Abstract
Background
Compared to the abundance of clinical and genomic information available on patients hospitalised with COVID-19 disease from high-income countries, there is a paucity of data from low-income countries. Our aim was to explore the relationship between viral lineage and patient outcome.
Methods
We enrolled a prospective observational cohort of adult patients hospitalised with PCR-confirmed COVID-19 disease between July 2020 and March 2022 from Blantyre, Malawi, covering four waves of SARS-CoV-2 infections. Clinical and diagnostic data were collected using an adapted ISARIC clinical characterization protocol for COVID-19. SARS-CoV-2 isolates were sequenced using the MinION™ in Blantyre.
Results
We enrolled 314 patients, good quality sequencing data was available for 55 patients. The sequencing data showed that 8 of 11 participants recruited in wave one had B.1 infections, 6/6 in wave two had Beta, 25/26 in wave three had Delta and 11/12 in wave four had Omicron. Patients infected during the Delta and Omicron waves reported fewer underlying chronic conditions and a shorter time to presentation. Significantly fewer patients required oxygen (22.7% [17/75] vs. 58.6% [140/239], p < 0.001) and steroids (38.7% [29/75] vs. 70.3% [167/239], p < 0.001) in the Omicron wave compared with the other waves. Multivariable logistic-regression demonstrated a trend toward increased mortality in the Delta wave (OR 4.99 [95% CI 1.0–25.0 p = 0.05) compared to the first wave of infection.
Conclusions
Our data show that each wave of patients hospitalised with SARS-CoV-2 was infected with a distinct viral variant. The clinical data suggests that patients with severe COVID-19 disease were more likely to die during the Delta wave.
Similar content being viewed by others
Introduction
There is limited COVID-19 genomic surveillance data from low income countries such as Malawi [1]. Genomic surveillance data supports the development of contextually relevant and effective national, regional and international public health interventions [2]. For patients with severe disease, little is known about the impact of viral variants on disease severity in these resource constrained settings where there is frequently a high prevalence of concomitant HIV-infection. Early data from South Africa suggested that the emergence of the SARS-CoV-2 omicron variant of concern (VOC) was associated with reduced disease severity [3], but there is a paucity of data from neighbouring countries in the region.
Genomic sequencing is a vital tool to inform strategies for an effective COVID-19 care and treatment response. The early release of the Wuhan-1 genome sequence [4] enabled the development of specific diagnostic tests [5] and the design of mRNA vaccines, used to great success in high-income countries [6, 7]. The evolution of the virus has led to the emergence of lineages designated as VOCs, which are defined using genome sequencing and the widespread use of genomic surveillance to inform public health strategy has been a defining feature of the pandemic [8, 9]. Early data on the emergence of VOCs has enabled policy makers to rapidly implement public health responses to constrain disease spread; prepare health systems (e.g. increased oxygen provision; opening more hospital beds; and increasing testing); and to select optimal vaccines and therapies [10]. In Malawi, Blantyre is the commercial hub with high detected rates of COVID-19 disease [11]. We previously deployed the WHO-accredited International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) clinical characteristation protocol at Queen Elizabeth Central Hospital (QECH) to patients admitted with suspected COVID-19 disease [12]. However, this cohort completed in September 2020; and did not include pathogen genome sequencing.
In this study we determined SARS-CoV-2 genome sequences from swabs collected from adult patients admitted to Queen Elizabeth Central Hospital (QECH) with PCR-confirmed and symptomatic COVID-19 during four sequential waves of the pandemic. Our aim was to explore the relationship between viral lineage and patient outcome in southern Malawi using an international clinical characterisation protocol. Based on emerging data from other settings [13,14,15,16], we hypothesised that there would be increased disease severity for patients with confirmed Delta disease.
