Abstract
Background
Migraine is a complex neurological disorder that is characterized by a "lower threshold of neuronal hyperexcitability" with distinctive periodicity and complex vascular dysfunction. Genetic factors have impacted incredibly on the susceptibility of migraine and one such example is the TNF-α 308G > A.
Aim
Therefore, we aim to provide a glimpse of the association of the TNF-α 308G > A risk on the susceptibility of migraine.
Method
The pooled odds ratio with the associated 95% of confidence interval were calculated using different genetic models. Heterogeneity was accessed by using Cochran's Q Test and I2 statistics and Begg's and Egger's tests were used for finding the publication bias, tests were two-sided, and a p-value of < 0.05 was considered statistically significant. The Trial Sequential Analysis with Meta-regression Analysis were also utilized to find out the sample size requirement for meta-analysis to avoid type I error and source of heterogeneity respectively.
Result
A total of 13 studies with cases: 7193 and controls: 23,091 were included and after using different genetic models, no overall association with migraine and its clinical subtype migraine with aura was observed (Allele model “OR: 1.28, 95% C.I. [0.96–1.69] and OR: 0.99,95% C.I. [0.69–1.42]) respectively. Interestingly, after sub-grouping using the “ethnicity criteria” in the migraine group, it was observed that the allelic genetic model and the dominant model were found to be significantly associated with the Asian ethnic group (OR: 1.79, 95% C.I. [1.13–2.84], and OR: 1.85, 95% C.I. [1.0927; 3.1580].
Conclusion
In conclusion, the present meta-analysis has provided evidence that 308G > A increases the risk of migraine only in the Asian population.
Similar content being viewed by others
Introduction
Lower neuronal hyperexcitability is used to define migraine, which is a complex neurological disorder characterized by distinctive periodicity and complex vascular dysfunction [1]. According to the International Classification of Headache Disorders (ICHD-3), migraine is classified into episodic migraine which is further sub-classified into Migraine without Aura (MO), Migraine with Aura (MA), and Chronic Migraine (CM). The overall prevalence rate of the diseases is roughly 21.7% with a 12–15% average variance between nations [2,3,4] where it impacted every age group, including younger children (2.7 to 10.0%), where both sexes are equally affected, while adult females (12–17%) are more likely to experience them than males (4–7%) [5, 6].
Neurogenic neuro-inflammation, defined as "inflammatory reactions in central and peripheral parts of the trigeminovascular system in response to neuronal activity," has piqued the interest of migraine researchers in recent years [7] due to its critical role in the processing, integration, and transmission of sensory information in migraine pathogenesis [1, 8, 9]. TNF-α is an astonishing example of a proinflammatory cytokine involved in inflammation initiation and is secreted by the microglial cell upon activation [10].
TNF-α (NCBI Entrez Gene: 7124) encodes for a cytokine that promotes a diverse range of proinflammatory reactions and is responsible for various conditions including migraine (MalaCards—human disease database and OMIM—Online Mendelian Inheritance in Man). There are diverse numbers of Single Nucleotide Variations (SNVs) presented in the upstream region of TNF-α (Fig. 1) [11]. One such variant is located -308 upstream of the gene and is known for its regulatory activity and is named “-308 G > A polymorphism/ rs1800629”. This G to A polymorphism shows different allele frequencies within the different populations (Ensembl.org). Greater baseline/constitutive and inducible TNF-α expression has been linked to the uncommon 308A allele both in-vivo and in-vitro studies [12] and is associated with elevated plasma levels [13]. Multiple researchers have found an increased risk of diseases with the presence of TNF-α 308A on a wider scale, across different populations [14,15,16,17].
Given that the -308 G > A polymorphism is thought to affect the TNF-α gene's promoter activity and that the gene is part of the major histocompatibility complex, this variant could interfere with immunologic equilibrium and impact migraine genesis, development, or progression. Considering the aforementioned data, numerous case–control and cohort research have been performed, to investigate the association between migraine risk and the -308G/A variant but, the results were found inconsistent. Diversity might be due to the low power of the individual study, diverse ethnic groups, and possible selection bias. Numerous meta-analyses have been published in the past to help overcome the constraints of individual investigations [18,19,20,21], but showed the controversial outcome. Therefore, we conducted an updated meta-analysis featured with Trial sequential analysis (TSA) and Meta-Regression Analysis (MRA) in order to enhance statistical power and derive a more precise result of the association between the rs1800629 and the risk of migraine.
