Abstract
Background
Osteogenesis imperfecta (OI) is a collagen-related bone dysplasia leading to a susceptibility to fractures. OI can be caused by mutations in several genes including BMP1. It encodes two isoforms, bone morphogenetic protein 1 (BMP1) and mammalian tolloid (mTLD); both have proteolytic activity to remove the C-propeptide from procollagen.
Case presentation
We report a Thai OI patient who had his first fracture at the age of three months. Using next generation sequencing, we successfully identified two novel compound heterozygous BMP1 mutations. One mutation, c.796_797delTT (p.Phe266Argfs*25) affects both BMP1 and mTLD isoforms, while the other, c.2108-2A > G, affects only the BMP1 isoform. Preservation of the mTLD may explain the relatively less severe clinical phenotype in this patient. Intravenous bisphosphonate was given from the age of 8 months to 5 years. He was free from fractures for 9 months before discontinuation.
Conclusion
This case expands the mutation spectrum of BMP1, strengthens the correlation between genotype and phenotype, and supports the benefits of bisphosphonate in OI patients with BMP1 mutations.
Similar content being viewed by others
Background
Osteogenesis imperfecta (OI), a brittle bone disease, is a collagen-related bone dysplasia characterized by bone fragility leading to a susceptibility to fractures. Clinical manifestations of OI vary from intrauterine fractures and perinatal death to a mild form with few or no fractures [1]. Other features include short stature, bone deformities, joint laxity, dentinogenesis imperfecta, and blue sclerae. OI has been classified into autosomal dominant and recessive forms. More than 90% of OI patients are caused by mutations in COL1A1 (Collagen Type I Alpha Chain) and COL1A2 (Collagen Type II Alpha Chain), inherited in an autosomal dominant manner. COL1A1 and COL1A2 encode alpha 1 and alpha 2 chains of type I collagen, respectively. Other inherited forms are rare and can be caused by mutations in different genes including IFITM5 (Interferon Induced Transmembrane Protein 5) responsible for a dominant form, 14 genes for the recessive form, BMP1 (Bone Morphogenic Protein 1), CRTAP (Cartilage Associated Protein), CREB3L1 (CAMP Responsive Element Binding Protein 3 Like 1), FKBP10 (FKP506 Binding Protein 10), LEPRE1 (Leucine- And Proline-Enriched Proteoglycan 1), PLOD2 (Procollagen-Lysine, 2 –Oxoglutarate 5-Dioxygenase 2), PPIB (Peptidylprolyl Isomerase B), SEC24D (SEC24 Homolog A, COPII Coat Complex Component), SERPINF1 (Serpin Family F Member 1), SERPINH1 (Serpin Family H Member 1), SP7 (Sp7 Transcription Factor), SPARC (Secreted Protein Acidic And Cysteine Rich), TMEM38B (Transmembrane Protein 38B) and WNT1 (Wnt Family Member 1) [2–4] and MBTPS2 (Membrane Bound Transcription Factor Peptidase, Site 2) for the X-linked form [5].
Mutations in COL1A1 and COL1A2 result in primary structural or quantitative defects of type I collagen. The recessive forms of OI arise from defects in proteins that are responsible for post-translation modification of collagen, including defects in proteins responsible for folding and processing of collagen, and from defects in proteins that are necessary for ossification, mineralization or osteoblast development.
Proα1 and proα2 chains of type I collagen are post-translationally modified in the endoplasmic reticulum lumen into triple helix and then secreted into the extracellular matrix. After cleavage of their N and C-terminal propeptides, trimeric collagen molecules will be arranged into highly ordered collagen fibrils. BMP1, which is located on chromosome 8p21.3, encodes a protein with a role in proteolytic removal of the C-propeptide from procollagen. This crucial step is needed for the assembly of mature collagen monomers into fibrils [6]. Here we describe a Thai boy with OI who harbored two novel compound heterozygous BMP1 mutations, c.796_797delTT (p.Phe266Argfs*25) and c.2108-2A > G.
