (56.8 ± 8.6 vs. 66.4 ± 5.8, p = 0.0001) and CM (54.9 ± 9.1 vs. 67.5 ± 5.2, p = 0.0001) (Supplementary Fig. 2).
Comparison of the therapeutic effects on HIT-6 score among CGRP mAbs
All three CGRP mAbs significantly reduced the HIT-6 score after 3 doses (galcanezumab: 54.3 ± 10.2 vs. 66.9 ± 5.9, p = 0.0001; fremanezumab: 58.8 ± 6.8 vs. 67.2 ± 5.5, p = 0.0003; erenumab: 56.4 ± 7.5 vs. 65.5 ± 5.8, p = 0.0023, Fig. 7A). Two-way ANOVA revealed that there were significant effects of time (F (3, 218) = 20.09, p < 0.0001) and CGRP mAb type (F (2, 218) = 5.643, p = 0.0041), without an interaction between them (F (6, 218) = 0.8083, p = 0.5644). Multiple comparisons detected significant differences between galcanezumab and fremanezumab at V2 (mean differences: -5.3 [95% CI: -10.2 to -0.3], p = 0.0338) and V3 (mean differences: -5.8 [95% CI: -11.0 to -0.7], p = 0.0242 (Fig. 7B).
Effects of each CGRP mAb on HIT-6 score. Temporal profiles of HIT-6 score in patients treated with galcanezumab (n = 31, A), fremanezumab (n = 24, B), and erenumab (n = 13, C). Data are shown as mean ± SD. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test for galcanezumab and erenumab and the Kruskal–Wallis test with Dunn’s post hoc test for erenumab. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. D Overlay line graph depicting the temporal profiles of each CGRP mAb. Between-group comparisons at each timepoint were performed with Dunnett’s post hoc test. #p < 0.05, galcanezumab vs. fremanezumab
Temporal profiles of the distributions of MMDs and HIT-6 score
There was no correlation between MMDs and HIT-6 score at baseline (Supplementary Fig. 3A). We examined the correlations between the MMD difference from baseline and the HIT-6 score differences from baseline after the initiation of CGRP-targeted antibody treatment. The scatter plots showed that there was a tendency for the distribution to shift toward the lower left part (Supplementary Fig. 3B–D). There was a statistically significant correlation between the change in MMDs and the change in the HIT-6 score from baseline to V2 (p = 0.0189).
Prediction of therapeutic response at V3
To explore demographic and clinical factors associated with the achievement of ≥ 50% therapeutic response at V3, age, disease duration, baseline MMDs, and baseline HIT-6 score were compared between the ≥ 50% responders and nonresponders at V3. There were no significant between-group differences in any of these factors (Supplementary Table 1). These factors were not found to be significant contributors to ≥ 50% therapeutic response at V3 by univariate or multivariate logistic regression analysis (Supplementary Table 2).
Complete data on MMDs at baseline and the first three visits were available for 65 participants (Supplementary Fig. 4). Based on their data, we asked whether the therapeutic response at V3 could be predicted from the response status at V1 or V2 by calculating positive and negative predictive values. The ≥ 50% responders at both V1 and V2 were significantly more likely to be ≥ 50% responders at V3 than nonresponders at both V1 and V2 were (crude odds ratio: 3.474 [95% CI: 1.037 to 10.4], p = 0.0467, Supplementary Table 3).
Adverse events
The treatment-emergent adverse events reported during the study period are shown in Table 2. Constipation was the most frequent adverse event (7.4%). None of the adverse events were serious, and treatment discontinuation was not needed.
Discussion
Our two-center real-world study demonstrated that CGRP mAbs were effective in migraine prophylaxis in Japanese patients with a history of treatment failure with at least one TOMP in terms of the changes from baseline in MMDs and HIT-6 score. Significant reductions in MMDs were achieved by galcanezumab and fremanezumab. The HIT-6 score was significantly decreased by all CGRP mAbs. There was no significant correlation between MMDs and the HIT-6 score at baseline. After CGRP mAb treatment, a significant positive correlation between the changes in MMDs and HIT-6 score from baseline was observed at V2. For achieving a ≥ 50% reduction in MMDs at V3, the positive therapeutic response at V1 and V2 was found to be a significant predictor. With respect to safety, there were no serious treatment-emergent adverse events. Taken together, our real-world data confirm that CGRP mAbs provide excellent migraine prophylaxis with favorable safety and tolerability profiles in Japanese patients with migraine. Unlike previous Japanese real-world studies [23,24,25,26], the present study was conducted at two independent institutions, extending the generalizability of the data.