Method
Literature survey
Using the approach of “systematic way of literature survey” according to the PRISMA (Preferred Reporting Items for Systematics Reviews and Meta-Analysis) guidelines [22] from the online database such as PubMed and research article search engine i.e., Google scholar, potential studies that examine the relationship between -308G/A variant and migraine risk were identified by our three authors (S.S, M.B, & A.C.P). Multiple key terms were used in our search strategy, including “TNF-α OR TNF-α gene OR Tumor necrosis factor-alpha gene AND variant OR mutation OR polymorphism OR AND -308 OR rs1800629 AND Migraine disorder”. The search procedure lasted up to May/10/ 2022, and the language restrictions were English. Since PubMed has more than 34 million citations for scientific publications from sources including MEDLINE, life science journals, and e-books, we didn't search any additional databases.
Inclusion and exclusion features
The following inclusion features have to be satisfied by the studies included in this meta-analysis such as (1) research must have investigated the correlation between the -308G/A and migraine predisposition (2) studies must be cohort designed or a case–control design, (3) research need to yield sufficient information to compute ORs and the related CIs of 95% and also other factors described in (Table 1).
Data extraction and quality assessment
Different features from each study were extracted including the demographic characteristic including the country name and ethnicity, the numbers of patients and healthy groups, also the cohort data, genotypic frequency from both cases and controls, and first authors with years of publication and lastly what type of technique utilized to determine the genotype? Each published research study's quality was evaluated using the Newcastle–Ottawa quality assessment scale (NOS), and a score of six points was considered to be a good study (Ottawa Hospital Research Institute (ohri.ca).
Statistical analysis
To find out the strength of an association between the rs1800629 and migraine susceptibility, the pooled Odds Ratio (OR) (OR > 1: the odds of exposure among case are greater than odds of exposure among controls & OR < 1: the odds of exposure among case are lower than the odds of exposure among controls) with associated 95% of Confidence Interval (CI) were calculated. In order to evaluate the Hardy–Weinberg equilibrium (HWE) in the control groups, the Chi-square statistic was used. To find out the impact of the risk factor on disease susceptibility, four different genetic models including allele model (A vs. G), AA vs. GG + GA (recessive model), AA + GA vs. GG (dominant model), and GA vs AA + GG (over-dominant model). To evaluate how each study affected the total OR and 95% CI, a sensitivity analysis was also performed.
Using the Cochran's Q Test and I2 statistics as a test for heterogeneity of the research studies included in this meta-analysis, different level of heterogeneity was defined which includes low (up to 25%), moderate (> 25% to 75%), and (above 75%) degrees of heterogeneity. The random effect model was only used when the test of heterogeneity i.e., I2 will be above 75%, and notably, the publication bias including reporting bias was assessed using Begg's and Egger's tests. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant. Due to easy graphical user interphase (GUI), the Meta-Genyo online Statistical Analysis System software was used for all statistical analyses (MetaGenyo: Meta-Analysis of Genetic Association Studies).
Meta-Regression analysis
Subgroup analysis and Bayesian meta-regression analysis based on years of population, ethnicity, diagnostic criteria, and genotyping technique were carried out to find specified sources of heterogeneity across included research. Using Microsoft Excel-2019 with Analysis ToolPak, data on parameters like R-square, intercept coefficient, standard error, t-value, confidence interval, and p-value were retrieved for the Bayesian meta-regression.
Trial sequential analysis
A unique approach known as Trial Sequential analysis (TSA) has been employed in the current meta-analysis to limit random mistakes by determining whether the studies contained in the meta-analysis have exceeded the required sample size or not. Because chances of random error increase in the meta-analysis due to the repeated significance tests and continuous data distribution which will ultimately cause the Type I error [23]. TSA tool (Copenhagen Trial Unit, Denmark) was used to calculate the needed information size based on a 5% overall risk and a relative risk reduction of 20% (with 80 percent power) for assessing meta-analysis reliability (TSA – ctu.dk). However, there are two main possibilities: if the cumulative Z value/curve crosses the RIS (Required Information Size), no further studies are required,and if the Z curve does not exceed the RIS threshold, the sample size is insufficient and more reliable studies are required.