Methods
Study design and recruitment
We prospectively recruited adult patients (> 18 years old) using the tier one sampling strategy from the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Clinical Characterisation Protocol (CCP) [17], as previously described [12]. Patients were recruited at Queen Elizabeth Central Hospital (QECH), Blantyre, Malawi, the largest referral hospital in southern Malawi (Additional file 2: Fig. S6). For this study, only patients admitted to hospital with severe acute respiratory infection and a positive SARS-CoV-2 PCR test (defined as a Ct < 40) were included. Patients (or personal consultee if the patient lacked capacity) with a severe acute respiratory infection (SARI) were consecutively approached for informed consent with an aim to recruit within 72 h of hospital admission. Respiratory samples (combined nasopharyngeal and oropharyngeal swab) and peripheral venous blood samples were collected at recruitment. SARS-CoV-2 PCR diagnostic testing was carried out on the swab samples, as previously described [12]. Waves (W) of SARS-CoV-2 were defined with reference to nationally reported COVID-19 figures (W1: 04/2020-10/2020, W2: 11/2020-03/2021; W3: 04/2021-08/2021; and W4: 12/2021-03/2022). COVID-19 vaccine became available in Malawi from 10th March 2021 [18].
During the recruitment period, patients with COVID-19 were treated on wards capable of providing continuous oxygen therapy, but without capacity for invasive mechanical ventilation, intensive care facilities, continuous positive airways pressure (CPAP) or high flow oxygen. All patients received protocolised standard care depending on the severity, including oxygen, steroids and antibiotics as previously described [19]. Clinical and treatment parameters were recorded using the ISARIC standardised case report form. Participants were followed up until death, discharge or transfer to another facility.
Study protocols were approved by the Malawi National Health Science Research Committee (NHSRC, 20/02/2518 and 19/08/2246) and Liverpool School of Tropical Medicine Research Ethics Committee (LSTM REC, 20/026 and 19/017). We have included a reflexivity statement detailing how equitable partnership was promoted within our collaboration in the Additional Material.
SARS-CoV-2 molecular biology and genome sequencing
Samples were extracted using the Qiasymphony-DSP mini kit 200 (Qiagen, UK) with offboard lysis or manually using the Qiagen mini viral extraction kit. Samples were then tested using the CDC N1 assay to confirm the Ct values before sequencing. ARTIC protocol V2 sequencing protocol was used until June 2021, after which we switched to the V3 protocol. ARTIC version 3 primers were used for the tiling PCR until we switched to the University of Zambia (UNZA) primer set that provided better results for Delta VOC in August 2021 (data not shown) [20]. Initially two primer pools were used, however a third pool was made for primer pairs that commonly had lower depth compared to the average (details Additional file 1: Table S1). PCR cycling conditions were adapted to the new sequencing primers, with annealing temperature changed to 60 °C. Sequencing was carried out with the Oxford Nanopore Technologies MinION sequencer. Samples that had poor coverage (< 70%) with the ARTIC primer set were repeated with the UNZA primer set.
Analysis of SARS-CoV-2 sequencing data
Raw FAST5 data produced by the MinION were processed with Guppy v5.0.7. FAST5s were basecalled with guppy_basecaller, basecalled FASTQs were assigned to barcodes using guppy_barcoder, including the ‘_require_barcodes_both_ends’ flag. The per-sample FASTQ files were processed with the artic pipeline using the ‘medaka’ option [21]. The lineage of each consensus genome was identified using pangolin with the following versions; pangolin v3.1.17, pangolearn 2021-12-06, constellations v0.1.1, scorpio v0.3.16, pango-designation used by pangoLEARN/Usher v1.2.105, pango-designation aliases v1.2.122 [22]. Samples were re-analysed when the Pangolin database was updated. The run was repeated if there was contamination in the negative control.
To set reasonable Ct thresholds for selecting samples to sequence in future work, we plotted the true positive rate versus the false positive rate (i.e. ROC curves) for a range of Ct thresholds from 15 to 40, where the true positive rate was defined as the proportion of samples with a genome coverage ≥ 70% that had a Ct below the threshold. The false-positive rate was defined as the proportion of samples with a genome coverage < 70% that had a Ct below the threshold. Code to calculate the values for the ROC curves is available here—https://gist.github.com/flashton2003/bb690261106dc98bb1ae5de8a0e61199. The lineage/VOC of samples in GISAID was obtained via the GISAID website (https://www.epicov.org/epi3/start).