The present study evaluated the temporal changes in MMDs for each CGRP mAb. Unlike galcanezumab and fremanezumab, erenumab did not significantly decrease MMDs from baseline at V3. However, we must interpret our findings cautiously due to the low number of patients treated with erenumab. With more patients, the results with erenumab would also have been statistically significant. The comparison of efficacy in reducing MMDs among CGRP mAbs is a clinically relevant topic, which should be explored with a much larger population [25, 27,28,29].
All the CGRP mAbs used in the present study improved the HIT-6 score from baseline at V3. HIT-6 score is a patient-reported outcome measure reflecting the negative impact of migraine attacks on normal daily activity [30]. Hence, the present study provides evidence that CGRP mAbs abate migraine-associated disability, in line with previous reports [31,32,33,34,35]. In our data, there was no significant correlation between MMDs and HIT-6 scores in the study subjects at baseline, implying that migraine-associated disability was not determined simply by the number of MMDs. Other factors, such as headache intensity and duration of each migraine attack, contribute to the disability as well. Although both MMDs and HIT-6 score declined after CGRP mAb treatment, we found a significant correlation between the MMD difference from baseline and the HIT-6 score difference from baseline only at V2. HIT-6 scoring is useful in assessing the effect of migraine-associated disability factors other than MMDs. The number of MMDs alone does not seem to be sufficient to encompass the complexity of therapeutic benefits of CGPP Abs. Our data highlight the utility of implementing HIT-6 scoring along with MMD count to thoroughly evaluate the disease condition of migraineurs. To the best of our knowledge, this is the first real-world study to examine the effects of CGRP mAbs on both MMDs and HIT-6 score in Japanese patients with migraine.
The 50% RR is considered a useful index to compare the efficacy of prophylactic therapy for migraine among different studies. In RCTs, galcanezumab, fremanezumab, and erenumab yielded 50% RRs at 3 months in 39.7–62.3% of patients with EM and 27.5–50.0% of patients with CM [12,13,14, 36,37,38,39,40]. Real-world studies evaluating these CGRP mAbs for HFEM and CM reported 3-month 50% RRs of 39.5–61.5% [23, 24, 31, 34, 41, 42]. Hence, the results of our study are consistent with these previous findings. Our analysis revealed that the accomplishment of 50% RR at V1 and V2 increased the likelihood of a positive outcome at V3. This finding supports the therapeutic consistency of CGRP mAbs [26, 34, 35, 41, 43, 44].
Attempts have been made to detect predictive factors for a positive therapeutic response to CGRP mAbs in the real-world setting [45]. We explored whether demographic and clinical parameters could predict the therapeutic response at V3. However, age, disease duration, baseline MMDs, and baseline HIT-6 were not relevant to 50% RR at V3. Although previous studies found these clinical parameters to be predictors of clinical efficacy, it should be pointed out that there are clear discrepancies among such data in terms of age [24, 31, 46] and baseline MMDs [25, 46, 47]. Hence, there is the possibility that the effectiveness of these parameters as therapeutic predictors is influenced by the difference in study populations.
This was a real-world study in which the use of other prophylactic drugs was not restricted, so 61.8% of participants were concomitantly taking at least one TOMP. Many TOMPs can act in the central nervous system [48]. Amitriptyline and lomerizine were often used in our study subjects. Currently, lomerizine is used exclusively in Japan. This agent is a centrally acting calcium channel blocker similar to flunarizine, a globally used migraine preventive drug [49,50,51]. Migraine is a complex disorder involving the central nervous system as well as the trigeminovascular system [1, 7, 8]. CGRP mAbs are likely to rectify abnormalities in the trigeminovascular system [9]. Hence, the combination of CGRP mAbs and centrally acting migraine prophylaxis may confer a potent therapeutic effect by affecting complementary pathways (Fig. 8), although there is still insufficient evidence to support this paradigm [52].
