Abstract
Background
Lingering symptoms after acute COVID-19 present a major challenge to ambulatory care services. Since there are reservations regarding their optimal management, we aimed to collate all available evidence on the effects of rehabilitation treatments applicable in ambulatory care for these patients.
Methods
On 9 May 2022, we systematically searched articles in COVID-19 collections, Embase, MEDLINE, Cochrane Library, Web of Science, CINAHL, PsycArticles, PEDro, and EuropePMC. References were eligible if they reported on the clinical effectiveness of a rehabilitation therapy applicable in ambulatory care for adult patients with persisting symptoms continuing 4 weeks after the onset of COVID-19. The quality of the studies was evaluated using the CASP cohort study checklist and the Cochrane Risk of Bias Assessment Tool. Summary of Findings tables were constructed and the certainty of evidence was assessed using the GRADE framework.
Results
We included 38 studies comprising 2,790 participants. Physical training and breathing exercises may reduce fatigue, dyspnoea, and chest pain and may improve physical capacity and quality of life, but the evidence is very weak (based on 6 RCTs and 12 cohort studies). The evidence underpinning the effect of nutritional supplements on fatigue, dyspnoea, muscle pain, sensory function, psychological well-being, quality of life, and functional capacity is very poor (based on 4 RCTs). Also, the evidence-base is very weak about the effect of olfactory training on sensory function and quality of life (based on 4 RCTs and 3 cohort studies). Multidisciplinary treatment may have beneficial effects on fatigue, dyspnoea, physical capacity, pulmonary function, quality of life, return to daily life activities, and functional capacity, but the evidence is very weak (based on 5 cohort studies). The certainty of evidence is very low due to study limitations, inconsistency, indirectness, and imprecision.
Conclusions
Physical training, breathing exercises, olfactory training and multidisciplinary treatment can be effective rehabilitation therapies for patients with persisting symptoms after COVID-19, still with high uncertainty regarding these effects. These findings can guide ambulatory care practitioners to treat these patients and should be incorporated in clinical practice guidelines. High-quality studies are needed to confirm our hypotheses and should report on adverse events.
Similar content being viewed by others
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for coronavirus disease 2019 (COVID-19), has caused considerable morbidity and mortality at an unprecedented global scale [1]. Evidence on the subacute and longer-term effects of COVID-19 is evolving worldwide [1]. Persisting symptoms following COVID-19 can be defined as ‘ongoing symptomatic COVID-19’ (symptoms lasting 4 to 12 weeks) and ‘post-COVID-19 syndrome’ (symptoms beyond 12 weeks) according to the National Institute for Health and Care Excellence (NICE)’s terms [2], ‘post-COVID-19 condition’ as named by the World Health Organisation (WHO) [3], and ‘post-COVID conditions’ as referred to by the United States Center for Disease Control and Prevention’s (CDC) group [4]. It is estimated that at an average follow-up time of 4 months, 45% of COVID-19 survivors exhibit at least one unresolved symptom [5]. The incidence is even higher among previously hospitalised patients, reaching 53% [5]. The most commonly reported symptoms are fatigue, dyspnoea, (muscle) pain, affected sleep, impaired usual activity, and loss of smell and taste [5]. Therefore, this is a complex, multifaceted condition affecting multiple organ systems [5]. Also its pathogenesis is likely multifactorial and it includes prolonged inflammation, immune-mediated vascular dysfunction, thromboembolism, and nervous system dysfunction [6]. Risk factors may include female sex, increasing age, having two or more comorbidities, a more severe acute COVID-19 illness, and a higher number of symptoms during the acute illness [7,8,9]. Increased levels of D-dimer or C-reactive protein or reduced lymphocyte count during the acute illness may also be prognostic factors [7]. Further research is needed to better define these risk factors, to understand the underlying mechanisms, and to address the neuropsychological components and its impact on this new clinical disease entity [6, 7]. Currently, there are reservations regarding the optimal management of patients with persisting symptoms after COVID-19, as there is insufficient evidence on the mechanisms that underpin this condition [10]. The WHO suggests that rehabilitation for these patients requires a person-centred, comprehensive, and multidisciplinary approach [11]. Interventions for rehabilitation may include education, skills training on self-management strategies, advice on paced return to activities, breathing techniques (including respiratory muscle training), physical exercise therapy, psychological interventions, cognitive training, rehabilitation for communication and swallowing difficulties, and occupational therapy [11]. These interventions should be provided in close collaboration with primary healthcare and several medical specialties [11].
We aimed to collate all available evidence on the effects of rehabilitation treatments, applicable in ambulatory care, for patients with persisting symptoms after COVID-19. This review is part of the development of a guideline on the follow-up and rehabilitation of patients with persisting symptoms after COVID-19 in primary care, which was commissioned by the Belgian government.