Result
Study characteristics
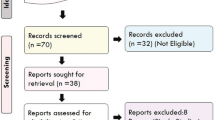
The studies used for this meta-analysis meet the PRISMA requirements and the selection flow diagram depicted in (Fig. 2). We found a total of 18 studies but after founding one duplicated study [24] only 17 were found to be eligible studies with the pooled case numbers of 7692 and 23,570 controls concerning 308G > A (Table 2) were included in the meta-analysis. But after finding the studies that were not found in HWE [22,23,24,25], only 13 studies (cases: 7193 and controls: 23,091) were determined to be suitable for further examination.
Meta-analysis
This currently updated meta-analysis utilizes the different genetic models to observe the effect of the rs1800629 variant of TNF-α on migraine susceptibility. In the first model i.e., the allelic model, polled results from the experiment group (n = 14,278) and control (n = 46,074) showed the non-significant association value of “OR: 1.28, 95% C.I. [0.96–1.69]. The impact of the mutant allele was seen after using the random effect model as the “test of heterogeneity (I2) was 88% (Fig. 3A). After adjusting for the dominant model and utilizing the random effect model (I2), a non-significant strength of association was found OR: 1.29, 95% C.I. [0.95–1.75]. As the value for the test of heterogeneity was found below the moderate threshold (55%), a fixed effect model was then utilized to check out the effect of the recessive genetic model on migraine susceptibility and found an OR: 1.00, 95% C.I. [0.84–1.19]. For the analysis of the over-dominant genetic model with an I2 = 84%, the random effect model showed the associated value OR: 1.22 [0.91–1.64].
A Forest plot representing the Odds ratio (OR) utilizing the 95% of Confidence interval (C.I.) for all the individual studies featured with different sample size and also the pooled odds ratio (OR) with 95% C.I. for Allelic model. B Funnel plot of TNF-alpha -308 G > A and susceptibility of migraine in diverse population utilizing Allele model
Using Egger's test, which is based on the relationship between standard error and strength of association, the publication bias was evaluated. The plotted articles with high accuracy on top and low accuracy down in the plot formed the perfect funnel-shaped structures. Such symmetrical funnel plots were formed for all genetic models which direct that there was no publication bias (Fig. 3B). A sensitive analysis was also performed for all genetic models by removing each research one at a time. It was shown that none of the pooled ORs were significantly impacted, demonstrating the strong stability of the meta-analysis findings (Fig. 4).
After sub-grouping using the criteria of ethnicity for the migraine group, it was observed that the allelic genetic model and the dominant model were found to be significantly associated with the Asian ethnic group (OR: 1.79, 95% C.I. [1.13–2.84], and OR: 1.85, 95% C.I. [1.09; 3.15] (Table 3). Also, it was observed that the recessive genetic model, an over-dominant genetic model was found to increase the risk but did not reach statistical significance. A highly statistically significant association was found in the Egyptian population for all genetic models with recessive showed the highest (OR: 6.88, 95% C.I. [1.53–30.90]) (Table 3).
Apart from the ethnicity sub-grouping, clinical feature subgroup analysis of migraine i.e., migraine with aura was also carried out to see if there is any significant association between the rs1800629 and the susceptibility of MA. We observed that after excluding the studies, with no MA cases/ no inclusion of MA cases and the studies which don’t fall under HWE, a total of 8 studies were found with a total sample size of 25,475 (case = 3249 and control = 22,226) (Table 4). For the allelic model, as the I2 was found 86% and after using the random effect model, no association was found OR: 0.99,95% C.I. [0.69–1.42] (Fig. 5A). For the dominant model, the I2 was above the high threshold, therefore, after using the random effect model the association value was OR: 1.00, 95% C.I. [0.68–1.45]. The recessive and the over-dominant model show a non-significant association with an association value i.e., OR: 0.96, 95% C.I. [0.74–1.24], and (OR: 0.99, 95% C.I. 0.71–1.38] after adjusting for the fixed effect model (I2: 36%) and the random model respectively. Sub-grouping of MA based on the ethnicity criteria revealed no association between rs1800629 and migraines in Asian and Caucasian populations (Table 5). To evaluate the publishing bias, all funnel plots seemed symmetrical, indicating a negligible publication bias (Fig. 5B). Sensitivity analysis also indicated the high stability of the meta-analysis results (Fig. 6).
