Abstract
Objective
The relative effects of monoclonal antibody against calcitonin gene-related peptide (CGRP) or its receptor for adult migraine patients with prior treatment failure remains uncertain. Therefore, this study systematically assessed the comparative effectiveness of different CGRP binding monoclonal antibodies (mAbs) for these patients.
Methods
Several online databases including Ovid MEDILNE, Ovid EMBASE, Cochrane Library, and ClinicalTrials.gov were systematically searched from inception to June 15, 2022. We included randomized clinical trials (RCT) of adult migraine patients with previous treatment failure that assessed any CGRP monoclonal antibody. The primary efficacy outcome was change in monthly migraine days (MMDs), and the primary safety outcome was treatment-emergent adverse events (TEAEs).
Results
Overall, seven studies totaling 3, 052 patients were included. Three-node analysis showed that CGRP mAbs was superior to CGRP receptor mAbs in reducing MMDs (MD: -1.55, 95% CrI: − 2.43 to − 0.44) and improving at least 50% response rates (RR: 1.52, 95% CrI: 1.04 to 2.21). Nine-node analysis showed galcanezumab 240 mg ranked first in reducing MMDs (MD -4.40, 95% CrI − 7.60 to − 1.19) and improving 50% response rates (RR: 4.18, 95% CrI: 2.63 to 6.67). Moreover, treatment with fremanezumab or eptinezumab 300 mg provides a significant advantage over erenumab 140 mg regarding an improved response rate of at least 50%. The analysis did not show difference in incidences of TEAEs and serious adverse events in any of the comparisons.
Conclusions
It appears that CGRP mAbs, especially galcanezumab 240 mg, monthly fremanezumab, and eptinezumab 300 mg, seem to be the best choice for the treatment of migraine patients with previous treatment failures. This finding also calls for future research that examine the associations between these medications in migraine therapy among the same patient group to testify the present findings.
Similar content being viewed by others
Introduction
Migraine is considered as one of the most important causes of disease-related disability worldwide, contributing to functional impairment as well as substantial social and economic burden [1,2,3]. Although there are several drugs used for migraine patients, many patients either cannot tolerate the side effects, or do not respond to oral migraine preventive medications. At last, they had to switch, re-initiate or discontinue on-going therapies. Up to 78% of patients with migraine have been reported to experience treatment failure [4, 5]. The burden is even higher for patients who have failed previous migraine preventive treatment [6, 7]. Therefore, developing novel drugs with favorable tolerability and sustained efficacy is urgent needed for migraine patients who failed previous treatments.
Monoclonal antibodies (mAbs) related to the calcitonin gene-related peptide (CGRP) are new therapeutic biologics to prevent migraine [8, 9]. Generally, this class of drugs can be divided into two types, mAbs targeting CGRP including eptinezumab, fremanezumab and galcanezumab, and mAbs targeting CGRP receptor including erenumab [10]. However, in most of the available evidences derived from phase II and phase III RCTs associated with these novel agents, participants who had previously failed prophylactic medication for migraine were excluded [11,12,13]. This suggests that effects of this class of medicine may be different for patients with treatment-resistant migraine. Moreover, the relative safety and efficacy of these drugs in patients with prior migraine treatment failures have not been investigated in depth due to lack of direct comparison of different types of mAbs against CGRP or its receptor in these patients.
Therefore, we performed a bayesian network meta-analysis to assess safety and efficacy of various types of CGRP related mAbs in migraine patients with prior treatment failures. We also conducted a comprehensive ranking of various medications to determine which medications were the most effective in safely reducing monthly migraine headache days.
Methods
Search strategy and guideline
We searched bibliographic databases from inception until June 15, 2022 in several databases including Ovid MEDLINE, Cochrane CENTRAL database, and Ovid EMBASE (Supplementary Table A1). Clinical Research registry portal (ClinicalTrials.gov) and reference lists from the previous systematic review on the same field were also searched to identify additional studies. No language restrictions were adopted. The following MeSH terms and free-text terms such as “migraine”, “calcitonin gene-related peptide binding monoclonal antibody”, “erenumab”, “galcanezumab”, “fremanezumab”, “eptinezumab”, and “randomized controlled trial” were used to identify any eligible publications.