Methods
We performed a systematic review according to the methods as described by Cochrane [12] and reported it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Checklist (Additional File 1) [13].
Search strategy
First, we screened existing COVID-19 libraries, namely Research Aid Networks Long Covid Library [14] and Resources LongCovid (Care) [15] and web-based COVID-19 collections, i.e. Cochrane COVID-19 Study Register [16], Epistemonikos [17], and WHO COVID-19 database [18]. In addition, we searched the following literature databases: Embase, MEDLINE, Cochrane Library, Web of Science Core Collection, CINAHL, PsycArticles, PEDro, and EuropePMC (preprints). The search string was based on two concepts: ‘persisting symptoms after COVID-19’ and ‘rehabilitation’. Full search strategies for each database are included in Additional File 1. We also screened the references included in NICE’s COVID-19 rapid guideline: ‘managing the long-term effects of COVID-19’ [2]. Experts from the Belgian guideline working group were also requested to verify the list of retrieved publications and add publications when missing.
Inclusion and exclusion criteria
References were eligible if they reported on the clinical effectiveness of a rehabilitation therapy applicable in ambulatory care for patients with persisting symptoms after COVID-19 (i.e., new or ongoing symptoms continuing after 4 weeks from the onset of acute COVID-19). Studies investigating a rehabilitation therapy contiguous to hospitalisation were excluded because this was not the population of interest (i.e., these patients were often more seriously ill and often had specific problems). We included randomised controlled trials (RCTs), non-randomised trials, prospective and retrospective cohort studies, cross-sectional studies, case–control studies, and case series with at least 10 patients. Systematic reviews, case reports, letters, editorials, qualitative studies, conference abstracts, posters, and protocols were excluded. Preprints were not included in the final analysis, but it was checked whether the corresponding articles had already been published. We only considered studies on adults (≥ 18 years) who had experienced symptomatic and confirmed (by polymerase chain reaction, antibodies, or chest CT) COVID-19. We excluded studies on nursing home residents and people with specific comorbidities (e.g., heart failure, diabetes), except if these populations constituted less than 20% of the sample size. There were no restrictions in terms of language, country, race, or gender. We excluded studies investigating the effectiveness of individual molecules and synthetic drugs, except for over the counter nutritional supplements (such as vitamins). If molecules were evaluated on top of a rehabilitation therapy, the study was included.
Selection and data extraction
Search results were imported into a reference management program (Endnote 20.2 (Bld 15,709), Clarivate Analytics) and duplicate citations were removed [19]. Based on inclusion and exclusion criteria, pairs of two reviewers (11 reviewers in total) independently screened all records by title and abstract, using Rayyan (Rayyan Systems Inc.) [20] and Covidence [21] software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.). Next, pairs of two reviewers independently reviewed the full text of all potentially relevant records, using the same selection criteria. Data were extracted by one reviewer (HD) and checked by another reviewer (GB, DP) using standardised data extraction forms in Covidence and Microsoft Excel (Version 2202). Any queries or disagreements in either of the steps above were resolved through discussion or, if necessary, another reviewer. The following data items were extracted: title, authors, publication year, journal, publication status, country, timeframe of patient recruitment, study design, population characteristics (i.e., inclusion and exclusion criteria, setting, sample size, age, sex, follow-up time since acute COVID-19, acute COVID-19 disease severity, hospitalisation or intensive care unit use during acute COVID-19 illness), rehabilitation therapy, comparator(s), primary and secondary endpoints, and the main results. Data were sought for the following outcome measures: fatigue, dyspnoea, muscle pain, chest pain, physical capacity (i.e., physical fitness and muscle performance), pulmonary function, cognitive function, sensory function (i.e., smell and taste), psychological well-being, quality of life, return to normal daily life activities, functional capacity (i.e., ability to perform ‘activities of daily living’), and adverse events. All results that were compatible with each outcome were extracted (i.e., all measures, time points, and analyses), except for pulmonary function for which we only extracted data for the maximal voluntary ventilation (MVV), vital capacity (VC), and maximal inspiratory pressure (MIP) as these were considered as measurable in an ambulatory care setting. If needed, corresponding authors were contacted for additional study information.
Risk of bias assessment
The methodological quality of the selected studies was evaluated independently by pairs of two reviewers (HD, GB, SG) using the CASP cohort study checklist for cohort studies [22] and the Cochrane Risk of Bias Assessment Tool 2.0 for RCTs [23].