There are limitations to the present study. First, the relatively small number of study subjects and inequality of assignment to each CGRP mAb lowered the statistical power. Because the clinical data were not collected in a prospective manner, 15 cases (18%) were excluded due to missing baseline data. In Japan, galcanezumab was approved earliest, so more patients were treated with this antibody than any other. Second, in accordance with the general Japanese insurance policy, patients must cover 30% of their drug costs. Hence, we are able to apply CGRP mAb therapy only to those who can afford it, generating a selection bias stemming from the economic status of patients. Third, we did not collect detailed information about the clinical features of migraine attacks, such as the nature of the headaches and their laterality and the presence of accompanying symptoms. Unilaterality, pulsatile nature, vomiting, cranial autonomic symptoms, allodynia, osmophobia, and good response to triptans have been identified as predictive factors for therapeutic response [25, 43, 53, 54]. Conversely, concomitant depression and obesity are known to predict poor outcomes [43, 53]. Hence, our failure to collect detailed clinical information may have led to our inability to find effective predictors for therapeutic response at V3. Lastly, the short follow-up duration may have limited our study, because it has been pointed out that the effectiveness of CGRP mAbs might be evident up to 6 months of consecutive treatment [52].
Conclusions
The present study provides new real-world evidence of the efficacy and safety of CGRP mAbs in Japanese migraine sufferers. In particular, our data first demonstrated the utility of concomitant monitoring of MMDs and the HIT-6 score to evaluate the efficacy of CGRP mAbs in Japanese patients. In addition, each CGRP mAb exhibited distinct improving actions on MMDs and the HIT-6 score.
Availability of data and materials
Not applicable.
Change history
09 May 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12883-024-03665-5
Abbreviations
- AED:
-
Antiepileptic drug
- CGRP:
-
Calcitonin gene-related peptide
- CI:
-
Confidence interval
- CM:
-
Chronic migraine
- EM:
-
Episodic migraine
- GAD-7:
-
General Anxiety Disorder-7
- HFEM:
-
High-frequency episodic migraine
- HIT-6:
-
Headache Impact Test-6
- ICHD-3:
-
International Classification of Headache Disorders 3rd edition
- MDAS:
-
Migraine Disability Assessment Test
- MMD:
-
Monthly migraine day
- MOH:
-
Medication-overuse headache
- OR:
-
Odds ratio
- PHQ-9:
-
Patient Health Questionnaire-9
- RCT:
-
Randomized clinical trial
- RR:
-
Responder rate
- SD:
-
Standard deviation
References
Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–76. https://doi.org/10.1056/NEJMra1915327.
Vincent M, Viktrup L, Nicholson RA, Ossipov MH, Vargas BB. The not so hidden impact of interictal burden in migraine: A narrative review. Front Neurol. 2022;13:1032103. https://doi.org/10.3389/fneur.2022.1032103.
Lampl C, Thomas H, Stovner LJ, Tassorelli C, Katsarava Z, Lainez JM, et al. Interictal burden attributable to episodic headache: findings from the Eurolight project. J Headache Pain. 2016;17:9. https://doi.org/10.1186/s10194-016-0599-8.
Matsumori Y, Ueda K, Komori M, Zagar AJ, Kim Y, Jaffe DH, et al. Burden of Migraine in Japan: Results of the ObserVational Survey of the Epidemiology, tReatment, and Care Of MigrainE (OVERCOME [Japan]) Study. Neurol Ther. 2022;11(1):205–22. https://doi.org/10.1007/s40120-021-00305-9.
Shimizu T, Sakai F, Miyake H, Sone T, Sato M, Tanabe S, et al. Disability, quality of life, productivity impairment and employer costs of migraine in the workplace. J Headache Pain. 2021;22(1):29. https://doi.org/10.1186/s10194-021-01243-5.
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting The Burden: the Global Campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. https://doi.org/10.1186/s10194-020-01208-0.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–50. https://doi.org/10.1038/s41582-018-0003-1.
Puledda F, Silva EM, Suwanlaong K, Goadsby PJ. Migraine: from pathophysiology to treatment. J Neurol. 2023;270(7):3654–66. https://doi.org/10.1007/s00415-023-11706-1.
Labastida-Ramirez A, Caronna E, Gollion C, Stanyer E, Dapkute A, Braniste D, et al. Mode and site of action of therapies targeting CGRP signaling. J Headache Pain. 2023;24(1):125. https://doi.org/10.1186/s10194-023-01644-8.
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14(11):1289–303. https://doi.org/10.1016/j.jpain.2013.03.010.