The study was registered in OSF platform (https://osf.io/tr8wh), and implemented following the Cochrane Handbook for Systematic Reviews of Interventions. Reporting the study was conforming to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension Statement for network Meta-analyses (PRISMA-NMA) guideline [14].
Eligible criteria
The eligible criteria were based on the patients, intervention, control, outcome, and study (PICOS) principles. We included randomized controlled trials (RCTs) of adult migraine patients (age ≥ 18 yeas) with previous preventive treatment failures (including studies with an identifiable subset of migraine patients with previous preventive treatment failures). The diagnostic criteria are based on the third version of the International Classification of Headache Disorders (ICHD-III) [15]. We defined intervention as any types of CGRP related mAbs, including mAbs targeting CGRP (galcanezumab, eptinezumab, and fremanezumab) and mAbs targeting CGRP receptors (erenumab). The control group was treatment with placebo or a different type of CGRP related mAb. The primary efficacy outcome was defined as change in monthly migraine days (MMDs). Secondary efficacy outcomes were 50%, 75% response rates (defined as a reduction of the frequency of headache attacks by at least given percentage). The primary safety outcome was defined as treatment-emergent adverse events (TEAEs), and secondary safety outcome was serious adverse events.
Besides, the following studies were excluded, 1) RCTs that compared CGRP related mAb with other pharmacological active agents.
Selection process and data extraction
After removal of duplicates, two reviewers independently filtered publications that were deemed as ineligible based on reading titles and abstracts. The articles in full text were then reviewed and further excluded based on the inclusion and exclusion criteria. Two reviewers independently accomplished this process. Discrepancies in selection were resolved by a third independent reviewer.
Two authors independently extracted data on study characteristics from the eligible trials using a predesigned table. The following data item were noted, characteristics of study including primary author, year of publication, duration of follow-up; patient characteristics including age, sex, type, and dosage of the therapy agent. Two reviewers independently extracted data from the eligible studies. In case of unclear information and additional information for which no relevant results are reported, the corresponding author of the study would be contacted to request the information. Discrepancies in extraction were resolved by a third independent reviewer.
Evaluation of risk of Bias and quality of evidence
Risk of bias of each trial was assessed using the Cochrane Statistical Methods Group tool across seven domains [16]. In brief, each domain was judged as low, unclear, or high risk of bias. If each domain is assessed as low risk of bias, then a trial will be rated as having an overall low risk of bias. If necessary data were required, we contacted the corresponding author of the original study for more information.
Besides, the quality of evidence for outcomes would be judged using the Grading of Recommendations Assessment, Development, and Evaluation working group (GRADE) tool across five domains, including publication bias, imprecision, overall risk of bias, indirectness, and inconsistency [17]. The overall quality of evidence of each estimate was rated “high”, “moderate”, “low” or “very low”.
Statistical analysis
The relevant analyses were a 3-node NMA (CGRP mAbs vs. CGRP receptor mAbs vs. placebo) and a 9-node NMA (eptinezumab 100 mg vs. eptinezumab 300 mg vs. erenumab 70 mg vs. erenumab 140 mg vs. monthly fremanezumab vs. quarterly fremanezumab vs. galcanezumab 120 mg vs. galcanezumab 240 mg vs. placebo). We performed a bayesian network meta-analysis model in R software to incorporate indirect comparisons using the consistency model. The point estimates [mean difference (MD) or relative risk (RR)] and the corresponding 95% credible intervals (CrIs) were obtained from the Markov chain Monte Carlo model. In brief, model was set to be with 40,000 simulated draws after a burn-in of 20,000 iterations. The probability for each treatment agent in each outcome was also estimated to rank the intervention levels in the network meta-analysis. Besides, we used the surface under the cumulative ranking curve (SUCRA) to obtain the probability of ranking from worst to best for each treatment agent. In the case of continuous variables that provided incomplete results, we used the formula recommended by Cochrane Handbook for Systematic Reviews of Interventions [18]. Heterogeneity of treatment effects among included studies was examined using the Cochrane Q test and I2statistic. I2 of 25%, 50% and 75% represent low, moderate and high heterogeneity. The publication bias was checked via Harbord regression test, Egger regression test, and Begg’s test if ten or more trials were pooled.
