Abstract
Background
Despite current broad natural and vaccine-induced protection, a substantial number of patients infected with emerging SARS-CoV-2 variants (e.g., BF.7 and BQ.1.1) still experience severe COVID-19. Real-life studies investigating the impact of these variants on clinical outcomes of severe cases are currently not available. We performed a prospective multicenter observational cohort study. Adult patients with acute respiratory failure admitted between December 7, 2021 and December 15, 2022, in one of the 20 participating intensive care units (17 from the Greater Paris area and 3 from the North of France) were eligible for inclusion if they had SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR). Full-length SARS-CoV-2 genomes from all included patients were sequenced by means of next-generation sequencing. The primary endpoint of the study was day-28 mortality.
Results
The study included 158 patients infected with three groups of Omicron sublineages, including (i) BA.2 variants and their early sublineages referred as “BA.2” (n = 50), (ii) early BA.4 and BA.5 sublineages (including BA.5.1 and BA.5.2, n = 61) referred as “BA.4/BA.5”, and (iii) recent emerging BA.5 sublineages (including BQ.1, BQ.1.1, BF.7, BE.1 and CE.1, n = 47) referred as “BQ.1.1”. The clinical phenotype of BQ1.1-infected patients compared to earlier BA.2 and BA.4/BA.5 sublineages, showed more frequent obesity and less frequent immunosuppression. There was no significant difference between Omicron sublineage groups regarding the severity of the disease at ICU admission, need for organ failure support during ICU stay, nor day 28 mortality (21.7%, n = 10/47 in BQ.1.1 group vs 26.7%, n = 16/61 in BA.4/BA.5 vs 22.0%, n = 11/50 in BA.2, p = 0.791). No significant relationship was found between any SARS-CoV-2 substitution and/or deletion on the one hand and survival on the other hand over hospital follow-up.
Conclusions
Critically-ill patients with Omicron BQ.1.1 infection showed a different clinical phenotype than other patients infected with earlier Omicron sublineage but no day-28 mortality difference.
Similar content being viewed by others
Background
Since summer 2022, an unprecedented diversification of SARS-CoV-2 Omicron variant sublineages has followed the emergence and global spread of Omicron lineage BA.2 and subsequently BA.5. Currently, BF.7, followed by BQ.1.1 and the XBB.1.5 recombinant appear to be among the fastest growing variants in the world [1]. BF.7 is also one of the dominant circulating variants in China since restrictions policies were lifted in the country at the end of 2022 [2]. These variants differ from the original BA.2 and BA.5 variants by several amino acid substitutions in the receptor-binding domain (RBD) of the Spike protein that induce key antigenic shifts with altered antibody evasion properties [3, 4].
Although infection with the Omicron variant was recently demonstrated to yield less severe disease than other variants in critically ill patients [5], the relationship between Omicron sublineages and the severity of COVID-19 is not well understood. Preliminary animal model studies suggest that some BA.2 descendants, such as BA.5 and BA.2.75, might cause more severe disease than their parental BA.2 [6, 7]. Conversely, the intrinsic pathogenicity of variant BQ.1.1 was reported to be equivalent or reduced, as compared to that of BA.5 in a hamster model [8]. No real-life studies investigating the relationship between the newly emerging SARS-CoV-2 variants, including BF.7 and BQ.1.1, and clinical outcomes have been reported. During the first variant Omicron wave in early 2022, almost 50% of patients admitted to the intensive care unit (ICU) for acute respiratory failure were immunocompromised and they had a poor antibody response to vaccination [9]. The more recent BA.2 and BA.5 Omicron sublineages (e.g., BF.7, BQ.1.1, and XBB) have shown in vitro resistance to monoclonal antibodies [10], a treatment option for immunocompromised patients with severe COVID-19 [11]. Whether emerging substitutions conferring resistance to monoclonal antibodies are associated with different clinical outcomes has not yet been investigated.