Statistical analysis
Clinical data were analysed using Stata V15.1 (StataCorp, Stata Statistical Software: Release 15, College Station, Texas, USA). Categorical variables were compared using Fisher’s exact test. Continuous variables were tested for normality and appropriate statistical tests were applied; non-normally distributed measurements are expressed as the median [IQR] and were analysed by the Kruskal–Wallis test to compare clinical parameters across the four waves. The primary outcome variable was survival to hospital discharge. We selected the following covariates a priori to determine potential predictors of mortality: pandemic infection wave; vaccine status; age; sex; HIV infection status; prior diagnosis of cardiac disease; prior diagnosis of diabetes mellitus; time from symptoms to hospital admission; respiratory rate; and oxygen saturation (SpO2). This information was obtained from the patients admission files, health passport, medical chart or other documents. HIV was not independently confirmed, but was determined from patient medical records. All the above variables were included within the multivariable model and were collected at, or shortly after, hospital admission (selected as clinically relevant parameters that could reasonably be used by clinicians to influence treatment decisions). Univariable and multivariable logistic regression analyses were fitted using the STATA “logistic” command to generate odds ratios and confidence intervals (see supplementary materials). In addition, we conducted an exploratory sensitivity analysis, excluding patients who did require supplemental oxygen (indicative of less severe disease) at the time of enrolment. The overall statistical significance of the difference in mortality between waves was assessed using a likelihood ratio test, comparing the univariable model against a null, intercept-only model and the full multivariable model against a null model with all covariates except for the categorical variable encoding the epidemic wave. Statistical analysis and plotting of genomic results was done using R v4.1.0 [23]. Exact binomial confidence intervals for the proportion of each genotype during each wave were calculated using the binom.test function. Statistical analysis STATA code is available here https://gist.github.com/flashton2003/c241f1153a6a9cb76a26f5857fe53976).
Results
Patient recruitment and SARS-CoV-2 genomic analysis
Between July 2020 and March 2022, we recruited 314 adults with PCR confirmed COVID-19 disease, using the ISARIC Clinical Characterisation Protocol (Table 1). Recruitment spanned four distinct waves of COVID-19 in Malawi; 1st wave n = 48 (July–November 2020), 2nd wave n = 94 (December 2020–March 2021), 3rd wave n = 97 (June 2021–October 2021) and 4th wave n = 75 (December 2021–March 2022). The higher number of participants recruited in waves 2 and 3 reflected the epidemiology of COVID-19 in Malawi (Additional file 2: Fig. S1). Overall, 89.5% of patients survived to hospital discharge (per wave numbers can be seen in Table 1).
The sequencing laboratory received viral material from 161 of 314 participants. RT-PCR Ct values were available for 156 cases. There was no difference between Ct values from the different waves (Additional file 2: Fig. S2, Kruskal–Wallis test p-value 0.24). There was no significant difference between Ct values from patients who were HIV positive, HIV negative, or whose HIV status was unknown (Additional file 2: Fig. S3, Kruskal–Wallis test p-value = 0.22), although measures of the degree of immunosuppression were unavailable.
We sequenced all samples with a Ct below 27 (this cut-off was selected based on Additional file 2: Fig. S4), and as many samples with a Ct above 27 as sequencing capacity allowed. Of the 161 cases for which we received viral material, we sequenced 126 samples from 126 patients and obtained 55 genomes with greater than 70% coverage at 20 × depth (Additional file 1: Table S2). Low coverage of the genome (< 70%) was associated with low viral load (i.e., high Ct). This was true for both ARTIC v3 and UNZA tiling PCR primer sets (Fig. 1). Overall, the median Ct value of samples with < 70% coverage at 20 × depth was 32.0, compared with a Ct 25.9 for samples with ≥ 70% coverage (Additional file 1: Tables 2 and 3).
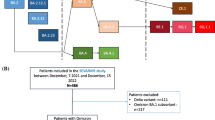
We observed three lineages among the 11 SARS-CoV-2 samples from wave 1 (Fig. 2, Additional file 1: Table S2), with the most frequently identified pangolin lineage being B.1 (n = 8), followed by B.1.1 (n = 2) and B.1.1.448 (n = 1). All 6 samples from wave 2 were VOC Beta (exact binomial 95% CI of the estimate in the untested population = 54–100%) and 96% (25/26) of samples from wave 3 were VOC Delta (95% CI 80–100%) (Fig. 2). One sample received at the beginning of June 2021 was VOC Beta. We observed seven pangolin lineages among the 25 VOC Delta samples sequenced during wave 3; AY.75.1 (n = 11), B.1.617.2 (n = 8), AY.75 (n = 2) and 1 each of AY.50, AY.59, AY.122 and AY.72 (Additional file 2: Fig. S5). Of the 12 successfully sequenced samples from wave 4, 100% (95% CI 73.5–100%) were Omicron VOC. Eleven of twelve were BA.1 with the remaining sample belonging to BA.2. The BA.2 sample came from a patient enrolled in February 2022. Due to low numbers of successfully sequenced isolates during the second wave, we also obtained the genotype of samples from Malawi submitted to GISAID during this time, for which explicit permission could be obtained for re-use from the data depositor; Beta VOC accounted for 100 of the 104 (96%, 95% CI: 90–98%) SARS-CoV-2 genomes from Malawi in GISAID which were sampled.