A Migraine with aura: Forest plot representing the Odds ratio (OR) utilizing the 95% of Confidence interval (C.I.) for all the individual studies featured with different sample size and also the pooled odds ratio (OR) with 95% C.I. for Allele model. B Migraine with aura: Funnel plot of TNF-alpha -308 G > A and susceptibility of migraine in diverse population utilizing Allele model
Trial sequential analysis
The necessary sample size and the reliability of the meta-analysis were calculated using TSA statistics and it was noted that the cumulative Z score did not cross the Required Information Size for allele models in migraine (Fig. 7). Therefore, the TSA has shown that more sample size will still be needed to find out the precise association.
Migraine (Allele Model): Trial sequential analysis (TSA) of the studies included in the meta-analysis of TNF-alpha 308 G > A polymorphism allele model based on a 5% overall risk of false positive error and a relative risk reduction of 20% (with 80 percent power). The cumulative Z-score did not cross the TSA monitoring threshold and the RIS line therefore indicates more studies are needed to find out the precise association
Bayesian meta-regression analysis
To identify possible causes of variation among acceptable papers based on different factors such as ethnicity (Fig. 8A), year (Fig. 8B), criteria (Fig. 8C), and genotyping technique (Fig. 8D), we used meta-regression analysis. The meta-regression analysis showed that the majority of the predicted heterogeneity parameters were not responsible for such heterogeneity (Table 6) in contrast to the ethnicity in the over-dominant genetic model where a significantly (p value = 0.02) strong linear relationship was observed (R = 0.6184) (Table 6).
Meta regression A Regression Analysis Plot (RAP) showing the ethnicity as an independent variable for over dominant model’s OR. B RAP showing the year independent variable for allelic model’s OR. C RAP showing the diagnostic criteria as independent variable for the prediction of dominant model’s OR. D RAP showing the genotyping method as independent variable for allelic model’s OR
Discussion
TNF-alpha is a pro-inflammatory cytokine that appears to play a role in migraine pathogenesis by simulating Calcitonin gene-related peptide (CGRP) [41]. The expression of the protein has been found to be regulated by the presence of common functional SNP i.e., -308 G/A both in vivo and in vitro [12], and is responsible for the altered levels [13] such as in Cerebrospinal fluid (CSF), plasma, and urine concentrations [37, 42,43,44,45,46,47] in migraine patients. Diverse independent research studies and meta-analyses [18,19,20,21], have found the conflicting result on the association between -308 G/A variant and the risk of migraine. Therefore, we conducted an updated meta-analysis with TSA and meta-regression to understand the relationship between -308 G > A transition and migraine susceptibility more precisely.
The present meta-analysis did not show any significant association between the rs1800629 and migraine and its clinical phenotype (MA) after utilizing different genetic models. Interestingly, after sub-grouping using the “ethnicity criteria” in the migraine group, it was found that the dominant model showed a slightly significantly higher risk of migraine than the allelic model (Table 3) in the Asian population. Also, the recessive, and over-dominant models increase the risk of the condition in Asian ethnic groups but the association did not reach statistical significance (Table 3). In addition, we didn’t observe any significant association in the Caucasian population in contrast to the Egyptian population which shows a considerably higher risk of migraine utilizing different genetic models (Table 3).