Case presentation
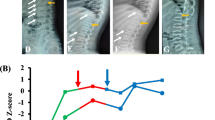
Our patient was a 6-year-old boy, who was the second child of a non-consanguineous Thai couple. There was no family history of OI or other bone disorders. He was born at term with a birth weight of 3600 g (60th centile). His early development was normal. He sustained the first fracture of his right arm at the age of three months from a minor trauma. Since then he had multiple fractures of various bones including both humeri, femora, tibiae, ulnae and radii, leading to a diagnosis of OI Sillence type III. Intravenous bisphosphonate was initiated at the age of 8 months; however, the fractures continued to occur once every few months and required many surgeries. At the age of 5 years, after he was free from fractures for 9 months, bisphosphonate was discontinued. His mental development was appropriate. He was able to walk until the age of six years, when a nonunion and muscle wasting of lower extremities occurred. Physical examination revealed deformities of both upper and lower extremities. However, no blue sclerae or dentinogenesis imperfecta was observed. Investigations revealed normal serum calcium, phosphorus and alkaline phosphatase levels. Plain radiographs of his long bones at the age of nine are shown in Fig. 1.
AP plain radiographs of lower extremities at the age of nine. a Both femoral AP radiographs reveal a nonunion at the shaft with shortening and varus deformity of the left femur. Intramedullary rods were used to stabilize the fractures. Generalized osteopenia is noted. b Both tibial AP radiographs reveal a nonunion, varus deformity, and shortening of the right tibia. Intramedullary rods were used to stabilize the fractures
After informed consent was obtained, genomic DNA was extracted from leukocytes using a Puregene blood kit (Qiagen, Hilden, Germany). Sixteen known OI genes, BMP1, COL1A1, COL1A2, CREB3L1, CRTAP, FKBP10, IFITM5, LEPRE1, PLOD2, PPIB, SERPINF1, SERPINH1, SP7, TMEM38B, WNT1, and MBTPS2, were amplified from 200 ng of genomic DNA using the Truseq Amplicon Sequencing kit (Illumina, San Diego, CA). 286 amplicons which covered all the 226 exons (28 kb) of the target genes were sequenced by Miseq (Illumina, San Diego, CA) using 2x250 paired-end reads. All reads were aligned against the University of California Santa Cruz human genome assembly hg19 using Burrows-Wheeler Alignment software [7]. SNVs and Indel were detected by Miseq reporter software. Finally, possible disease causing variants were confirmed by PCR and Sanger sequencing. Segregation analysis was subsequently performed.
The targeted gene panel study using next generation sequencing of the proband revealed compound heterozygous mutations, c.796_797delTT (p.Phe266Argfs*25) and c.2108-2A > G in the BMP1 gene (NM_006129.4). PCR followed by Sanger sequencing using genomic DNA of leukocytes from the patient and his parents confirmed that the patient was compound heterozygous for the mutations, while his father had c.796_797delTT (p. Phe266Argfs*25) and his mother had c.2108-2A > G (Fig. 2). Both mutations have never been previously described in Human Gene Mutation Database [8] or the Exome Aggregation Consortium database [9].
Discussion
We identified a Thai patient with OI who had his first fracture at the age of three months. He was found to be compound heterozygous for c.796_797delTT (p. Phe266Argfs*25) and c.2108-2A > G mutations in the BMP1 gene (Fig. 2). Both have never been reported. Adding this patient to the literature, there are currently 15 patients from 12 families (Table 1) who have an autosomal recessive form of OI resulted from mutations in the BMP1gene. Eight patients of six families were homozygous while the other seven patients of six families were compound heterozygous. Of these 12 families, 12 different mutations have been identified (Fig. 3).
Processing of procollagen I C-terminal propeptide (PICP) is accomplished byfour BMP1-like proteinases: BMP1, mammalian tolloid (mTLD), and mammalian tolloid-like 1 and 2 (mTLL1 and 2). BMP1 and mTLD are resulted from the alternative splicing of the BMP gene, which is located on chromosome 8p21.3. The other two remaining members of this protein family, mTLL1 and mTLL2, are encoded by Tll1 and Tll2 located on chromosome 4q32.3 and 10q24.1, respectively. BMP1 has the highest PICP cleavage efficiency in vitro, followed by mTLD and mTLL1, while mTLL2 has no cleavage activity [10].