We hypothesized that emerging Omicron sublineages could be associated with more severe COVID-19 and different clinical presentation in critically ill patients. In the present study, we thus compared the characteristics of critically ill patients with acute respiratory failure infected with the latest emerging SARS-CoV-2 sublineages circulating in France in autumn and winter 2022, including BF.7 and BQ.1.1 shown to have acquired antibody neutralization escape capacity [10, 12, 13] referred as “BQ.1.1” group, with those of patients infected with earlier BA.2 referred as “BA.2”, and earlier BA.4 and BA.5 variants referred as “BA.4/BA.5”. The main objective of this study was to determine the association between Omicron sublineages, categorized into three groups (i.e., BA.2, BA.4/BA.5, and BQ.1.1 groups), and day-28 mortality. Secondary objectives were to explore the association (1) between Omicron sublineages and clinical features upon ICU admission and outcomes during ICU stay; and (2) between specific viral mutations/mutational patterns and day 28 mortality.
Patients and methods
Study design and patients
The current study is a substudy of the SEVARVIR study. SEVARVIR is a prospective multicenter observational cohort study. Patients admitted between December 7, 2021 and December 15, 2022, in one of the 20 participating ICUs (17 from the Greater Paris area and 3 from the North of France, see Additional file 1: Table S1 for the list of participating centers) were eligible for inclusion in the SEVARVIR cohort study (NCT05162508) if they presented with the following inclusion criteria: age ≥ 18 years, SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) in nasopharyngeal swab samples, admission in the ICU for acute respiratory failure (i.e., peripheral oxygen saturation (SpO2) ≤ 90% and need for supplemental oxygen or any kind of ventilator support, including high flow oxygen therapy, continuous-positive airway pressure, and non-invasive or invasive mechanical ventilation), patient or next of kin informed of study inclusion. Patients with SARS-CoV-2 infection but no acute respiratory failure or with a RT-PCR cycle threshold (Ct) value > 32 in nasopharyngeal swabs were not included. The study was approved by the Comité de Protection des Personnes Sud-Méditerranée I (N° EudraCT/ID-RCB: 2021-A02914-37). Informed consent was obtained from all patients or their relatives.
For this substudy focused on BA.2, BA.4 and BA.5-infected patients (including sublineage BQ.1.1), we decided to stop the inclusion period when the BQ.1.1 sublineage epidemic dynamic started to decrease in France (i.e., week 51, 2022). The inclusion period started when the first patients infected with the BA.2 Omicron sublineage, which we thought was the most relevant control group, were detected (i.e., week 12, 2021). Omicron sublineages were categorized into three groups: “BA.2”, “BA.4/BA.5” and “BQ.1.1 group” (Fig. 1A).
A Diagram representing all SARS-CoV-2 Omicron lineages included in the study (starting from BA.2 and their descendant sublineages), as designated by PANGOLIN (https://cov-lineages.org/). B Study flow chart. A total of 486 patients were included in the SEVARVIR study between December, 7 2021 and December, 15 2022, with 158 patients infected with BA.2, BA.4, and BA.5 Omicron sublineages, who were included in this substudy
Demographics, clinical and laboratory variables were recorded upon ICU admission and during ICU stay. Patients’ frailty was assessed using the Clinical Frailty Scale [14]. The severity of the disease upon ICU admission was assessed using the World Health Organization (WHO) 10-point ordinal scale [15], the sequential organ failure assessment (SOFA [16]) score, and the simplified acute physiology score (SAPS) II score [17]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [18]. Ventilator-acquired pneumonia was defined according to current French guidelines [19]. COVID-19-associated pulmonary aspergillosis (CAPA) was defined according to ECMM/ISHAM consensus criteria [20]. Immunosuppression was defined as solid-organ transplant, active onco-hematological malignancy (within the past three years), HIV infection, long-term corticosteroid treatment (i.e., more than three months of > 0.5 mg/kg/day prednisone equivalent), and exposure to any other immunosuppressive treatment. Obesity was defined as body mass index > 30 kg/m2.