Comparing our meta-analytic data with the pre-existing meta-analysis, the results were found consistent [18,19,20,21] in contrast to Chen and group [20]. This difference might be due to the inclusion of studies [27,28,29,30, 34, 35] belonging to the Asian population only by Chen and group (Chen et al., 2015). Concerning the risk association between MA and -308 A variant, the present study didn’t find any significant association after utilizing different genetics models which were not consistent with the pre-existing meta-analysis results [18,19,20]. Interestingly, it is important to note that the risk variant showed a strong association in females regarding both migraines as well in MA phenotype [18, 20], which was not observed in the present study. Apart from such conflicting and diverse results, all meta-analyses confirmed that the risk of migraine was greater in the Asian population than in the Caucasian population concerning the risk allele of TNF alpha (rs1800629). In addition, we also observe a significant association in the Egyptian population which shows a considerably higher risk of migraine utilizing a different genetic model. But this large effect might be due to the presence of a single study and might be changed if more studies from Egyptian ethnicity will be included.
There are many reasons why a lack of association occurs and the prime reason might be the limited number of studies in the meta-analysis as shown (Fig. 7), also there might be due high degree of heterogeneity among the studies investigating, inherent biases such as publication, sampling, and selection bias (only English published literature) can’t be avoided in a meta-analysis of observational studies. The disparity between the different meta-analysis studies which we observe in the present study related to the risk of MA in presence of -308A rare variant might result from diverse reasons which might be the varied sample size, population stratification, utilization of the diagnostic criteria, and also the criteria utilized for the selection of research studies for meta-analysis. Varied sample sizes could impact the association and frequency and one such example is the cohort study [32] which was much more powered than the other studies. But, after using sensitivity analysis, there was no such indication for the dominance of a large study on the pooled result. According to population stratification theory, systematic differences in the frequency of alleles between sub-populations are caused by several processes including distinct ancestry, non-random mating, and geographic isolation [48]. Utilization of the strict or modified IHS classification criteria and updated ICHD-3, there is a chance for the misclassification of migraine types due to its biological heterogeneity and wide clinical spectrum [49]. But using a “Bayesian meta-regression” analysis, the present study did not find any significant cause of heterogeneity which mighty be the factor for the heterogeneity (Table 6).
Regarding the strength of the present meta-analysis from the previous meta-analysis [18,19,20,21] first, this meta-study included a large number of subjects (cases: 7193 and controls: 23,091) and featured with the TSA and meta-regression. Second, the NOS quality scale was utilized for the inclusion of studies based on their quality. Thirdly and notably, only the studies were included that were found to be in the HWE, and this resulted in the inclusion of only 13 studies after 18 studies were selected (Table 2). Additionally, we have also incorporated the meta-regression to find out the variable responsible for the heterogeneity, which was not previously mentioned in any meta-analysis of TNF alpha -308 G > A and migraine risk. Apart from the strengths, a major limitation of our meta-analysis was that we did not observe any relationship between migraine and -308G/A utilizing the gender variable which was observed by [18, 20]. Also, we did not observe any gene–gene or gene-environmental interaction and risk of migraine. Because of the possibility of population stratification and the small number of studies (Fig. 7), the results of these subgroup analyses should be interpreted with caution. Enclosing the section, interestingly, all meta-analysis to date including the present study supports the fact that the rare variant i.e., -308A increases the risk of migraine significantly in the Asian ethnic group. Therefore, this study might explain the effect of risk variation on migraine susceptibility based on ethnicity.
Availability of data and materials
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Abbreviations
- TNF-α :
-
Tumor Necrosis Factor- Alpha
- ICHD-3:
-
International Classification of Headache Disorders-3
- MA:
-
Migraine with Aura
- MO:
-
Migraine without Aura
- CM:
-
Chronic Migraine
- CNS:
-
Central Nervous System
- G > A:
-
Guanine > Adenine
- OR:
-
Odds Ratio
- CI:
-
Confidence Interval
- NOS:
-
Newcastle–Ottawa quality assessment scale
- GUI:
-
Graphical User Interphase
- MRA:
-
Meta-Regression Analysis
- TSA:
-
Trial Sequential Analysis
- PRISMA:
-
Preferred Reporting Items for Systematics Reviews and Meta-Analysis
- GABA:
-
Gamma-Aminobutyric Acid
- SNPs:
-
Single Nucleotide Polymorphisms
- RIS:
-
Required Information Size
- CSF:
-
Cerebrospinal fluid
- GCRP:
-
Calcitonin gene-related peptide
- SNV:
-
Single Nnucleotide Variations
References
Sudershan A, Mahajan K, Singh K, Dhar MK, Kumar P. The complexities of migraine: a debate among migraine researchers: a review. Clin Neurol Neurosurg. 2022;214:107136. https://doi.org/10.1016/j.clineuro.2022.107136
Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8(6):e00950. https://doi.org/10.1002/BRB3.950
Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 2017;372:307–15. https://doi.org/10.1016/J.JNS.2016.11.071.