Clinical characteristics
There were no significant differences in sex or median age between participants between waves (Table 1), however, there was a significant reduction (p = < 0.001) in time from symptom onset to presentation in Delta (median two days [IQR 1–5]) and Omicron waves (median two days [IQR: 0–4]) compared to the B.1 (median five days [IQR: 2–8]) or Beta waves (median four days [IQR: 2–9]). There was a lower percentage of patients with cardiac disease (30.0% and 23.4% vs 4.1% vs 5.3%, p < 0.001) and diabetes (40% vs 19.2% vs 17.5% vs 6.7% p ≤ 0.001) in later waves. There was a significant reduction in the numbers of patients requiring oxygen at enrolment during the Omicron wave, with the highest proportion during Delta wave (50% vs 58.5% vs 63.9% vs 22.7% p ≤ 0.001). Similarly, fewer patients were given steroids during Omicron wave, with the highest numbers receiving steroids in Delta wave (60.4% vs 59.6% vs 84.5% vs 38.7% p ≤ 0.001). Overall, few patients were vaccinated; in this cohort 21/97 (21.7%) Delta wave participants and 15/75 (20%) Omicron wave participants had received at least one dose of any vaccine. For both unvaccinated and vaccinated groups survival was just under 90% (p = 0.9).
Univariable logistic regression analysis demonstrated that age ≥ 70 (OR7.21 CI:1.48–35.07), respiratory rate ≥ 30 (OR 14.87 CI: 3.09–71.71) and SpO2 ≤ 87% (OR 15.4 CI: 5.66–41.93) were associated with mortality, although with wide confidence intervals (Table 2). Multivariable analysis showed a statistically significant increase in case fatality rate in the whole cohort during the Delta wave (OR 4.99 CI 1.00–25.02) (Table 2). However, the likelihood ratio test for the presence or absence of wave within the model was not significant (Chi2 = 5.91, p = 0.116). Therefore, these exploratory findings within our limited cohort should not be overinterpreted. HIV infection; presence of co-morbidities; days from symptoms to admission; and respiratory rate were not associated with survival within the multivariable model. We conducted an exploratory sensitivity analysis including only participants who required oxygen at study enrolment as a marker of disease severity (n = 157, of whom 26 [16.6%] died).
This demonstrated that admission during Delta wave was independently associated with mortality within a multivariable analysis (OR 13.91 [CI: 1.56–125.06, p = 0.018) (Additional file 1: Table S4).
Discussion
Using genomic sequencing we were able to define the viral sub-types or VOCs associated with four distinct waves of patients hospitalised with COVID-19. The first wave was predominantly B.1, all sequenced samples from the second wave were Beta VOC, the sequenced samples from the third wave were predominantly Delta, whilst the samples from the fourth wave were largely Omicron BA.1. Infection with Delta variant was associated with a higher risk of mortality, particularly in patients requiring oxygen during admission. This study reports clinical differences in outcome between SARS-CoV-2 variants in a low-income southern African setting in a population with a high burden of infectious disease, including HIV.