Data analysis and certainty of evidence assessment
Due to a high heterogeneity between studies, results were summarised narratively. We constructed Summary of Findings (SoF) tables to summarize all available evidence by intervention type (i.e., physical training program, breathing exercises, nutritional supplements, olfactory training, and multidisciplinary treatment) and subsequently by study design. For each outcome measure, we assessed certainty of evidence using the methods of the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) framework [24, 25]. GRADEpro was used to create the SoF tables (GRADEpro Guideline Development Tool. McMaster University and Evidence Prime, 2022. Available from gradepro.org.).
We performed vote counting to determine by outcome domain the number of studies demonstrating beneficial results, no significant improvement, and mixed results (i.e., some measures showed beneficial results, while other measures within the same study did not show a significant improvement).
Protocol registration
We published and prospectively registered the protocol for this systematic review on PROSPERO (CRD42022330205).
Results
Identification of studies
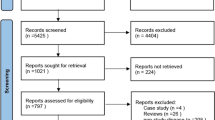
From the database search on 9 May 2022, 45,479 records were identified, of which 28,322 were screened for title and abstract. Of those, 415 references were screened based on full-text, whereof 38 unique studies were retained for this review (Fig. 1).
Physical training programs were evaluated by 14 studies, breathing exercises by 10 studies, nutritional supplements by four studies, olfactory training by seven studies, and multidisciplinary treatment programs were assessed by five studies. Seven studies evaluated other interventions which do not fall within these categories (i.e., narrative exposure therapy, aromatherapy, hydrogen inhalation, massage techniques, hyperbaric oxygen therapy, enhanced external counterpulsation). Characteristics of included studies are presented in Table 1.
Risk of bias
Of the 27 cohort studies assessed with the CASP cohort study checklist, 14 had a good overall score (i.e., 7 or 8), 12 had a moderate score (i.e., 4–6), and one study had a low score (i.e., ≤ 3). Of the 13 RCTs assessed with the Cochrane Risk of Bias Assessment Tool 2.0, two had a low risk of bias, three had some concerns, and eight had a high risk of bias. Full details on the risk of bias assessment can be found in Additional file 2.
Effects of rehabilitation interventions
Figure 2 graphically summarises the results of the included studies. Full details and SoF tables can be found in Additional File 1.
Physical training program
Three RCTs [33, 41, 42] and 11 cohort studies [26,27,28,29,30,31,32, 38,39,40, 43] evaluated physical training as part of a rehabilitation program in patients with persisting complaints after COVID-19. Beneficial effects have been reported for the following outcomes (however, certainty of evidence is very low): dyspnoea, physical capacity, pulmonary function, quality of life, fatigue, chest pain, cognitive function, psychological well-being, and functional capacity. An improvement could not be demonstrated for muscle pain and return to work (very low certainty of evidence). One RCT [33] found that, in men with post-COVID-19 sarcopenia, low intensity training resulted in an increased handgrip strength and a better quality of life compared to high intensity training. Two studies [32, 41] reported adverse events, and when detected, none were related to the intervention.
Breathing exercises
Five RCTs [35,36,37, 41, 42] and five cohort studies [34, 38,39,40, 43] evaluated breathing exercises as part of a rehabilitation program in patients with prolonged symptoms after COVID-19. Beneficial effects (all with very low certainty of evidence) have been reported for the following outcomes: dyspnoea, physical capacity, pulmonary function, psychological well-being, quality of life, functional capacity, fatigue, muscle pain, chest pain, cognitive function, and return to normal daily life activities. Two studies reported adverse events such as hospitalisations [41] and dizziness [36]. One study reported that participation could trigger exacerbation of symptoms such as fatigue [36].
Nutritional supplements
Four RCTs assessed the effects of nutritional supplements in patients with persisting symptoms after COVID-19. The following supplements were evaluated: palmitoylethanolamide and luteolin (two studies [44, 45]), systemic enzymes (ImmunoSEB) and probiotics (one study [46]), and acetyl-carnitine (one study [42]).
Beneficial effects have been reported for the following outcomes (all with very low certainty of evidence): fatigue (systemic enzymes and probiotics), dyspnoea (acetyl-carnitine), muscle pain (acetyl-carnitine), sensory function (palmitoylethanolamide and luteolin), psychological well-being (acetyl-carnitine), quality of life (acetyl-carnitine), and functional capacity (acetyl-carnitine, mixed results). Two studies [45, 46] reported on adverse events, of which none were detected.
Olfactory training
Four RCTs [44, 45, 47, 50] and three cohort studies [48, 49, 51] evaluated olfactory training in patients with persisting olfactory complaints after COVID-19. For the RCTs, we only used control group data, so these were all considered as observational studies. Five studies [44, 47, 48, 50, 51] reported beneficial results on olfactory function, while two studies [45, 49] reported no difference (very low certainty of evidence). One study [51] reported beneficial effects on quality of life (very low certainty of evidence). One RCT [50] suggested that advanced olfactory training (i.e., increasing the number of essences) does not show superiority over classical olfactory training. Two studies reported adverse events: one study [50] observed that 18 out of 80 participants had side effects with olfactory training, while the other study [45] did not detect any adverse effect.