Lukacs M, Haanes KA, Majlath Z, Tajti J, Vecsei L, Warfvinge K, Edvinsson L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J Headache Pain. 2015;16:564. https://doi.org/10.1186/s10194-015-0564-y.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018;75(9):1080–8. https://doi.org/10.1001/jamaneurol.2018.1212.
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA. 2018;319(19):1999–2008. https://doi.org/10.1001/jama.2018.4853.
Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, et al. A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med. 2017;377(22):2123–32. https://doi.org/10.1056/NEJMoa1705848.
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54. https://doi.org/10.1177/0333102420905132.
Ashina M, Lanteri-Minet M, Pozo-Rosich P, Ettrup A, Christoffersen CL, Josiassen MK, et al. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022;21(7):597–607. https://doi.org/10.1016/S1474-4422(22)00185-5.
Mulleners WM, Kim BK, Lainez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25. https://doi.org/10.1016/S1474-4422(20)30279-9.
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–40. https://doi.org/10.1016/S0140-6736(19)31946-4.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, Klatt J. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7. https://doi.org/10.1016/S0140-6736(18)32534-0.
Fumihiko S, Ozeki A, Skljarevski V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: A phase 2 randomized controlled clinical trial. Cephalalgia Report. 2020;3:1–10.
Sakai F, Suzuki N, Kim BK, Tatsuoka Y, Imai N, Ning X, et al. Efficacy and safety of fremanezumab for episodic migraine prevention: Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache. 2021;61(7):1102–11. https://doi.org/10.1111/head.14178.
Takeshima T, Sakai F, Hirata K, Imai N, Matsumori Y, Yoshida R, et al. Erenumab treatment for migraine prevention in Japanese patients: Efficacy and safety results from a Phase 3, randomized, double-blind, placebo-controlled study. Headache. 2021;61(6):927–35. https://doi.org/10.1111/head.14138.
Takizawa T, Ohtani S, Watanabe N, Miyazaki N, Ishizuchi K, Sekiguchi K, et al. Real-world evidence of galcanezumab for migraine treatment in Japan: a retrospective analysis. BMC Neurol. 2022;22(1):512. https://doi.org/10.1186/s12883-022-03041-1.
Ihara K, Ohtani S, Watanabe N, Takahashi N, Miyazaki N, Ishizuchi K, et al. Predicting response to CGRP-monoclonal antibodies in patients with migraine in Japan: a single-centre retrospective observational study. J Headache Pain. 2023;24(1):23. https://doi.org/10.1186/s10194-023-01556-7.
Suzuki K, Suzuki S, Shiina T, Tatsumoto M, Fujita H, Haruyama Y, Hirata K. Effectiveness of three calcitonin gene-related peptide monoclonal antibodies for migraine: A 12-month, single-center, observational real-world study in Japan. Cephalalgia. 2023;43(5):3331024231177649. https://doi.org/10.1177/03331024231177649.
Suzuki S, Suzuki K, Shiina T, Haruyama Y, Hirata K. Real-world experience with monthly and quarterly dosing of fremanezumab for the treatment of patients with migraine in Japan. Front Neurol. 2023;14:1220285. https://doi.org/10.3389/fneur.2023.1220285.
Shi M, Guo J, Li Z, Sun H, Yang X, Yang D, Zhao H. Network meta-analysis on efficacy and safety of different anti-CGRP monoclonal antibody regimens for prophylaxis and treatment of episodic migraine. Neurol Res. 2021;43(11):932–49. https://doi.org/10.1080/01616412.2021.1940672.
Sacca F, Braca S, Sansone M, Miele A, Stornaiuolo A, De Simone R, Russo CV. A head-to-head observational cohort study on the efficacy and safety of monoclonal antibodies against calcitonin gene-related peptide for chronic and episodic migraine. Headache. 2023;63(6):788–94. https://doi.org/10.1111/head.14528.
Wang X, Wen D, He Q, You C, Ma L. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine patients with prior preventive treatment failure: a network meta-analysis. J Headache Pain. 2022;23(1):105. https://doi.org/10.1186/s10194-022-01472-2.
Houts CR, Wirth RJ, McGinley JS, Gwaltney C, Kassel E, Snapinn S, Cady R. Content Validity of HIT-6 as a Measure of Headache Impact in People With Migraine: A Narrative Review. Headache. 2020;60(1):28–39. https://doi.org/10.1111/head.13701.