The increased risk of mortality in this cohort was associated with increased age (≥ 70 years) and low oxygen at recruitment (SpO2 < 87%), in line with other cohorts (ISARIC, [24]). While our small sample size necessitates caution in interpretation, there was an increased risk of death associated with Delta VOC, particularly in those patients requiring oxygen. Increased mortality with Delta VOC has been reported elsewhere [13,14,15,16], but not consistently in Africa [25], where robust clinical data has not commonly been linked with SARS-CoV-2 sequencing data. Patients with severe disease were managed with oxygen, steroids and beta-lactam antibiotics, consistently applied within the hospital between waves. We did not observe an excess of deaths in people living with HIV, however the sample size was low and we did not assess level of immune-suppression in these patients [26]. Patients admitted during the Omicron wave required less oxygen at enrolment, suggesting they were less unwell at presentation, although overall mortality was not significantly lower. This is consistent with other studies in sub-Saharan Africa where patients admitted with COVID-19 during Omicron waves had comparatively less severe disease [16, 27, 28]. There is a high burden of HIV and a low SARS-CoV-2 vaccine coverage in Malawi [29], this provides a plausible environment for the emergence of novel VOCs [30,31,32,33]. It is crucial to identify potential VOCs rapidly and report these internationally. The continuation of in-country genomic surveillance in Malawi is therefore important locally and globally.
Throughout the study there was no invasive and very limited non-invasive ventilatory support available for COVID-19 patients and no access to newer therapies such as interleukin-6 antagonists. Therapeutic options for COVID-19 in high income settings are developing rapidly, with genomic viral sequencing used to guide treatments (NICE). This study thus highlights significant inequity in availability of globaly recommended therapeutics for COVID-19 despite relatively high rates of in-patient mortality. It is unclear from this study whether the reduction in severity seen in the Omicron wave was affected by immunity—either vaccine derived or naturally acquired. Overall, 20.9% of the recruited patients in waves three and four were vaccinated with at least one dose (predominantly Astra-Zeneca ChAdOx1-S and J&J Ad26.COV2.S), which is higher than the background population overall, but similar to rates seen in urban Blantyre (25% at least one dose by Feb 2022, Personal Communication, Blantyre District Health Office). However there were already high rates of sero-positivity amongst blood donors in Malawi with 70% of adults SARS-CoV-2 sero-positive in July 2021 during the Delta wave [34] suggesting high population exposure with naturally acquired immunity.
A strength of our study is that we carried out sequencing and analysis in Malawi directly linked with robust and systematically collected clinical data. In country analysis allowed us to report our findings to clinical and public health partners rapidly. Vital to our success in establishing sequencing in Malawi was the portability of the MinION sequencer; the public lab protocols (18); bioinformatics software from the scientific community (13); and the infrastructure and funding available to us as an international research institution. The MinION platform has become integral to outbreak response, as demonstrated for SARS-CoV-2 (19, 20), Ebola (21) and Zika (22). However, even with this portable and low-maintenance sequencer (with no service contracts or engineer visits required); experienced molecular biologists and bioinformaticians; and considerable international support, it was still very difficult to establish sequencing capability. In particular, we found it extremely challenging to procure reagents, and this was exacerbated by border closures and travel restrictions. As there is no existing policy framework within Malawi for the integration of sequencing data into public health decision making, the utility of our data to decision makers was limited.
Our study has several limitations. We produced a relatively small number of sequences. This was partly due to the limited number of patients recruited into the study during each wave but also because patients frequently presented with Ct values too high to generate good quality sequence data. Secondly, our observations are limited to a sample of hospitalised patients in a single centre in the southern region of Malawi. Our relatively low sample size impairs our ability to draw firm conclusions on the association between wave and patient outcome. Finally, we recognize that we may not be capturing the full diversity of SARS-CoV-2 circulating in the community, as our sampling of hospitalised patients represents a considerable bias towards people with severe disease, and there is likely to be significant under ascertainment of cases [34].
In conclusion, pragmatic clinical research protocols coupled with portable sequencing capacity enabled us to improve our understanding of the clinical characteristics and impact of the multiple waves of COVID-19 pandemic in Malawi. We recommend that funders support the development of capacity in genomic surveillance of agents of communicable disease, focussing their strategies on endemic diseases, which can pivot to pandemics and outbreak scenarios as the need arises. A key part of this is the development of robust networks for the production and distribution of molecular biology reagents, mirroring what is being developed for vaccines, as this would enable a more rapid and sustained response to future pandemics. Challenges and opportunities arising from this work are detailed in Box 1. Data and sample collection was enabled by collaboration with the ISARIC consortium. This enabled us to enrol patients very quickly using tools already developed for pandemic response. We were also able to contribute valuable clinical data from a low income setting to global analyses.
Availability of data and materials
All genome sequences are available in GISAID (https://gisaid.org/) and INSDC (e.g. https://www.ncbi.nlm.nih.gov/) databases—accessions are available in Additional file 1: Table S2.