Statistical analyses were completed in R (release version 4.0.5) and RevMan (release version 5.4.1; The Cochrane Collaboration) software. Bilateral P values less than 0.05 were considered statistically significant.
Results
Study characteristics
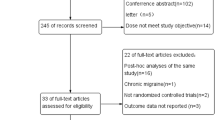
The initial searching identified 1814 potentially relevant articles (Fig. A1 in the Supplement). At last, seven studies (derived from nine trials) totaling 3052 patients were included in this network meta-analysis [19,20,21,22,23,24,25]. One trial evaluated effects between eptinezumab and placebo [19]; three trials evaluated effects between erenumab and placebo [20,21,22]; one trial evaluated effects between fremanezumab and placebo [23]; and two trials assessed galcanezumab vs. placebo [24, 25]. Participants in each trial ranged from 11 to 890. All the included trials mostly enrolled female patients, and the median of proportion of female was 88%. The median age of the included studies was 45.7 years. Five trials (55.6%) completed follow-up visit until 24 weeks, the others completed follow-up visit until 12 weeks. Most trials (78%) were conducted in multiple countries. Study characteristics was summarized and presented in the Table 1.
Efficacy outcomes
Regarding primary efficacy outcome, the network of comparison of different types of CGRP related mAbs was reported in all eligible trials totaling 3052 participants (Fig. 1A). According to the three-node analysis (Table 2), both CGRP mAbs (MD: -3.29, 95% CrI: − 3.97 to − 2.76) and CGRP receptor mAbs (MD: -1.74, 95% CrI: − 2.72 to − 1.11) resulted in greater reduction in mean monthly migraine days than placebo. CGRP mAbs also showed better efficacy than CGRP receptor mAbs in reducing MMDs (MD: -1.55, 95% CrI: − 2.43 to − 0.44). Nine-node analysis (Fig. 1B-C) showed that galcanezumab 240 mg had the highest probability of rating first to reduce MMDs (MD -4.40, 95% CrI − 7.60 to − 1.19, SUCRA 0.84), followed by monthly fremanezumab (MD -3.50, 95% CrI − 6.00 to − 0.98, SUCRA 0.76), eptinezumab 300 mg (MD -3.20, 95% CrI − 5.72 to − 0.70, SUCRA 0.66), galcanezumab 120 mg (MD -3.09, 95% CrI − 5.05 to − 1.07, SUCRA 0.61), quarterly fremanezumab (MD -3.11, 95% CrI − 5.60 to − 0.54, SUCRA 0.60), eptinezumab 100 mg (MD -2.70, 95% CrI − 5.15 to − 0.20, SUCRA 0.47), erenumab 140 mg (MD -1.81, 95% CrI − 3.99 to − 0.38, SUCRA 0.30), and erenumab 70 mg (MD -1.64, 95% CrI − 3.63 to 0.18, SUCRA 0.24). Comparisons of drugs with each other did not show significant difference (Fig. A2 in the Supplement).
Summary of the primary efficacy outcome. A Network plot of change in MMDs. The width of the lines is proportional to the number of studies comparing every pair of treatments, and the size of each circle is proportional to the number of participants. B The forest plot shows the risk ratio (RR) and credible interval (CrI). C Ranking probabilities graph (blue bars) of each treatment agent. The SUCRA values (red bars) for each treatment are as follows: 84% for galcanezumab 240 mg; 76% for monthly fremanezumab; 66% for eptinezumab 300 mg; 61% for galcanezumab 120 mg; 60% for quarterly fremanezumab; 47% for eptinezumab 100 mg; 30% for erenumab 140 mg; 24% for erenumab 70 mg; 2% for placebo. MMDs: monthly migraine days; SUCRA: surface under the cumulative ranking curve