Of all the 12 BMP1 mutations identified to date, nine affect both BMP1 and mTLD while the other three: c.2108-2A > G, Gln730Prof*294, and c*241 T > C affect only the BMP1 (Fig. 3). No mutations have been found in positions affecting only the mTLD. Those mutant alleles affecting only the BMP1 are expected to synthesize an intact mTLD, which has some PICP activity leading to a relatively milder form of OI. This hypothesis is true for the 11 families except Family 11 of Table 1, which harbored the c.34G > C (p.Gly12Arg) and c.2188dupC (p.Gln730Profs*294) mutations. Although the latter mutation affects only BMP1, two siblings of this family had severe bone fragility with more than a hundred fractures [11].
The conditions of the eight patients with both mutant alleles affecting both isoforms, no matter they are homozygous (Families 1, 2, and 3 of Table 1) or compound heterozygous (Families 8, 9, and 10 of Table 1), are relatively more severe. They had high frequency of fractures and severe bone deformities. For instance, the two patients with homozygous p.Phe249Leu mutation (Family 1 of Table 1), which is in the enzymatically active astacin domain affecting both BMP1 and mTLD, had very severe clinical presentation including severe bone fragility with fracture rate at 10-15/year, decreased bone mass, bone deformities, marked short stature and dysmorphic facial features.
Patients who were compound heterozygous with one mutant allele affecting only the BMP1 resulting in an intact mTLD (Families 7, 11, and 12 of Table 1) had relatively milder phenotypes. Our patient was compound heterozygous. One mutation, c.796_797delTT (p. Phe266Argfs*25) is an out-of-frame deletion in the astacin domain affecting both BMP1 and mTLD isoforms. The other mutation, c.2108-2A > G, is predicted to affect only the BMP1 isoform but preserve the mTLD (Fig. 3). This may explain the milder disease severity of our patient who had his first fracture at the age of three months, compared with patients whose mutations affect both BMP1 and mTLD.
Three patients (Families 4, 5, and 6 of Table 1) were homozygous for the c.*241 T > C. This mutation is predicted to affect only the BMP1 and preserve the activity of mTLD. They had the mildest phenotype. Their total number of fractures was 12, 16, and 18 times at the ages of 17, 22, and 28, respectively. In addition, two of them had the latest onset of the first fracture, which occurred at the ages of 2.5 and 4 years. Compared with a Canadian-French patient with compound heterozygous mutations, c.*241 T > C and p.Glu703Gln (Family 7 of Table 1) who had first fracture at birth and 12 total fractures at the age of eight, the three patients with homozygous c.*241 T > C had a later onset and lower rates of fractures. This finding could be explained by the fact that the p.Glu703Gln mutation in Family 7 affects the activity of both BMP1 and mTLD.
Interestingly, most of the OI patients with BMP1 mutations have increased or normal bone mineral density (BMD), which is unusual for OI. Despite an increased BMD, some patients received bisphosphonate. After the treatment with bisphosphonate, two siblings with homozygous p.Gly12Arg had an increased BMD, an elevated urinary deoxypyridinoline excretion (a marker for osteoclastic activities) and a reduced fracture rate [12]. Intravenous bisphosphonate therapy in two Canadian-French patients (Families 6 and 7 of Table 1) with an elevated BMD also exhibited a similar result. One of them showed improvement in the shape of compressed vertebral bodies and a reduction in fracture rate [13]. Our patient was also given intravenous bisphosphonate and a decrease in fracture frequency was observed. Unfortunately, he did not undergo a BMD measurement. Whether bisphosphonate therapy in OI patients with BMP1 mutations is useful awaits further studies.
Conclusion
We described an OI patient with two novel compound heterozygous mutations in BMP1. One of the two is expected to preserve the mTLD isoform, which may lead to his relatively mild phenotype.