Multidisciplinary treatment
Five cohort studies [52,53,54,55,56,57] evaluated the effects of multidisciplinary treatment in patients with persisting complaints after COVID-19. All five studies included physiotherapy, four included psychological support, four included nutritional counselling and three included occupational therapy. Beneficial effects have been reported for the following outcomes (all with very low certainty of evidence): fatigue, dyspnoea, physical capacity, pulmonary function, cognitive function, psychological well-being, quality of life, return to work, and functional capacity. Two studies [52, 53, 56] reported adverse events and none were detected.
Other interventions
Three RCTs and four cohort studies investigated other interventions in patients with persisting symptoms after COVID-19. Narrative exposure therapy can have a positive effect on post-traumatic stress symptoms (one RCT [59], very low certainty of evidence). Aromatherapy can improve energy levels among women who are experiencing fatigue after recovering from COVID-19 (one RCT [60], low certainty of evidence). Hydrogen inhalation may increase the tolerance to physical activity (one RCT [64], very low certainty of evidence). Massage techniques may reduce fatigue and may improve cognitive function and psychological well-being (two cohort studies [58, 61], very low certainty of evidence). Hyperbaric oxygen therapy can have beneficial effects on fatigue (one cohort study [62], very low certainty of evidence). Enhanced external counterpulsation may reduce fatigue and brain fog and may improve physical capacity, psychological well-being, and functional capacity (one cohort study [63], very low certainty of evidence). Adverse events were reported for the following interventions: aromatherapy (one participant experienced headache; no other adverse events), hydrogen inhalation (none were detected), and hyperbaric oxygen therapy (none were detected).
Discussion
Main findings
Physical training programs and breathing exercises may reduce fatigue, dyspnoea, and chest pain and may improve physical capacity and quality of life, but the supporting evidence is very weak. Their effects on muscle pain, pulmonary function, cognitive function, psychological well-being, return to normal daily life activities, and functional capacity are still unclear. The evidence underpinning the effect of nutritional supplements on fatigue, dyspnoea, muscle pain, sensory function, psychological well-being, quality of life, and functional capacity is considered to be very poor. Also, the evidence is very uncertain about the effect of olfactory training on sensory function and quality of life. Multidisciplinary treatment may have beneficial effects on fatigue, dyspnoea, physical capacity, pulmonary function, quality of life, return to normal daily life activities, and functional capacity, but the evidence is very uncertain. Its effect on cognitive function and psychological well-being is still unclear. The certainty of evidence is very low due to study limitations (the majority of studies have a cohort design), inconsistency (e.g., some studies found positive results while others showed negative or mixed results), indirectness (e.g., physical training programs and breathing exercises were often evaluated simultaneously), and imprecision (low number of participants). For instance, some rehabilitation programs consisted of a combination of exercises (e.g., aerobic and strength training, with or without breathing techniques), so we are unable to derive from these data the most effective type of training.
Strengths and limitations
A key strength of this systematic review is a rigorous methodology, including an extensive search and the use of the GRADE framework [24, 25] to assess the certainty of evidence, which was not performed in similar reviews [65,66,67,68,69]. Also, various ambulatory care professionals (i.e., general practitioners, physiotherapists, occupational therapists, psychologists, and dieticians) as well as patients with persisting symptoms after COVID-19 were involved in the literature search, which allowed them, from the perspective of their discipline and/or experience, to verify the list of retrieved publications and to add missing ones. Further, the use of existing COVID-19 libraries limited the chance that relevant literature was not identified.
However, this review has some limitations. Given persistent symptoms after COVID-19 is a relatively new condition, the majority of included studies are single-group cohort studies with a short follow-up period, which had an impact on the certainty of evidence. Additionally, the range of outcomes for the same intervention as well as the heterogeneity in interventions might limit the generalizability of our findings. We were also not able to summarize the results quantitatively. Moreover, this review did not consider the effect of the interventions on some other important symptoms that are commonly reported by these patients, such as general pain or discomfort, affected sleep, impaired walking, joint pain, and cough, with heterogeneity in number and severity experienced [5]. Last, few studies included in this review reported on the side effects or adverse events with the therapies applied. Reports on post exertional malaise or post exertional symptom exacerbation after (exercise) training have been made available and this may require tapering down or even stopping of the training program [70,71,72]. Further research is needed on the place of other interventions (such as occupational therapy) in this context [73].