Regarding secondary efficacy outcomes, three-node analysis demonstrated that both CGRP mAbs (RR: 3.66, 95% CrI: 3.01 to 4.49) and CGRP receptor mAbs (RR: 2.40, 95% CrI: 1.76 to 3.35) achieved a 50% or greater reduction in the monthly number of migraine days than placebo (Table 2). CGRP mAbs were superior to CGRP receptor mAbs to achieve at least 50% response rates (RR: 1.52, 95% CrI: 1.04 to 2.21). According to nine-node analysis (Fig. 2A-B), galcanezumab 240 mg (RR: 4.18, 95% CrI: 2.63 to 6.67, SUCRA 0.79) had the highest probability of rating first in reducing the frequency of headache attacks by at least 50%, followed by quarterly fremanezumab (RR: 4.02, 95% CrI: 2.71 to 6.25, SUCRA 0.76), monthly fremanezumab (RR: 4.01, 95% CrI: 2.71 to 6.22, SUCRA 0.76), eptinezumab 300 mg (RR: 3.81, 95% CrI: 2.83 to 5.31, SUCRA 0.74), eptinezumab 100 mg (RR: 3.25, 95% CrI: 2.38 to 4.55, SUCRA 0.50), galcanezumab 120 mg (RR: 3.14, 95% CrI: 2.28 to 4.43, SUCRA 0.48), erenumab 70 mg (RR: 2.40, 95% CrI: 1.69 to 3.46, SUCRA 0.24), and erenumab 140 mg (RR: 2.37, 95% CrI: 1.72 to 3.34, SUCRA 0.22). Comparison between different treatment agents showed monthly and quarterly fremanezumab was superior to erenumab 140 mg (RR: 0.59, 95% CrI: 0.34 to 1.00; RR: 0.59, 95% CrI: 0.34 to 1.00, respectivley), and treatment with eptinezumab 300 mg was superior to erenumab 140 mg (RR: 1.61, 95% CrI: 1.02 to 2.56). The results were presented in Fig. A3 in the Supplement.
Summary of the secondary efficacy outcomes. Network plot of (A) The forest plot for 50% response rates; (B) The SUCRA value of each treatment for 50% response rates. (C) The forest plot for 75% response rates; (D) The SUCRA value of each treatment for 75% response rates. SUCRA: surface under the cumulative ranking curve
In addition, three-node analysis demonstrated that both CGRP mAbs (RR:6.29, 95% CrI: 4.07 to 10.29) and CGRP receptor mAbs (RR: 5.38, 95% CrI: 2.58 to 13.21) achieved a 75% or greater reduction in the monthly number of migraine days than placebo. According to nine-node analysis (Fig. 2C-D), eptinezumab 300 mg (RR: 9.93, 95% CrI: 4.61 to 25.73, SUCRA 0.88) had the highest probability of rating first in reducing the frequency of headache attacks by at least 75%, followed by eptinezumab 100 mg (RR: 8.27, 95% CrI: 3.82 to 21.74, SUCRA 0.73), monthly fremanezumab (RR: 6.01, 95% CrI: 2.72 to 15.91, SUCRA 0.62), erenumab 140 mg (RR: 5.39, 95% CrI: 2.56 to 13.31, SUCRA 0.52), erenumab 70 mg (RR: 5.39, 95% CrI: 2.34 to 14.08, SUCRA 0.52), galcanezumab 120 mg (RR: 4.34, 95% CrI: 2.15 to 9.99, SUCRA 0.39), and quarterly fremanezumab (RR: 4.03, 95% CrI: 1.73 to 10.94, SUCRA 0.34). Comparisons of drugs with each other did not show significant difference (Fig. A4 in the Supplement).
Safety outcomes
Regarding primary safety outcome, the network of comparison of different types of mAbs targeting CGRP was reported in seven trials totaling 2921 participants (Fig. 3A). According to the three-node analysis, neither CGRP mAbs (RR: 0.99, 95% CrI: 0.88 to 1.12) nor CGRP receptor mAbs (RR: 0.99, 95% CrI: 0.87 to 1.13) associated with increased risk of treatmentemergent adverse events (Table 2). Nine-node analysis did not find significant difference. Erenumab 70 mg had the highest probability of rating first to reduce TEAEs (SUCRA 0.66), followed by galcanezumab 120 mg (SUCRA 0.60), erenumab 140 mg (SUCRA 0.57), monthly fremanezumab (SUCRA 0.56), eptinezumab 300 mg (SUCRA 0.43), quarterly fremanezumab (SUCRA 0.40), and eptinezumab 100 mg (SUCRA 0.29). The results were presented in Fig. 3B-C and Fig. A5 in the Supplement.