Sudershan A, Pushap AC, Younis M, Sudershan S, Bhagat S, Kumar H, Panjalyia RK, Kumar P. Neuroepidemiology study of headache in the region of Jammu of north Indian population: a cross-sectional study. Front Neurol. 2023;13:1030940. https://doi.org/10.3389/fneur.2022.1030940.
Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. https://doi.org/10.1186/s10194-019-1066-0.
Steiner TJ, Birbeck GL, Jensen RH, Katsarava Z, Stovner LJ, Martelletti P. Headache disorders are third cause of disability worldwide. J Headache Pain. 2015;16(1):1–3. https://doi.org/10.1186/S10194-015-0544-2.
Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–90. https://doi.org/10.1038/s41582-019-0216-y.
Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;154(Suppl):1. https://doi.org/10.1016/j.pain.2013.07.021.
Sudershan, A., Younis, M., Sudershan, S., & Kumar, P. (2022). Migraine as an inflammatory disorder with microglial activation as a prime candidate. Neurol Res, 1–16. https://doi.org/10.1080/01616412.2022.2129774. Advance online publication
De Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64(1):37–43. https://doi.org/10.1016/0165-5728(95)00148-4.
Elahi MM, Asotra K, Matata BM, Mastana SS. Tumor necrosis factor alpha -308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochem Biophys Acta. 2009;1792(3):163–72. https://doi.org/10.1016/j.bbadis.2009.01.007.
Kroeger KM, Steer JH, Joyce DA, Abraham LJ. Effects of stimulus and cell type on the expression of the -308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12(2):110–9. https://doi.org/10.1006/cyto.1999.0529.
Brinkman BM, Zuijdeest D, Kaijzel EL, Breedveld FC, Verweij CL. Relevance of the tumor necrosis factor alpha (TNF alpha) -308 promoter polymorphism in TNF alpha gene regulation. J Inflamm. 1995;46(1):32–41.
Guo XF, Wang J, Yu SJ, Song J, Ji MY, Cao Z, Zhang JX, Wang J, Dong WG. TNF-α-308 polymorphism and risk of digestive system cancers: a meta-analysis. World J Gastroenterol. 2013;19(48):9461–71. https://doi.org/10.3748/wjg.v19.i48.9461.
Tiongco RE, Aguas IS, Cabrera FJ, Catacata M, Flake CC, Manao MA, Policarpio A. The role of the TNF-α gene -308 G/A polymorphism in the development of diabetic nephropathy: an updated meta-analysis. Diabetes Metab Syndr. 2020;14(6):2123–9. https://doi.org/10.1016/j.dsx.2020.10.032.
Cao Y, Song Y, Ning P, Zhang L, Wu S, Quan J, Li Q. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: a meta-analysis update. BMC Pulm Med. 2020;20(1):215. https://doi.org/10.1186/s12890-020-01253-0.
Wang, D., He, L., & Zhang, X. (2021). -308G/A polymorphism of tumor necrosis factor alpha (TNF-α) gene and metabolic syndrome susceptibility: a meta-analysis. Sci Rep, 11(1). https://doi.org/10.1038/s41598-021-83321-x
Schurks M, Rist PM, Zee RY, Chasman DI, Kurth T. Tumour necrosis factor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia. 2011;31(13):1381–404. https://doi.org/10.1177/0333102411419022.
Gu L, Yan Y, Long J, Su L, Hu Y, Chen Q, Xie J, Wu G. The TNF-α-308G/A polymorphism is associated with migraine risk: a meta-analysis. Exp Ther Med. 2012;3(6):1082–6. https://doi.org/10.3892/etm.2012.533.