Comparison to existing literature
Previous reviews on rehabilitation interventions for patients with persisting symptoms after COVID-19 focussed on pulmonary rehabilitation [65] or only included RCTs [66]. Other reviews covered all or other (i.e., earlier) stages of rehabilitation of COVID-19 patients [67,68,69]. Their evidence suggests that, in line with our results, physical training and breathing exercises can be useful in patients with persisting symptoms after COVID-19. A review by Décary et al. summarized the current literature on care models and pathways for post COVID-19 condition and advises that rehabilitation care for these patients should include multiple professionals at different levels [74], which is in accordance with our results on the effects of multidisciplinary treatment. Since the closing of this review at least two RCTs were published. One RCT reported significant improvements in physical fitness, fatigue, quality of life, and symptoms of depression for using an 8-week supervised exercise training program compared to the WHO self-management brochure [75] in patients with mild initial COVID-19 [76]. The other RCT investigated the effect of respiratory muscle training and confirmed its effects on improvements of dyspnoea [77]. These newer studies provide further support to our findings.
Implications for clinical practice and further research
These findings can guide ambulatory care practitioners for treating patients with persisting symptoms after COVID-19. This evidence should therefore be incorporated in clinical practice guidelines for the care of these patients, as already partially implemented in England [2], Germany [78], the Netherlands [79] and Belgium [80].
Given the paucity of evidence, high-quality and rigorous studies are needed to confirm our hypotheses. Future studies preferably have a controlled design and should include a sufficient number of participants. Also, adverse events of rehabilitation programs in ambulatory care must be studied more. Moreover, evidence is particularly scarce for the following interventions: advice on how to self-manage symptoms and on return to activities, occupational therapy, rehabilitation for communication and swallowing difficulties, cognitive training, and psychological interventions including coping and post-traumatic stress management strategies. Besides, standardized structured questionnaires specific to qualitative olfactory dysfunction should be routinely used, since the Sniffin’ Sticks test, which was mainly used in the olfactory training studies included in this review [44, 45, 47,48,49,50,51], focuses on quantitative loss of olfactory function and therefore can underestimate the prevalence of persistent COVID-related parosmia [81]. Finally, research into the prevalence, risk factors, and pathophysiology of persisting symptoms after COVID-19 will give more insight into which rehabilitation interventions may be most beneficial in this population, along with a tailored treatment according to the subtype of the condition.
Conclusions
Physical training programs, breathing exercises, olfactory training, and multidisciplinary treatment can be effective rehabilitation therapies for patients with persisting symptoms after COVID-19. They have already shown marked effects on fatigue, dyspnoea, physical capacity, and quality of life. However, the certainty of evidence is very low, which means there is still a lot of uncertainty about these effects.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- GRADE:
-
Grading of Recommendation Assessment, Development, and Evaluation
- RCT:
-
Randomized controlled trial
- SoF:
-
Summary of Findings
- WHO:
-
World Health Organisation
References
Nalbandian A, Sehgal K, Gupta A, Madhavan MVM, McGroder C, Stevens JS, Cook JR, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15.
NICE. COVID-19 rapid guideline: Managing the long-term effects of COVID-19. 2020.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021;22(4):102–7.
Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 27 September 2022.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55:101762.
Maltezou HC, Pavli A, Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines (Basel). 2021;9(5):497.
International Primary Care Respiratory Group. What are the risk factors for long-COVID-19 disease/post-COVID syndrome (PCS)? 2021. Available from: https://www.ipcrg.org/resources/search-resources/what-are-the-risk-factors-for-long-covid-19-diseasepost-covid-syndrome. Accessed 10 February 2022.
Menges D, Ballouz TA, Anagnostopoulos A, Aschmann HED, Domenghino A, Fehr JS, Puhan M. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One. 2021;16(7):e0254523.
Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):13.
Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–42.
World Health Organisation. Clinical management of COVID-19: Living guideline, 15 September 2022 2022. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-2022.2. Accessed 21 December 2022.
The Cochrane Collaboration. Cochrane Training 2022. Available from: https://training.cochrane.org/. Accessed 2 May 2022.
PRISMA. PRISMA - transparent reporting of systematic reviews and meta-analyses 2021. Available from: http://www.prisma-statement.org/. Accessed 2 May 2022.
Research Aid Networks Long Covid Library. Available from: https://www.zotero.org/groups/4411227/research_aid_networks_long_covid_library/items/9ITDFM6W. Accessed 9 May 2022.
Resources LongCovid. Available from: https://docs.google.com/spreadsheets/d/1jy354stmCE30zYoE5Ou3lz0O1hZSbvuLfvxcUGoBroQ/htmlview?fbclid=IwAR1RFFF7YnmeNzGQIH9uf3RcgWzmHkRARzj_oIfu4F00qGiHLe4yH0bP3L4. Accessed 9 May 2022.
Cochrane. Available from: https://covid-19.cochrane.org/. Accessed 9 May 2022.