Summary of the primary safety outcome. A Network plot of change in TEAEs. The width of the lines is proportional to the number of studies comparing every pair of treatments, and the size of each circle is proportional to the number of participants. B The forest plot shows the risk ratio (RR) and credible interval (CrI). C Ranking probabilities graph (blue bars) of each treatment agent. The SUCRA values (red bars) for each treatment are as follows: 66% for erenumab 70 mg; 60% galcanezumab 120 mg; 57% erenumab 140 mg; 56% for monthly fremanezumab; 48% for placebo; 43% for eptinezumab 300 mg; 40% for quarterly fremanezumab; 29% for eptinezumab 100 mg. TEAEs: treatment-emergent adverse events; SUCRA: surface under the cumulative ranking curve
Regarding secondary safety outcomes, three-node analysis demonstrated that neither of the treatments increased risk of serious adverse events (Table 2). Nine-node analysis did not show significant difference in any of the comparisons (Fig. 4; Fig. A6 in the Supplement). Quarterly fremanezumab (SUCRA 0.84) had the highest probability of rating first, followed by monthly fremanezumab (SUCRA 0.59), galcanezumab 120 mg (SUCRA 0.58), eptinezumab 100 mg (SUCRA 0.51), eptinezumab 300 mg (SUCRA 0.33), erenumab 140 mg (SUCRA 0.27), and erenumab 70 mg (SUCRA 0.23).
Risk of Bias and certainty of evidence
Six trials were judged as overall low risk of bias. Only one trial was regarded as unclear risk of bias. Supplementary fig. A7–8 presented the full details of risk of bias assessment for each study. The quality of the evidence for primary outcomes was summarized in Supplementary fig. A9. In general, the certainty of evidence for each agent vs. placebo was judged to be moderate to high.
Discussion
To our knowledge, this is the first time that different CGRP related mAbs for migraine patients with prior treatment failures have been compared in a network meta-analysis. Our network meta-analysis focused on four CGRP related mAbs involving 3052 patients by pooling data derived from nine RCTs. Pooled results from three-node analysis showed that CGRP mAbs was superior to CGRP receptor mAbs in reducing monthly migraine days and improving at least 50% response rates. Nine-node analysis showed all the treatment agents were similarly efficient in reducing monthly migraine days. Moreover, treatment with fremanezumab or eptinezumab 300 mg provides a significant advantage over erenumab 140 mg in terms of improving at least 50% response rates. All the treatment agents were well tolerated which did not show difference in incidences of TEAEs and serious adverse events in any of the comparisons.
Based on the results of the present analysis, it appears that CGRP mAbs, especially galcanezumab 240 mg, monthly fremanezumab, and eptinezumab 300 mg, seem to be the best choice for the treatment of migraine patients with previous treatment failures. These findings also call for future studies that investigate differences in the efficacy and safety of these drugs in the treatment of migraine in the same patient population.
Comparison with other studies
This bayesian network meta-analysis compared the relative effects of different mAbs targeting CGRP or its receptor for the treatment of migraine patients with prior treatment failures. In our study we synthesized data across 10 trials to perform three-node and nine-node analysis, and showed the relative ranking of different treatment agents in terms of each outcome.
To the best of our knowledge, most analyses on the same field evaluate the comparative effects of different GCRP related mAbs in patients with migraine regardless of prior medication failure. Therefore, it remains unclear whether these previous findings could be directly applicable to headache problems in the specific population of patients [26, 27]. An additional limitation of previous studies is that most of them used direct method to compare the effectiveness of this medication class with placebo, unable to show the comparative efficacy of different agents in the lack of direct comparison of different types of CGRP related mAbs [28].
Strengths and limitations of the study
Since network meta-analysis enables different interventions to be evaluated both directly and indirectly even if direct comparison is lacking, this approach has a unique strength over conventional pairwise meta-analysis to provide a more comprehensive analysis of available evidences. A key strength of our study is the use of a network approach to investigate the relative effects of different kinds of CGRP related mAbs for migraine patients with prior treatment failures. By using this method, we were able to compare different therapy agents with placebo both directly and indirectly which give a more precise estimate of the relative efficacy and safety over the pairwise analyses. This method also allowed us to rank the efficacy and safety of different treatment agents. In addition, we reasonably used GRADE tool to judge the quality of evidence for the primary outcomes. These methods are helpful to clinicians in making clinical decisions. Overall, we provided more up-to-date information regarding the reported efficacy and safety of CGRP related mAbs in treating adult migraine patients with prior treatment failures.