Chen M, Tang W, Hou L, Liu R, Dong Z, Han X, Zhang X, Wan D, Yu S. Tumor Necrosis Factor (TNF) -308G>A, Nitric Oxide Synthase 3 (NOS3) +894G>T polymorphisms and migraine risk: a meta-analysis. PloS one. 2015;10(6):e0129372. https://doi.org/10.1371/journal.pone.0129372.
Kesavan P, Satheesh A. P, Husain R. S. R. A, Veerappan U, Kannaian S, Ahmed S. S, Veerabathiran R. Genetic predisposition of TNFα gene polymorphism in South-Indian Migraineurs and meta-analysis. Front Biosci (Elite Edition). 2021;13(2):226–36. https://doi.org/10.52586/e880.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A., … Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed.), 372, n71. https://doi.org/10.1136/bmj.n71
Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39. https://doi.org/10.1186/s12874-017-0315-7.
Poscente M, Brioli G, Lulli P, Morellini M, Martelletti P, Giacovazzo M, Trabace S. TNFA gene: the -308 promoter polymorphism in migraine. J Headache Pain. 2000;1(S2):S169–71. https://doi.org/10.1007/s101940070013.
Trabace S, Brioli G, Lulli P, Morellini M, Giacovazzo M, Cicciarelli G, Martelletti P. Tumor necrosis factor gene polymorphism in migraine. Headache. 2002;42(5):341–5. https://doi.org/10.1046/j.1526-4610.2002.02104.x.
Rainero I, Grimaldi LM, Salani G, Valfre W, Rivoiro C, Savi L, Pinessi L. Association between the tumor necrosis factor- -308 G/A gene polymorphism and migraine. Neurology. 2004;62(1):141–3. https://doi.org/10.1212/01.wnl.0000101717.16799.8f.
Herken H, Erdal ME, Yilmaz M, Savasoglu K, Bayazit YA. The –308 G/A polymorphism of tumor necrosis factor alpha gene is not associated with migraine. Pain Clinic. 2005;17(4):389–93. https://doi.org/10.1163/156856905774482814.
Mazaheri S, Hajilooi M, Rafiei A. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with migraine without aura. J Neurol. 2006;253(12):1589–93. https://doi.org/10.1007/s00415-006-0270-4.
Lee KA, Jang SY, Sohn KM, Won HH, Kim MJ, Kim JW, Chung CS. Association between a polymorphism in the lymphotoxin?Apromoter region and migraine. Headache. 2007;47(7):1056–62. https://doi.org/10.1111/j.1526-4610.2007.00847.x.
Ghosh J, Joshi G, Pradhan S, Mittal B. Investigation of TNFA 308G > A and TNFB 252G > A polymorphisms in genetic susceptibility to migraine. J Neurol. 2009;257(6):898–904. https://doi.org/10.1007/s00415-009-5430-x.
Asuni C, Stochino ME, Cherchi A, Manchia M, Congiu D, Manconi F, Squassina A, Piccardi MP, Zompo M. Migraine and tumour necrosis factor gene polymorphism. J Neurol. 2009;256(2):194–7. https://doi.org/10.1007/s00415-009-0961-8.
Schürks M, Kurth T, Buring JE, Zee RY. A candidate gene association study of 77 polymorphisms in migraine. J Pain. 2009;10(7):759–66. https://doi.org/10.1016/j.jpain.2009.01.326.
Pappa S, Hatzistilianou M, Kouvatsi A, Pantzartzi C, Sakellaropoulou A, Pavlou E, Mavromichales I, Athanassiadou F. Tumour necrosis factor gene polymorphisms and migraine in Greek children. Arch Med Sci. 2010;6(3):430–7. https://doi.org/10.5114/aoms.2010.14267.
Yılmaz IA, Özge A, Erdal ME, Edgünlü TG, Çakmak SE, Yalın OZ. Cytokine polymorphism in patients with migraine: some suggestive clues of migraine and inflammation. Pain Med. 2010;11(4):492–7. https://doi.org/10.1111/j.1526-4637.2009.00791.x.