The primary endpoint of the study was day-28 mortality. Secondary endpoints included need for invasive mechanical ventilation during ICU stay, number of live ventilator-free days at day 28, and need for extracorporeal membrane oxygenation support (ECMO) during ICU stay.
SARS-CoV-2 variant determination
The full-length SARS-CoV-2 genomes were sequenced by means of next-generation sequencing. Briefly, viral RNA was extracted from nasopharyngeal swabs in viral transport medium using NucliSENS® easyMAG kit on EMAG device (bioMérieux, Marcy-l’Étoile, France). Sequencing was performed with the Illumina COVIDSeq Test (Illumina, San Diego, California), that uses 98-target multiplex amplifications along the full SARS-CoV-2 genome [21]. The libraries were sequenced with NextSeq 500/550 High Output Kit v2.5 (75 Cycles) on a NextSeq 500 device (Illumina). The sequences were demultiplexed and assembled as full-length genomes by means of the DRAGEN COVIDSeq Test Pipeline on a local DRAGEN server (Illumina). Lineages and clades were interpreted using Pangolin and NextClade [22]. For mutational pattern analysis at the amino acid level, only high-quality sequences, i.e., sequences covering ≥ 90% of nucleotides of the full-length viral genome and 95% of the spike gene, were considered.
Key amino acid substitutions in spike in the BQ.1.1 group spike were defined compared to its direct progenitor BA.5. BQ.1.1 has indeed some additional spike mutations in some key antigenic sites, which confer further immune escape ability over pre-existing lineages (e.g., deletion (Del)69/70, Del140, other N-terminal domain amino acid mutations, R346T/I, K444T/R, L4552R, N460K, A484T/V, F486V, other S1/Receptor Binding Domain substitutions and S2 substitutions). Full-length viral genome sequence analysis yielding high coverage have been deposited in Genbank (GenBank accession numbers OQ423331-OQ423468; https://www.ncbi.nlm.nih.gov/genbank/).
Statistical analysis
Descriptive results are presented as mean (± standard deviation [SD]) or median (1st–3rd quartiles) for continuous variables, and as numbers with percentages for categorical variables. Two-tailed p-values < 0.05 were considered statistically significant. Unadjusted comparisons between patients infected with three groups of Omicron sublineages defined a priori (including parental early BA.2 sublineages, early BA.4 and BA.5 sublineages, and emerging BA.5 sublineages harboring resistance-associated substitutions to available monoclonal antibodies, including BQ.1, BQ.1.1, BF.7, BE.1 and CE.1 assigned as the “BQ.1.1 group”) were performed using Chi square or Fisher’s exact tests for categorical variables, and ANOVA or Kruskal–Wallis tests for continuous variables, as appropriate. We also performed a sensitivity analysis on BQ.1.1 sublineage, excluding BF.7 and BE.1 sublineages and comparing BQ.1.1-infected patients to the two first groups (BA.2- and early BA.4/BA.5-infected patients) because BQ.1.1 was one of the most prevalent sublineage circulating in France and the world during fall 2022 [23, 24]. Missing data were not imputed. The overall sample size of the SEVARVIR study was a priori defined (n = 2000). The sample size of this substudy was not predefined. Indeed, we had anticipated that data could be sequentially extracted from the prospective database based on epidemiological surges. Results have been reported according to the STROBE guidelines for cohort studies.
Adjusted analyses of the association between Omicron sublineages and 28-day mortality relied on multivariable logistic regression models, entering variables previously shown to be important confounding factors, including age, gender, SOFA score and immunosuppression. Adjusted odds ratios (aOR) along with their 95% confidence intervals (CI) were computed. An exploratory evaluation of the associations between hospital mortality and results from mutational pattern analysis at the amino acid level was performed by unadjusted Cox proportional hazard regression modeling.