Epistemonikos. Available from: https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d/advanced-search. Accessed 9 May 2022.
World Health Organisation. Available from: https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/. Accessed 9 May 2022.
Falconer J. Removing duplicates from an EndNote library 2018. Available from: https://blogs.lshtm.ac.uk/library/2018/12/07/removing-duplicates-from-an-endnote-library/. Accessed 9 May 2022.
Rayyan. Available from: https://www.rayyan.ai/. Accessed 9 May 2022.
Covidence. Available from: https://www.covidence.org/. Accessed 9 May 2022.
CASP UK. CASP CHECKLISTS 2021. Available from: https://casp-uk.net/casp-tools-checklists/. Accessed 11 January 2022.
Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4898.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
GRADE working group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 12 October 2021.
Barbara C, Clavario P, De Marzo V, Lotti R, Guglielmi G, Porcile A, et al. Effects of exercise rehabilitation in patients with long COVID-19. Eur J Prev Cardiol. 2022;29(7):258–60.
Betschart M, Rezek S, Unger I, Beyer S, Gisi D, Shannon H, et al. Feasibility of an outpatient training program after COVID-19. Int J Environ Res Public Health. 2021;18(8):3978.
Bouteleux B, Henrot P, Ernst R, Grassion L, Raherison-Semjen C, Beaufils F, et al. Respiratory rehabilitation for Covid-19 related persistent dyspnoea: a one-year experience. Respir Med. 2021;189: 106648.
Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition – a cohort study. Chron Respir Dis. 2021;18:14799731211015692.
Hameed F, Palatulan E, Jaywant A, Said R, Lau C, Sood V, et al. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: a prospective cohort study. PM&R. 2021;13(6):609–17.
Kireyev IV, Zhabotynska NV, Bakumenko MG, Khyzhnyak VM, Knizhenko IB. Rehabilitation in Post COVID-19 neurological syndrome. Acta Balneologica. 2022;64(1):11–5.
Martin I, Braem F, Baudet L, Poncin W, Fizaine S, Aboubakar F, et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir Med. 2021;183: 106438.
Nambi G, Abdelbasset WK, Alrawaili SM, Elsayed SH, Verma A, Vellaiyan A, et al. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID 19 sarcopenia: a randomized controlled trial. Clin Rehabil. 2022;36(1):59–68.
Cahalan RM, Meade C, Mockler S. SingStrong-A singing and breathing retraining intervention for respiratory and other common symptoms of long COVID: a pilot study. Canadian Journal of Respiratory Therapy. 2022;58:20–7.
Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39: 101166.
Philip KEJ, Owles H, McVey S, Pagnuco T, Bruce K, Brunjes H, et al. An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial. Lancet Respiratory Medicine. 2022;10(9):851–62.
Srinivasan V, Kandakurti PK, Alagesan J, Suganthirababu P, Jenifer Augustina S, Anitha A, et al. Efficacy of pursed lip breathing with bhastrika pranayama vs incentive spirometry in rehabilitating post Covid 19 follow up-a randomized control study. Turkish Journal of Physiotherapy and Rehabilitation. 2021;32(3):402–7.
Ahmed I, Inam AB, Belli S, Ahmad J, Khalil W, Jafar MM. Effectiveness of aerobic exercise training program on cardio-respiratory fitness and quality of life in patients recovered from COVID-19. European Journal of Physiotherapy. 2021;24(6):358–63.
Dalbosco-Salas M, Torres-Castro R, Leyton AR, Zapata FM, Salazar EH, Bastías GE, et al. Effectiveness of a primary care telerehabilitation program for post-covid-19 patients: a feasibility study. J Clin Med. 2021;10(19):4428.
Kokhan S, Romanova E, Nadeina L, Baatar B, Shagdarsuren O, Purevdorj D. Effect of physical rehabilitation on the functional state of post COVID-19 patients. Laplage em Revista. 2021;7(3A):675–81.
Li J, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. 2021;77(7):697–706.
Scaturro D, Vitagliani F, Di Bella VE, Falco V, Tomasello S, Lauricella L, et al. The role of acetyl-carnitine and rehabilitation in the management of patients with post-COVID syndrome: case-control study. Appl Sci. 2022;12(8):4084.
Stavrou VT, Tourlakopoulos KN, Vavougios GD, Papayianni E, Kiribesi K, Maggoutas S, et al. Eight weeks unsupervised pulmonary rehabilitation in previously hospitalized of SARS-CoV-2 infection. Journal of Personalized Medicine. 2021;11(8):806.
D’Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A. Randomized clinical trial “olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: preliminary results. Eur Rev Med Pharmacol Sci. 2021;25(11):4156–62.