There are several limitations need to be noted. First, the disease condition was heterogeneous across the trials, increasing heterogeneity. For example, five trials included patients with episodic migraine, while the others included both episodic and chronic migraine patients. We tried to conduct subgroup analysis based on different group of patients, but the analysis could not be performed due to limited studies. Future studies should report the effects of treatment agents in different types of patient groups.
Second, although galcanezumab 240 mg showed better efficacy in some of the comparisons, we could not evaluate its relative safety with other treatment agents since the original trials did not provide safety data. Future studies should consider this issue and focus on the safety of it in the same patients group.
Third, five trials completed follow-up visit until 24 weeks, while others followed patients up for 12 weeks in a double-blind visit. Given the fact that migraine need treatment for a long time, the dominance of short-term therapy might not be applicable in the actual patients. In fact, evidences from real-world observational studies and open- label extension phase of RCTs confirmed the effectiveness of those drugs.
Implications in practice
The latest guideline from European Headache Federation (EHF) recommended CGRP related mAbs as a third line treatment for migraine prevention in individuals with migraine [29, 30]. However, in most of the available evidences derived from phase II and phase III RCTs, participants with previous failure of preventive medication classes for migraine were excluded. This implies that efficacy can be different for patients with severe, treatment-resistant migraine. Our findings confirmed the efficacy and tolerability of CGRP related mAbs in the treatment of migraine patients with previous treatment failures. We also suggested CGRP mAbs, especially galcanezumab 240 mg, monthly fremanezumab, and eptinezumab 300 mg, seem to be the best choice for the treatment of these patients. However, cost effectiveness of these novel therapies might vary by intervention, which need to be considered in the same time. Clinicians should consider a comprehensive view based on efficacy, safety, and cost-effectiveness in developing treatment plans.
Conclusions
In summary, although most agents were well tolerated and showed similar effects, our analysis suggest that galcanezumab 240 mg, monthly fremanezumab, and eptinezumab 300 mg offer the first level in efficacy profile in terms of change in monthly migraine days and 50% response rates in migraine patients with previous treatment failures. These findings are helpful for guideline development and clinicians to make decisions as to which drug to use in the absence of head to-head trials. Future research that investigate differences in the efficacy and safety of these novel agents in the treatment of migraine in the same patient population are needed to validate the present findings.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- CGRP:
-
Calcitonin gene-related peptide
- RCT:
-
Randomized clinical trials
- MMDs:
-
Monthly migraine days
- TEAEs:
-
Treatment-emergent adverse events
- mAbs:
-
Monoclonal antibodies
- PICOS:
-
Patients, intervention, control, outcome, and study
- ICHD:
-
International Classification of Headache Disorders
- MD:
-
Mean difference
- RR:
-
Relative risk
- CrIs:
-
Credible intervals
- SUCRA:
-
Surface under the cumulative ranking curve
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation working group
- EHF:
-
European Headache Federation
References
MacGregor EA (2017) Migraine. Ann Intern Med 166(7):Itc49–itc64
Gaul C, Finken J, Biermann J, Mostardt S, Diener HC, Müller O et al (2011) Treatment costs and indirect costs of cluster headache: a health economics analysis. Cephalalgia 31(16):1664–1672
Messali A, Sanderson JC, Blumenfeld AM, Goadsby PJ, Buse DC, Varon SF et al (2016) Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache 56(2):306–322
González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM (2018) Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol 14(1):25–41
Ford JH, Jackson J, Milligan G, Cotton S, Ahl J, Aurora SK (2017) A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache 57(10):1532–1544
Martelletti P, Schwedt TJ, Lanteri-Minet M, Quintana R, Carboni V, Diener HC et al (2018) My migraine voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain 19(1):115
Pozo-Rosich P, Lucas C, Watson DPB, Gaul C, Ramsden E, Ritter S et al (2021) Burden of migraine in patients with preventive treatment failure attending European headache specialist centers: real-world evidence from the BECOME study. Pain Ther 10(2):1691–1708
Raffaelli B, Neeb L, Reuter U (2019) Monoclonal antibodies for the prevention of migraine. Expert Opin Biol Ther 19(12):1307–1317
Paemeleire K, MaassenVanDenBrink A (2018) Calcitonin-gene-related peptide pathway mAbs and migraine prevention. Curr Opin Neurol 31(3):274–280