Ates O, Kurt S, Altinisik J, Karaer H, Sezer S. Genetic variations in tumor necrosis factor alpha, interleukin-10 genes, and migraine susceptibility. Pain Med. 2011;12(10):1464–9. https://doi.org/10.1111/j.1526-4637.2011.01200.x.
Stuart S, Maher BH, Sutherland H, Benton M, Rodriguez A, Lea RA, Haupt LM, Griffiths LR. Genetic variation in cytokine-related genes and migraine susceptibility. Twin Res Hum Genet. 2013;16(6):1079–86. https://doi.org/10.1017/thg.2013.63.
Fawzi MS, El-Shal AS, Rashad NM, Fathy HA. Influence of tumor necrosis factor alpha gene promoter polymorphisms and its serum level on migraine susceptibility in Egyptian patients. J Neurol Sci. 2015;348(1–2):74–80. https://doi.org/10.1016/j.jns.2014.11.009.
Shaik MM, Abubakar MB, Tan HL, Gan SH. Influence of TNF-α and ESR1 polymorphisms on vascular, hormonal and inflammatory biomarkers in migraine. J Med Sci. 2018;18(2):76–86. https://doi.org/10.3923/jms.2018.76.86.
Hamad N, Alzoubi KH, Swedan SF, Khabour OF, El-Salem K. Association between tumor necrosis factor alpha and lymphotoxin alpha gene polymorphisms and migraine occurrence among Jordanians. Neurol Sci. 2021;42(9):3625–30.
Tatlisuluoglu D, Derle E, Ruhsen OCAL, Kibaroglu S, Ataç FB, Ufuk CAN. The relationship between tumor necrosis factor α-308 G/A poly-morphism and serum tumor necrosis factor α levels in patients with migraine without aura. Troia Medical Journal. 2021;2(3):73–8.
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–8. https://doi.org/10.1111/j.1526-4610.2006.00483.x.
Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47(7):1050–5. https://doi.org/10.1111/j.1526-4610.2006.00722.x.
Covelli V, Munno I, Pellegrino NM, Altamura M, Decandia P, Marcuccio C, Jirillo E. Are TNF-alpha and IL-1 beta relevant in the pathogenesis of migraine without aura? Acta neurologica. 1991;13(2):205–11.
Covelli V, Munno I, Pellegrino NM, Di Venere A, Jirillo E, Buscaino GA. Exaggerated spontaneous release of tumor necrosis factor-alpha/cachectin in patients with migraine without aura. Acta Neurol. 1990;12(4):257–63.
Perini F, D’Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, Toso V. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–31.
Mueller L, Gupta AK, Stein TP. Deficiency of tumor necrosis factor α in a subclass of menstrual migraineurs. Headache. 2001;41(2):129–37.
Martami F, RazeghiJahromi S, Togha M, Ghorbani Z, Seifishahpar M, Saidpour A. The serum level of inflammatory markers in chronic and episodic migraine: a case-control study. Neurol Sci. 2018;39:1741–9.
Hellwege JN, Keaton JM, Giri A, Gao X, Velez Edwards DR, Edwards TL. Population stratification in genetic association studies. Curr Protocols Hum Genets. 2017;95:1.22.1-1.22.23. https://doi.org/10.1002/cphg.48.
Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6(6):521–32. https://doi.org/10.1016/S1474-4422(07)70126-6.
Acknowledgements
Authors are highly thankful to the Institute of Human Genetics, University of Jammu and Department of Human Genetics (Sri Pratap College, Srinagar, Cluster University Srinagar) for support in present study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Detail of the author’s contribution, according to the CRediT (Contributor Roles Taxonomy) System: PK & AS conceptualized the study and provided supervision, SS, MB, & ACP downloaded and filtered the data, AS, PK conducted the statistical analysis, interpretation and drafted the manuscript, AS, SS, MY & ACP edited the pictures and tables, HK & PK edited the manuscript and PK finalize the manuscript. All authors provided critical feedback on drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sudershan, A., Sudershan, S., Younis, M. et al. Enlightening the association between TNF-α -308 G > A and migraine: a meta-analysis with meta-regression and trial sequential analysis. BMC Neurol 23, 159 (2023). https://doi.org/10.1186/s12883-023-03174-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03174-x