Analyses were performed using Stata V16.1 statistical software (StataCorp, College Station, TX, USA), and R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
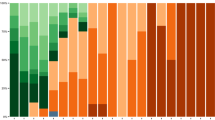
Between December 7, 2021, and December 15, 2022, 486 patients were admitted in one of the 20 participating ICUs and included in the prospective SEVARVIR study. The current analysis comprised 158 patients infected with Omicron sublineages included between February 4, 2022, and December 15, 2022. They split into 3 groups: (i) BA.2 variants and their early sublineages (n = 50); (ii) early BA.4 and BA.5 sublineages (including BA.5.1 and BA.5.2) (n = 61); and (iii) recent emerging BA.5 sublineages (including BQ.1, BQ.1.1, BF.7, BE.1 and CE.1), assigned as the BQ.1.1 group (n = 47) (Figs. 1B and 2).
Dynamics of infecting SARS-CoV-2 variants during the 40-week study period. Patient-related samples (n = 158) corresponding to BA.2, BA.4, BA.5 and emerging sublineages are displayed over time. BA.2 sublineages are in blue, BA.4/BA.5 sublineages are in orange, brown, green and yellow and BQ.1.1 and other sublineages are in red, burgundy and black. The x-axis displays the study time period in weeks (w) from week 12, 2021 to week 51, 2022; The y-axis displays the percentage of each variant/sublineage at each time-point
No statistically significant differences regarding age, gender and frequency of comorbidities were observed between patients infected with parental BA.2, BA.4/BA.5 and those infected with BQ.1.1 group sublineages, except more frequent obesity and less frequent immunosuppression in the latter group (Table 1). The proportion of patients who had received at least one dose of SARS-CoV-2 vaccine, as well as the median number of doses received, did not significantly differ between groups. However, the time elapsed since the third vaccine dose (for those who received it) and ICU admission was significantly longer for BQ.1.1 group-infected patients than for those from the two other groups of variants, including BA.2 and BA.4/BA.5 (307 days [190–508] vs 231 days [200–265] and 153 days [132–181], respectively; p = 0.013). The median time interval between the first symptoms and ICU admission was significantly shorter in the BQ.1.1 group than in early BA.4/BA.5 or BA.2-infected patients (3 [1–7] vs 4 [2–9] and 7 [3–13] days, respectively; p = 0.022). Other variables related to SARS-CoV-2 infection, including the median viral level in the upper respiratory tract, as measured by the cycle threshold in RT-PCR, and the prevalence of positive SARS-CoV-2 anti-S antibodies upon ICU admission, did not significantly differ between groups (Table 1).
There was no significant difference between BA.2, BA.4/BA.5, and BQ.1.1 groups regarding the severity of the disease at ICU admission, as reflected by the SOFA and SAPS II scores and the WHO 10-point ordinal scale (Table 1). Invasive mechanical ventilation support was required in 25.2% (n = 39/158) of patients within 24 h of ICU admission, with no significant difference between groups. Only one BA.2-infected patient required extracorporeal membrane oxygenation (ECMO) support upon ICU admission, while none did in the two other sublineage groups.
During ICU stay, 31.2% (n = 49/158) of patients required invasive mechanical ventilation, with no significant differences between the three subvariant groups. There was also no significant difference between groups regarding the need for other organ supports, including vasopressors, renal replacement therapy or ECMO (Table 2). Only 2 patients (1.3%), not belonging to the BQ1.1 group, required ECMO support for refractory acute respiratory distress syndrome, contrasting with older studies reporting a need for ECMO support in 10 to 20% of patients infected with the ancestral, Alpha or Delta SARS-CoV-2 variants [9, 25, 26]. Patients from the BQ.1.1 group had a significantly shorter duration of ICU stay than those from the two other groups (6 days [4–11] vs 7 days [3–15] and 11 days [5–22] days, respectively; p = 0.038). Day-28 mortality was not significantly different between the three groups. There were no significant between-group differences of COVID-19 management, with 72.0% (n = 113/157) of patients who received dexamethasone and 19.4% (n = 30/155) tocilizumab. Monoclonal antibodies were used in 13% (n = 18/158) of patients and nirmatrelvir-ritonavir in 8.9% (n = 14/157).