All methods were performed in accordance with the relevant guidelines and regulations. The study was reported in line with STROBE guidelines.
References
Brito AF, Semenova E, Dudas G, et al. Global disparities in SARS-CoV-2 genomic surveillance. medRxiv 2021; :2021.08.21.21262393.
Aborode AT, Hasan MM, Jain S, et al. Impact of poor disease surveillance system on COVID-19 response in africa: time to rethink and rebuilt. Clin Epidemiol Global Health. 2021;12: 100841.
Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. The Lancet. 2022;399:437–46.
Novel 2019 coronavirus genome—SARS-CoV-2 coronavirus. 2020. Available at: https://virological.org/t/novel-2019-coronavirus-genome/319. Accessed 28 October 2021.
CDC. CDC’s Diagnostic Test for COVID-19 Only and Supplies. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html. Accessed 28 October 2021.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16.
Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations—SARS-CoV-2 coronavirus / nCoV-2019 Genomic Epidemiology. 2020. Available at: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563. Accessed 28 October 2021.
Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. 2020: 2020.12.21.20248640. https://doi.org/10.1101/2020.12.21.20248640v1.
Why genomic sequencing is crucial in COVID-19 response. Available at: https://www.afro.who.int/news/why-genomic-sequencing-crucial-covid-19-response. Accessed 24 January 2022.
Nyasulu JCY, Munthali RJ, Nyondo-Mipando AL, et al. COVID-19 pandemic in Malawi: did public sociopolitical events gatherings contribute to its first-wave local transmission? Int J Infect Dis. 2021;106:269–75.
Morton B, Barnes KG, Anscombe C, et al. Distinct clinical and immunological profiles of patients with evidence of SARS-CoV-2 infection in sub-Saharan Africa. Nat Commun. 2021;12:3554.
Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42.
Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause TG. Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. Lancet Infect Dis. 2021;21:1351.
Bast E, Tang F, Dahn J, Palacio A. Increased risk of hospitalisation and death with the delta variant in the USA. Lancet Infect Dis. 2021;21:1629–30.
Hussey H, Davies M-A, Heekes A, et al. Assessing the clinical severity of the Omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between Omicron and Delta infections—a survival analysis. Int J Infect Dis. 2022;118:150–4.
Clinical Characterisation Protocol (CCP). Available at: https://isaric.org/research/covid-19-clinical-research-resources/clinical-characterisation-protocol-ccp/. Accessed 28 October 2021.
Malawi vaccine roll out: Chakwera first jab at Zomba Covid-19 field hospital, Chilima in Mzuzu—Malawi Nyasa Times—News from Malawi about Malawi. 2021. Available at: https://www.nyasatimes.com/malawi-vaccine-roll-out-chakwera-first-jab-at-zomba-covid-19-field-hospital-chilima-in-mzuzu/. Accessed 25 May 2022.
Banda NP, Hara W, Cocker D, et al. First case report of a successfully managed severe COVID-19 infection in Malawi. Malawi Med J. 2020;32:226–8.
Simulundu E, Mupeta F, Chanda-Kapata P, et al. First COVID-19 case in Zambia—comparative phylogenomic analyses of SARS-CoV-2 detected in African countries. Int J Infect Dis. 2021;102:455–9.
ARTIC field bioinformatics pipeline. Available at: https://github.com/artic-network/fieldbioinformatics. Accessed 28 October 2021.
O’Toole Á, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021; :veab064.
R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. Available at: https://www.R-project.org/.
ISARIC Clinical Characterisation Group, Baillie JK, Baruch J, et al. ISARIC COVID-19 Clinical Data Report issued: 27 March 2022. Infectious Diseases (except HIV/AIDS), 2020. https://doi.org/10.1101/2020.07.17.20155218.
Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–4.
Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19:3.
Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, South Africa. Int J Infect Dis. 2022;116:38–42.
Jassat W, Karim SSA, Mudara C, et al. Clinical severity of COVID-19 patients admitted to hospitals during the Omicron wave in South Africa. 2022. https://doi.org/10.1101/2022.02.22.21268475v1.
Malawi marks one year of COVID-19 vaccination, 828, 080 people receive full dose. Available at: https://www.afro.who.int/countries/malawi/news/malawi-marks-one-year-covid-19-vaccination-828-080-people-receive-full-dose. Accessed 1 July 2022.