Barbanti P, Egeo G, Aurilia C, d’Onofrio F, Albanese M, Cetta I, et al. Fremanezumab in the prevention of high-frequency episodic and chronic migraine: a 12-week, multicenter, real-life, cohort study (the FRIEND study). J Headache Pain. 2022;23(1):46. https://doi.org/10.1186/s10194-022-01396-x.
Gantenbein AR, Agosti R, Kamm CP, Landmann G, Meier N, Merki-Feld GS, et al. Swiss QUality of life and healthcare impact Assessment in a Real-world Erenumab treated migraine population (SQUARE study): interim results. J Headache Pain. 2022;23(1):142. https://doi.org/10.1186/s10194-022-01515-8.
Barbanti P, Egeo G, Aurilia C, Torelli P, Finocchi C, d’Onofrio F, et al. Early and sustained efficacy of fremanezumab over 24-weeks in migraine patients with multiple preventive treatment failures: the multicenter, prospective, real-life FRIEND2 study. J Headache Pain. 2023;24(1):30. https://doi.org/10.1186/s10194-023-01561-w.
Schiano di Cola F, Bolchini M, Ceccardi G, Caratozzolo S, Liberini P, Rao R, Padovani A. An observational study on monoclonal antibodies against calcitonin-gene-related peptide and its receptor. Eur J Neurol. 2023;30(6):1764–73. https://doi.org/10.1111/ene.15761.
Troy E, Shrukalla AA, Buture A, Conaty K, Macken E, Lonergan R, et al. Medium-term real-world data for erenumab in 177 treatment resistant or difficult to treat chronic migraine patients: persistence and patient reported outcome measures after 17–30 months. J Headache Pain. 2023;24(1):5. https://doi.org/10.1186/s10194-022-01536-3.
Ashina M, Tepper S, Brandes JL, Reuter U, Boudreau G, Dolezil D, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611–21. https://doi.org/10.1177/0333102418788347.
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–37. https://doi.org/10.1177/0333102418759786.
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. N Engl J Med. 2017;377(22):2113–22. https://doi.org/10.1056/NEJMoa1709038.
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–21. https://doi.org/10.1212/WNL.0000000000006640.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54. https://doi.org/10.1177/0333102418779543.
Alex A, Vaughn C, Rayhill M. Safety and Tolerability of 3 CGRP Monoclonal Antibodies in Practice: A Retrospective Cohort Study. Headache. 2020;60(10):2454–62. https://doi.org/10.1111/head.13956.
Torres-Ferrus M, Gallardo VJ, Alpuente A, Caronna E, Gine-Cipres E, Pozo-Rosich P. The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: a real-world evidence observational study. J Neurol. 2021;268(10):3789–98. https://doi.org/10.1007/s00415-021-10523-8.
Barbanti P, Egeo G, Aurilia C, Altamura C, d’Onofrio F, Finocchi C, et al. Predictors of response to anti-CGRP monoclonal antibodies: a 24-week, multicenter, prospective study on 864 migraine patients. J Headache Pain. 2022;23(1):138. https://doi.org/10.1186/s10194-022-01498-6.
Shibata M, Nihira A, Tanji Y, Ozeki A, Imagawa H, Komori M. Galcanezumab Efficacy Through the Dosing Interval in Japanese Patients with Episodic Migraine: Post Hoc Analysis of a Phase 2 Randomized Trial. Neurol Ther. 2023. https://doi.org/10.1007/s40120-023-00534-0.
Hong JB, Lange KS, Overeem LH, Triller P, Raffaelli B, Reuter U. A Scoping Review and Meta-Analysis of Anti-CGRP Monoclonal Antibodies: Predicting Response. Pharmaceuticals (Basel). 2023;16:7. https://doi.org/10.3390/ph16070934.
Lee HC, Cho S, Kim BK. Predictors of response to galcanezumab in patients with chronic migraine: a real-world prospective observational study. Neurol Sci. 2023;44(7):2455–63. https://doi.org/10.1007/s10072-023-06683-2.
Barbanti P, Aurilia C, Egeo G, Fofi L, Cevoli S, Colombo B, et al. Erenumab in the prevention of high-frequency episodic and chronic migraine: Erenumab in Real Life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache. 2021;61(2):363–72. https://doi.org/10.1111/head.14032.