A sensitivity analysis comparing patients with BQ.1.1 infection with the two other groups after excluding other emerging sublineages (n = 34) showed similar results (Additional file 1: Tables S2 and S3).
As expected, patients dead at day 28 were older and had more severe disease than survivors. They also more frequently had onco-hematological diseases (Additional file 1: Table S4). We found no significant association between Omicron sublineages and day 28 mortality in uni- and multivariable mortality adjusting on age, SOFA and immunosuppression (Table 3).
High-coverage full-length viral genome sequence analysis (> 90% full-length genome and > 95% full-length spike gene) was obtained in 140 of the 158 patients. A higher number of key amino acid substitutions, which confer further immune escape ability over pre-existing lineages, was found in the RBD of the BQ.1.1 group of patients than in those from the BA.4/BA.5 and BA.2 groups. Additional substitutions were detected in S2 in the three groups (Fig. 3). No significant association was found between any SARS-CoV-2 substitution and/or deletion on the one hand and survival on the other hand over hospital follow-up (Table 4).
Prevalence of amino acid substitutions and deletions in Spike RBD in BA.2, BA.4/BA.5, and BQ.1.1-related group viral sequences; The percentage of detected mutations (amino acid substitutions and deletions) per group is displayed on the y-axis, relative to the original Omicron BA.2 reference sequence (SARS-CoV-2/human/USA/FL-CDC-STM-77CPCCUR3/2022). Other individual NTD (N-terminal domain) mutations include H49Y, W64R, M153I, M177L, I197T, Del211, L212I, A222S, T250I, P251H, V289I and S316F; other S1/RBD mutations include N354K, I358L, T376S, T547K, T572I and N568S; other S2 mutations include T547K, T572I, H625R, A642G, A647V, E654K, N658S, N856S, V963F, A1020S, T1117I, P1143L, E1144Q and S1249P
Discussion
This study is the first describing the clinical phenotype associated with new emerging Omicron sublineages (e.g., BF.7 and BQ.1.1) in patients with severe COVID-19 requiring hospitalization in the ICU. Our data provide reassuring evidence that these emerging sublineages do not cause more severe outcomes than BA.2 and BA.5 variants that emerged and spread earlier in the population. We observed unexpected phenotype differences comparing Omicron sublineages, with more frequent obesity, less frequent immunosuppression and shorter duration of ICU stay in patients infected with BQ.1.1, as compared to those infected with other sublineages.
No significant difference was observed between Omicron sublineage groups regarding the severity of the disease at ICU admission, the need for organ failure support during ICU stay, or day 28 mortality. Notably, the emerging sublineages did not cause severe ARDS requiring ECMO support, in contrast with what has been observed during pre-Omicron COVID-19 waves [9, 25, 26]. In fact, with the emergence of new Omicron sublineages, the clinical phenotype of COVID-19-associated acute respiratory failure seems to be evolving towards less severe disease, as attested by only one third of patients of the current cohort who required invasive mechanical ventilation support, compared to almost 50% of Omicron BA.1-infected patients, 57% of Delta-infected patients [9] and 63% of patients infected with the Wuhan variant [25]. While immunosuppression remained associated with a high mortality, as previously shown with BA.1 [9], the high rate of BQ1.1-infected patients with cardiorespiratory comorbidities, together with a non-significant decrease in CT-scan lung parenchyma involvement, and the shorter ICU stay, as compared with previous series of critically ill COVID-19 patients [9, 26], suggests that a significant subset of patients presented with SARS-CoV-2-associated decompensated heart failure or acute exacerbation of chronic respiratory failure rather than true SARS-CoV-2-associated pneumonia.