Abbreviations
- BMD:
-
Bone mineral density
- BMP1:
-
Bone morphogenetic protein 1
- mTLD:
-
Mammalian tolloid
- mTLL1:
-
Mammalian tolloid-like 1
- mTLL2:
-
Mammalian tolloid-like 2
- OI:
-
Osteogenesis imperfect
References
Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16(2):101–16.
Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–71.
Garbes L, Kim K, Riess A, Hoyer-Kuhn H, Beleggia F, Bevot A, Kim MJ, Huh YH, Kweon HS, Savarirayan R, et al. Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. Am J Hum Genet. 2015;96(3):432–9.
Mendoza-Londono R, Fahiminiya S, Majewski J, Care4Rare Canada C, Tetreault M, Nadaf J, Kannu P, Sochett E, Howard A, Stimec J, et al. Recessive osteogenesis imperfecta caused by missense mutations in SPARC. Am J Hum Genet. 2015;96(6):979–85.
Lindert U, Cabral WA, Ausavarat S, Tongkobpetch S, Ludin K, Barnes AM, Yeetong P, Weis M, Krabichler B, Srichomthong C, et al. MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta. Nat Commun. 2016;7:11920.
Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118(Pt 7):1341–53.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–95.
The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff. http://www.hgmd.cf.ac.uk/ac/. Accessed 10 Mar 2016.
ExAC Browser (Beta) | Exome Aggregation Consortium. http://exac.broadinstitute.org/. Accessed 10 Mar 2016.
Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, et al. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213(2):283–300.
Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, Hermanns-Le T, Coucke PJ, De Paepe A, Malfait F. Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J Bone Miner Res. 2015;30(8):1445–56.
Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, Yigit G, Pohl E, Becker J, Frommolt P, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90(4):661–74.
Fahiminiya S, Al-Jallad H, Majewski J, Palomo T, Moffatt P, Roschger P, Klaushofer K, Glorieux FH, Rauch F. A polyadenylation site variant causes transcript-specific BMP1 deficiency and frequent fractures in children. Hum Mol Genet. 2015;24(2):516–24.
Martinez-Glez V, Valencia M, Caparros-Martin JA, Aglan M, Temtamy S, Tenorio J, Pulido V, Lindert U, Rohrbach M, Eyre D, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012;33(2):343–50.
Valencia M, Caparros-Martin JA, Sirerol-Piquer MS, Garcia-Verdugo JM, Martinez-Glez V, Lapunzina P, Temtamy S, Aglan M, Lund AM, Nikkels PG, et al. Report of a newly indentified patient with mutations in BMP1 and underlying pathogenetic aspects. Am J Med Genet A. 2014;164A(5):1143–50.
Cho SY, Asharani PV, Kim OH, Iida A, Miyake N, Matsumoto N, Nishimura G, Ki CS, Hong G, Kim SJ, et al. Identification and in vivo functional characterization of novel compound heterozygous BMP1 variants in osteogenesis imperfecta. Hum Mutat. 2015;36(2):191–5.
Acknowledgements
We thank the family members for participating in this study.
Funding
This study was supported by the Thailand Research Fund (BRG5980001), Chulalongkorn Academic Advancement into Its 2nd Century Project and the Newton Fund.
Availability of data and materials
All data are contained in the manuscript.
Authors’ contributions
AS participated in the molecular genetic studies and wrote the manuscript, CK participated in writing the manuscript, CS participated in molecular genetic studies, MP participated in the molecular genetic studies. KS assured the general supervision of the research group, VS assured the general supervision of the research group and raised funding. All authors revised and approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The parents gave permission for the publication of the patient’s clinical details and images.
Ethics approval and consent to participate
Ethical approval was obtained from the institutional review board, Faculty of Medicine, Chulalongkorn University. After explanation of the possible consequences of this study, the written informed consent and parental consent (for the proband) was obtained.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sangsin, A., Kuptanon, C., Srichomthong, C. et al. Two novel compound heterozygous BMP1 mutations in a patient with osteogenesis imperfecta: a case report. BMC Med Genet 18, 25 (2017). https://doi.org/10.1186/s12881-017-0384-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-017-0384-9