Edvinsson L, Warfvinge K (2019) Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia 39(3):366–373
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T et al (2018) Effect of Fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 319(19):1999–2008
Shibata M, Nakamura T, Ozeki A, Ueda K, Nichols RM (2020) Migraine-specific quality-of-life questionnaire (MSQ) version 2.1 score improvement in Japanese patients with episodic migraine by Galcanezumab treatment: Japan phase 2 study. J Pain Res 13:3531–3538
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR (2018) Evaluation of Galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 75(9):1080–1088
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
(2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33(9):629–808
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2019) Cochrane handbook for systematic reviews of interventions. New Jersey: Wiley
Ashina M, Lanteri-Minet M, Pozo-Rosich P, Ettrup A, Christoffersen CL, Josiassen MK et al (2022) Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 21(7):597–607
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392(10161):2280–2287
Goadsby PJ, Paemeleire K, Broessner G, Brandes J, Klatt J, Zhang F et al (2019) Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia 39(7):817–826
Hirata K, Sakai F, Takeshima T, Imai N, Matsumori Y, Yoshida R et al (2021) Efficacy and safety of erenumab in Japanese migraine patients with prior preventive treatment failure or concomitant preventive treatment: subgroup analyses of a phase 3, randomized trial. J Headache Pain 22(1):110
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 394(10203):1030–1040
Ailani J, Pearlman E, Zhang Q, Nagy AJ, Schuh K, Aurora SK (2020) Positive response to galcanezumab following treatment failure to onabotulinumtoxinA in patients with migraine: post hoc analyses of three randomized double-blind studies. Eur J Neurol 27(3):542–549
Mulleners WM, Kim B-K, Láinez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S et al (2020) Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 19(10):814–825
Deng H, Li GG, Nie H, Feng YY, Guo GY, Guo WL et al (2020) Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine - an updated systematic review and meta-analysis. BMC Neurol 20(1):57
Alasad YW, Asha MZ (2020) Monoclonal antibodies as a preventive therapy for migraine: a meta-analysis. Clin Neurol Neurosurg 195:105900
Yang Y, Chen M, Wu D, Sun Y, Jiang F, Chen Z et al (2022) Optimal dose of Erenumab for preventive treatment of episodic migraine: a systematic review and Meta-analysis. Curr Neuropharmacol 20(2):460–470
Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R et al (2022) European headache federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention - 2022 update. J Headache Pain 23(1):67
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas D-D et al (2019) European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache and Pain 20(1):6–38
Acknowledgements
None.
Funding
This research was funded by the National key Research & Development Program of China, grant number (2018YFA0108604 & 2018YFA0108603); Clinical Incubation Program of West China Hospital (grant number 2018HXFH008). The funders had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
XW and LM designed the meta-analysis, XW and CY searched for relevant studies, XW and DW selected the studies, extracted the relevant information, XW and QH synthesized the data, XW wrote the first draft of the paper. All authors revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval was not required for this type of study.
Competing interests
The authors declared that there was no any potential conflict of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table A1
. Search terms. Fig. A1. Study selection flowchart of randomized controlled trials. Fig. A2. League plot of different treatment regimens for the primary efficacy outcome from the network meta-analysis. Fig. A3. League plot of different treatment regimens for 50% response rates from the network meta-analysis. Fig. A4. League plot of different treatment regimens for 75% response rates from the network meta-analysis. Fig. A5. League plot of different treatment regimens for the primary safety outcome from the network meta-analysis. Fig. A6. League plot of different treatment regimens for the secondary safety outcome from the network meta-analysis. Fig. A7. Risk of bias summary of included trials. Fig. A8. Risk of bias graph of included trials. Fig. A9. GRADE summary for the primary outcomes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Wen, D., He, Q. et al. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine patients with prior preventive treatment failure: a network meta-analysis. J Headache Pain 23, 105 (2022). https://doi.org/10.1186/s10194-022-01472-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-022-01472-2