Weigang S, Fuchs J, Zimmer G, et al. Within-host evolution of SARS-CoV-2 in an immunosuppressed COVID-19 patient as a source of immune escape variants. Nat Commun. 2021;12:6405.
Karim F, Moosa MYS, Gosnell BI, et al. Persistent SARS-CoV-2 infection and intra-host evolution in association with advanced HIV infection. 2021. https://doi.org/10.1101/2021.06.03.21258228v1.
Cele S, Karim F, Lustig G, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe. 2022;30:154-162.e5.
Wilkinson SA, Richter A, Casey A, et al. Recurrent SARS-CoV-2 mutations in immunodeficient patients. 2022. https://doi.org/10.1101/2022.03.02.22271697v1.
Mandolo J, Msefula J, Henrion MYR, et al. SARS-CoV-2 exposure in Malawian blood donors: an analysis of seroprevalence and variant dynamics between January 2020 and July 2021. BMC Med. 2021;19:303.
Acknowledgements
The authors thank all study participants and the staff of the Queen Elizabeth Central Hospital (QECH) for their support and co-operation during the study. We would like to acknowledge the contribution of the following members of the Blantyre COVID-19 Consortium
Clinical—Wezzie Kalua9, Peter Mandala9, Barbara Katutula9, Rosaleen Ng'oma9, Steven Lanken9, Jacob Phulusa9, Mercy Mkandawire9, Sylvester Kaimba9, Sharon Nthala9, Edna Nsomba9, Lucy Keyala9, Beatrice Chinoko9, Markus Gmeiner1, Vella Kaudzu9, Bridget Freyne1, Todd D. Swarthout1 and Pui-Ying Iroh Tam1. Laboratory—Simon Sichone1, Ajisa Ahmadu1, Grace Stima1, Mazuba Masina1, Oscar Kanjewa1, Vita Nyasulu1, End Chinyama1, Allan Zuza1, Brigitte Denis1, Evance Storey1, Nedson Bondera1, Danford Matchado1, Adams Chande1, Arthur Chingota1, Chimenya Ntwea1, Langford Mkandawire1, Chimwemwe Mhango1, Agness Lakudzala1, Mphatso Chaponda1, Percy Mwenechanya1, Leonard Mvaya1 and Dumizulu Tembo1. Data and statistics—Marc Y. R. Henrion1, James Chirombo1, Paul Kambiya1, Clemens Masesa1 & Joel Gondwe1.
1Malawi-Liverpool-Wellcome Clinical Research Programme, Kamuzu University of Health Sciences, Blantyre, Malawi
9Department of Medicine, Queen Elizabeth Central Hospital, Blantyre, Malawi
Funding
This work was supported by the UK Foreign, Commonwealth and Development Office and Wellcome grants for SARS-CoV-2 diagnostics [220757/Z/20/Z] and the MLW Core Grant [206545/Z/17/Z]. KGB is supported by an NIH-Fogarty fellowship [TW010853].
Author information
Authors and Affiliations
Consortia
Contributions
CA, SL, HT, KGB, BM, PMA—Wrote the manuscript; JR, DD, MM, BK, CvdV, TP, NPB, KSM, KM, CP, JM, MN, GK, HM, SBG, KCJ, JC, NF—reviewed the manuscript and provided comments; PMA, BM—did analysis & prepared figures; CA, HB, SL, DD, MM, BK, CvdV, TP, NPB, KSM, KM, CP, JM, MN, GK, JC, KGB—generated or collected data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approcal and consent to participate
Written informed consent, was obtained from all subjects or their legal guardians. Study protocols were approved by the Malawi National Health Science Research Committee (NHSRC, 20/02/2518 and 19/08/2246) and Liverpool School of Tropical Medicine Research Ethics Committee (LSTM REC, 20/026 and 19/017).
Consent for publication
Not applicable.
Competing interests
We have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary tables.
Additional file 2.
Supplementary figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anscombe, C., Lissauer, S., Thole, H. et al. A comparison of four epidemic waves of COVID-19 in Malawi; an observational cohort study. BMC Infect Dis 23, 79 (2023). https://doi.org/10.1186/s12879-022-07941-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07941-y