Silberstein SD. Preventive Migraine Treatment. Continuum (Minneap Minn). 2015;21 4 Headache. 973–89. https://doi.org/10.1212/CON.0000000000000199.
Frenken CW, Nuijten ST. Flunarizine, a new preventive approach to migraine. A double-blind comparison with placebo. Clin Neurol Neurosurg. 1984;86(1):17–20. https://doi.org/10.1016/0303-8467(84)90273-7.
Mentenopoulos G, Manafi T, Logothetis J, Bostantzopoulou S. Flunarizine in the prevention of classical migraine: a placebo-controlled evaluation. Cephalalgia. 1985;5(Suppl 2):135–40. https://doi.org/10.1177/03331024850050S225.
Leone M, Grazzi L, La Mantia L, Bussone G. Flunarizine in migraine: a minireview. Headache. 1991;31(6):388–91. https://doi.org/10.1111/j.1526-4610.1991.hed3106388.x.
Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention - 2022 update. J Headache Pain. 2022;23(1):67. https://doi.org/10.1186/s10194-022-01431-x.
Raffaelli B, Fitzek M, Overeem LH, Storch E, Terhart M, Reuter U. Clinical evaluation of super-responders vs. non-responders to CGRP(-receptor) monoclonal antibodies: a real-world experience. J Headache Pain. 2023;24(1):16. https://doi.org/10.1186/s10194-023-01552-x.
Vernieri F, Brunelli N, Marcosano M, Aurilia C, Egeo G, Lovati C, et al. Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: The multicenter prospective cohort GARLIT study. Eur J Neurol. 2023;30(1):224–34. https://doi.org/10.1111/ene.15563.
Acknowledgements
The authors thank Dr. Shoko Takahashi at the Department of Neurology, Tokyo Saiseikai Central Hospital, Tokyo, Japan for her cooperation.
Funding
The APC of this article was supported by a research grant from Tokyo Dental College Ichikawa General Hospital.
Author information
Authors and Affiliations
Contributions
M.S. designed the study, M.S. drafted the manuscript, M.S. carried out data analysis, M.S., K.F., E.H., K.M., K.K., S.O., and F.S. performed data collection, and M.S., K.F., E.H., K.M., K.K., S.O. and F.S. revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Tokyo Dental College Ichikawa General Hospital Ethics Committee (Authorization number: I 23–02) and the Saitama Neuropsychiatric Institute Ethics Committee (SN I 23–002). We adopted opt-out procedures for obtaining consent in the present study. The need for informed consent was waived by the Tokyo Dental College Ichikawa General Hospital Ethics Committee and the Saitama Neuropsychiatric Institute Ethics Committee, in accordance with national regulations (Ethical Guidelines for Medical and Biological Research Involving Human Subjects). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
MS and FS received speaker honoraria from Daiichi Sankyo Company, Limited, Eli Lilly Japan, Otsuka Pharmaceutical Co., Ltd., and Amgen. The other authors do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: A couple of errors in numerical data were found under the headings “Effects of CGRP mAbs on MMDs”, “Comparison of the therapeutic effects on MMDs among CGRP mAbs” and Table 1.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Effects of all the CGRP mAbs on MMDs.
Additional file 2: Supplementary Figure 2.
Effects of all the CGRP mAbs on HIT-6 score in the EM and CM subgroups.
Additional file 3: Supplementary Figure 3.
Temporal profiles of the distributions of MMDs and HIT-6 score.
Additional file 4: Supplementary Figure 4.
Tree diagram depicting the numbers of ≥50% responders (green lines) and nonresponders (red lines) at each visit.
Additional file 5: Supplementary Table 1.
Comparison of demographic and clinical characteristics between ≥50% responders and non-responders.
Additional file 6: Supplementary Table 2.
Logistic regression analysis for 50% RR at V3.
Additional file 7: Supplementary Table 3.
Prediction of 50% response at V3 from the response status at V1 and V2.
Additional file 8: Supplementary file 8.
Visual abstract of the present study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shibata, M., Fujita, K., Hoshino, E. et al. Real-world experience with calcitonin gene-related peptide-targeted antibodies for migraine prevention: a retrospective observational cohort study at two Japanese headache centers. BMC Neurol 24, 32 (2024). https://doi.org/10.1186/s12883-023-03521-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03521-y