Patients infected with sublineage BQ.1.1 had more frequent obesity and were less frequently immunosuppressed than those infected with other Omicron sublineages in our study. Such finding was unexpected since immunosuppression has been the most frequently associated comorbidity in COVID-19 patients infected with the Omicron variant, since the BA.1 “ancestral” Omicron sublineage [9, 27], reported in almost 50% of cases. On the other hand, the higher prevalence of obesity, a previously reported risk factor of severity with previous SARS-CoV-2 variants, including the ancestral variant, is consistent with previous data reporting obesity as a risk factor of severity [28]. The re-emergence of a patient population with no immunosuppression and more classical comorbidities such as obesity, as encountered in pre-Omicron surges, might be consistent with a decrease in post-vaccinal protection, as suggested by a greater delay between the 3rd vaccinal dose and ICU admission in BQ.1.1 patients than in others in our cohort, and data showing a lower neutralization activity in patients infected with BQ.1.1 than with BA.5 or BA.1 subvariants, even after bivalent booster vaccination [29]. The median delay between the first symptoms and ICU admission was significantly shorter in the BQ.1.1 group, possibly accounting for the greater ability of these sublineages to increase cell–cell fusion compared to their parental variants [30].
Overall, the severity of the disease in patients hospitalized in the ICU and their day-28 mortality did not differ across different Omicron sublineage groups. However, the reported increased transmissibility and antibody neutralization escape capacity of sublineage BQ.1.1 [12] make the use of monoclonal antibodies inefficacious in this population [10], including Bebtelovimab, while Sotrovimab might remain weakly active [13]. In contrast, direct-acting antiviral agents, such as paxlovid, are active on new emerging sublineages [31]. The 20% mortality rate observed in our cohort of critically ill patients with COVID-19 corroborates the recent update of the World Health Organization living guideline on therapeutics and COVID-19 (https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4), which recommends the early use of nirmatrelvir-ritonavir (i.e., within the first five days of disease onset) in patients with non-severe COVID-19 at highest risk of hospitalization. This is particularly true in patients with lower exposure to vaccination, in whom the severity of infection is expected to be greater, as suggested by the COVID-19 surge during winter 2022–2023 in China, mainly driven by the BF.7 Omicron sublineage.
Our study has limitations, including a limited sample size in the BQ.1.1 group, limiting our statistical power to perform subgroup analyses and adjust for confounding variables. Indeed, our current multivariable analysis assessing the relationship between Omicron sublineages and day-28 mortality adjusted for key confounders such as age, gender, SOFA score and immunosuppression status, but the relatively low number of events (n = 36 deaths) precluded inflating the number of independent variables. Still, other confounders might have been relevant to include in the model and have been previously shown to be associated with mortality (e.g., diabetes, obesity, chronic respiratory and cardiac diseases [30], vaccination status). In this study, we did not define a priori the sample size of the study, making our study findings exploratory. This is because we aimed at capturing the dynamics of emerging SARS-CoV-2 sublineages and analyzing their phenotype and relationship with mortality in real time. The overall patient recruitment, in spite of a high number of participating centers (n = 20), reflects a lower epidemic activity during the study period than during previous COVID-19 waves. Moreover, we did not explore in depth the physiological mechanisms of acute respiratory failure (i.e., collecting hemodynamic and respiratory physiological data), precluding firm conclusions to be made regarding the contribution of SARS-CoV-2 pneumonia versus that of decompensated underlying cardiopulmonary comorbidities. In-depth mutation analysis could not be performed for all patients, because full-length viral genome sequences were analyzed only when the sequencing coverage was greater than 90% (> 95% in the Spike gene). However, our study also has major strengths, in particular the constitution of a unique national prospective multicenter cohort of well-phenotyped critically ill patients and the availability of full-length SARS-CoV-2 genome sequences for the vast majority of them, allowing for prospective exploration of the clinical consequences of all waves of infection related to emerging and spreading SARS-CoV-2 variants.