Di Stadio A, D’Ascanio L, Vaira LA, Cantone E, De Luca P, Cingolani C, et al. Ultramicronized palmitoylethanolamide and luteolin supplement combined with olfactory training to treat post-COVID-19 olfactory impairment: a multi-center double-blinded randomized placebo-controlled clinical trial. Curr Neuropharmacol. 2022;20(10):2001–12.
Rathi A, Jadhav SB, Shah N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines. 2021;8(9):47.
Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021;42(2): 102884.
Denis F, Septans A-L, Periers L, Maillard J-M, Legoff F, Gurden H, et al. Olfactory training and visual stimulation assisted by a web application for patients with persistent olfactory dysfunction after SARS-CoV-2 infection: observational study. J Med Internet Res. 2021;23(5): e29583.
Le Bon SD, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol. 2021;278(8):3113–7.
Pires ÍAT, Steffens ST, Mocelin AG, Shibukawa DE, Leahy L, Saito FL, et al. Intensive olfactory training in post-COVID-19 patients: a multicenter randomized clinical trial. Am J Rhinol Allergy. 2022;36(6):780–7.
Vandersteen C, Payne M, Dumas LÉ, Cancian É, Plonka A, D’Andrea G, et al. Olfactory training efficiency in Post-COVID-19 persistent olfactory disorders: value normalization for threshold but not identification. J Clin Med. 2022;11(12):3275.
Albu S, Rivas Zozaya N, Murillo N, García-Molina A, Figueroa Chacón CA, Kumru H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: a prospective, observational cohort study. Disabil Rehabil. 2021;44(22):6833–40.
García-Molina A, Espiña-Bou M, Rodríguez-Rajo P, Sánchez-Carrión R, Enseñat-Cantallops A. Programa de rehabilitación neuropsicológica en pacientes con síndrome post-COVID-19: una experiencia clínica. Neurología. 2021;36(7):565–6.
Everaerts S, Heyns A, Langer D, Beyens H, Hermans G, Troosters T, et al. COVID-19 recovery: benefits of multidisciplinary respiratory rehabilitation. BMJ Open Respir Res. 2021;8(1): e000837.
Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Nell C, Stenzel N, et al. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Research. 2021;7(2):00108–2021.
Hayden MC, Limbach M, Schuler M, Merkl S, Schwarzl G, Jakab K, et al. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: a prospective observational study. Int J Environ Res Public Health. 2021;18(17):9001.
Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101(6):593–601.
Babliuk L, Fediaeva S, Babova I, Mesoedova V, Tamazlykar S. Rehabilitation of post-COVID patients with chronic fatigue and cognitive disorders syndromes. Balneo and PRM Research Journal. 2022;13(1):497.
Fan Y, Shi Y, Zhang J, Sun D, Wang X, Fu G, et al. The effects of narrative exposure therapy on COVID-19 patients with post-traumatic stress symptoms: a randomized controlled trial. J Affect Disord. 2021;293:141–7.
Hawkins J, Hires C, Keenan L, Dunne E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: a randomized, double-blinded, placebo controlled clinical trial. Complement Ther Med. 2022;67: 102823.
Heald AH, Perrin R, Walther A, Stedman M, Hann M, Mukherjee A, et al. Reducing fatigue-related symptoms in Long COVID-19: a preliminary report of a lymphatic drainage intervention. Cardiovascular Endocrinology & Metabolism. 2022;11(2): e0261.
Robbins T, Gonevski M, Clark C, Baitule S, Sharma K, Magar A, et al. Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin Med. 2021;21(6):629–32.
Sathyamoorthy M, Verduzco-Gutierrez M, Varanasi S, Ward R, Spertus J, Shah S. Enhanced external counterpulsation for management of symptoms associated with long COVID. American Heart Journal Plus: Cardiology Research and Practice. 2022;13(9): e18398.
Shogenova LV, Tuet TT, Kryukova NO, Yusupkhodzhaeva KA, Pozdnyakova DD, Kim TG, et al. KalHydrogen inhalation in rehabilitation program of the medical staff recovered from COVID-19. Cardiovascular Therapy and Prevention. 2021;20(6):24–32.
Chen H, Shi H, Liu X, Sun T, Wu J, Liu Z. Effect of pulmonary rehabilitation for patients with post-COVID-19: a systematic review and meta-analysis. Front Med. 2022;9: 837420.
Fugazzaro S, Contri A, Esseroukh O, Kaleci S, Croci S, Massari M, et al. Rehabilitation Interventions for post-acute COVID-19 syndrome: a systematic review. Int J Environ Res Public Health. 2022;19(9):5185.
Debeuf R, Swinnen E, Plattiau T, De Smedt A, De Waele E, Roggeman S, et al. The effect of physical therapy on impairments in COVID-19 patients from intensive care to home rehabilitation: a rapid review. J Rehabil Med. 2022;54:jrm00242.
Goodwin VA, Allan L, Bethel A, Cowley A, Cross JL, Day J, et al. Rehabilitation to enable recovery from COVID-19: a rapid systematic review. Physiotherapy. 2021;111:4–22.
Negrini F, de Sire A, Andrenelli E, Lazzarini SG, Patrini M, Ceravolo MG. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane Rehabilitation Field as of December 31st, 2021. Eur J Phys Rehabil Med. 2022;58(2):328–31.
Twomey R, DeMars J, Franklin K, Culos-Reed SN, Weatherald J, Wrightson JG. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. 2022;102(4):pzac005.
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38: 101019.
Parker M, Sawant HB, Flannery T, Tarrant R, Shardha J, Bannister R, et al. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J Med Virol. 2022;95(1): e28373.
Hersche R, Weise A. Occupational therapy-based energy management education in people with post-COVID-19 condition-related fatigue: results from a focus group discussion. Occup Ther Int. 2022;2022:4590154.
Décary S, De Groote W, Arienti C, Kiekens C, Boldrini P, Lazzarini SG, et al. Scoping review of rehabilitation care models for post COVID-19 condition. Bull World Health Organ. 2022;100(11):13.
World Health Organisation. Support for rehabilitation: self-management after COVID-19-related illness, second edition 2021. Available from: https://www.who.int/europe/publications/i/item/WHO-EURO-2021-855-40590-59892. Accessed 21 December 2022.
Jimeno-Almazán A, Franco-López F, Buendía-Romero Á, Martínez-Cava A, Sánchez-Agar JA, Sánchez-Alcaraz Martínez BJ, Courel-Ibáñez JP, Pallarés JG. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. 2022;32(12):1791–801.
Del Corral T, Fabero-Garrido R, Plaza-Manzano G, Fernández-de-Las-Peñas C, Navarro-Santana M, López-de-Uralde-Villanueva I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: Randomized controlled trial. Ann Phys Rehabil Med. 2022;66(1): 101709.
Deutsche Gesellschaft für Neurorehabilitation e.V. (DGNR). SARS-CoV-2, COVID-19 und (Früh-) Rehabilitation. 2021.
Federatie Medisch Specialisten. Langdurige klachten en revalidatie na COVID-19. 2022.
Dillen H, Bekkering G, Bastiaens A, Li A, Van den Broeck A, Spiette A, et al. Richtlijn ‘Opvolging en revalidatie van patiënten met aanhoudende klachten na COVID-19 in de eerste lijn’. 2022.
Boscolo-Rizzo PH, Hopkins C, Menini A, Dibattista M, Cancellieri E, Gardenal N, Tofanelli M, et al. Parosmia assessment with structured questions and its functional impact in patients with long-term COVID-19-related olfactory dysfunction. Int Forum Allergy Rhinol. 2022;12(12):1570–4.
Acknowledgements
The authors wish to thank all the members of the Belgian guideline working group for reviewing the publication list: Ann Bastiaens, Anne-lies Van den Broeck, Anne-Sophie Spiette, Catharine Vander Linden, Chris Burtin, Daniel Langer, Dirk Bellemans, Dominique Van de Velde, Ellen Excelmans, Erika Vanhauwaert, Hadi Waelkens, Johan Wens, Joke Platteeuw, Paul Boon, Pierre Garin, Roy Remmen, Séverine Tibor, Stefan Teughels, Stijn De Baets, Thibault Coppens.
The authors also wish to thank Daan Pauwels (Master’s student biomedical sciences, KU Leuven) for his help with the data extraction.
GRADEpro was used to create the summary of findings tables: GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org.
Funding
This review is part of the project “Development of a guideline for the follow-up and rehabilitation of COVID-19 patients in primary care”, which is funded by the Belgian Federal Public Health Service (QPG-3D1907-VERBAKEL/TROOSTERS/GOSSELINK-FOD-COVID, 2021–2022). The design of the study and the collection, analysis, and interpretation of data are not influenced by the views or interests of the funding body.
Author information
Authors and Affiliations
Contributions
HD, GB, WJ, RG, TT and JV set up the protocol for this review. HD, GB, SG, YVW, MVH, SH, DB, AL, WJ, RG, TT and JV screened the records for their relevance. Data was extracted by HD and checked by GB. HD, GB, and SG performed the risk of bias assessment. HD constructed the summary of findings tables and interpreted the results. All authors contributed to writing and reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dillen, H., Bekkering, G., Gijsbers, S. et al. Clinical effectiveness of rehabilitation in ambulatory care for patients with persisting symptoms after COVID-19: a systematic review. BMC Infect Dis 23, 419 (2023). https://doi.org/10.1186/s12879-023-08374-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08374-x