In conclusion, critically-ill patients with Omicron BQ.1.1 infection showed a different clinical phenotype than other patients infected with earlier Omicron sublineage. However, there was no significant difference between Omicron sublineage groups regarding the severity of the disease at ICU admission, need for organ failure support during ICU stay, nor day 28 mortality.
Availability of data and materials
All the datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (S.F.).
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- Ct:
-
Cycle threshold
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- RBD:
-
Receptor-binding domain
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SAPS:
-
Simplified acute physiology score
- SD:
-
Standard deviation
- SOFA:
-
Sequential organ failure assessment
References
Varghese R, Kumar D, Sharma R (2023) Global threat from novel SARS-CoV-2 variants, BF.7, XBB.1.5, BQ.1, and BQ.1.1: variants of concern? Hum Cell 36:1218–1221
Novazzi F, Giombini E, Rueca M, Baj A, Fabeni L, Genoni A et al (2023) Genomic surveillance of SARS-CoV-2 positive passengers on flights from China to Italy, December 2022. Euro Surveill 28:2300008
Mykytyn AZ, Rosu ME, Kok A, Rissmann M, Amerongen G van, Geurtsvankessel C, et al. Antigenic mapping of emerging SARS-CoV-2 omicron variants BM.1.1.1, BQ.1.1, and XBB.1. Lancet Microbe [Internet]. 2023;0. Available from: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(22)00384-6/fulltext.
Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y et al (2023) Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186:279-286.e8
DP-EFFECT-BRAZIL investigators. Variants of concern and clinical outcomes in critically ill COVID-19 patients. Intensive Care Med. 2023;1–3.
Saito A, Tamura T, Zahradnik J, Deguchi S, Tabata K, Anraku Y et al (2022) Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant. Cell Host Microbe 30:1540–1555
Kimura I, Yamasoba D, Tamura T, Nao N, Suzuki T, Oda Y et al (2022) Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell 185:3992–4007
Ito J, Suzuki R, Uriu K, Itakura Y, Zahradnik J, Kimura KT et al (2023) Convergent evolution of SARS-CoV-2 Omicron subvariants leading to the emergence of BQ.1.1 variant. Nat Commun 14:2671
de Prost N, Audureau E, Heming N, Gault E, Pham T, Chaghouri A et al (2022) Clinical phenotypes and outcomes associated with SARS-CoV-2 variant Omicron in critically ill French patients with COVID-19. Nat Commun 13:6025
Cox M, Peacock TP, Harvey WT, Hughes J, Wright DW, Willett BJ et al (2023) SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol 21:112–124
RECOVERY Collaborative Group (2022) Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 399:665–676
Miller J, Hachmann NP, Collier AY, Lasrado N, Mazurek CR, Patio RC et al (2023) Substantial neutralization escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB.1. N Engl J Med 387:86
Planas D, Bruel T, Staropoli I, Guivel-Benhassine F, Porrot F, Maes P et al (2023) Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat Commun 14:824
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173:489–495
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection (2020) A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20:e192–e197
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E et al (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S et al (2018) Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann Intensive Care 8:104
Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M et al (2021) Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:e149–e162
Bhoyar RC, Jain A, Sehgal P, Divakar MK, Sharma D, Imran M et al (2021) High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing. PLoS ONE 16:e0247115
Rambaut A, Holmes EC, O’Toole Á, Hill V, McCrone JT, Ruis C et al (2020) A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 5:1403–1407
Ma KC, Shirk P, Lambrou AS, Hassell N, Zheng X-Y, Payne AB et al (2023) Genomic Surveillance for SARS-CoV-2 Variants: circulation of Omicron Lineages—United States, January 2022-May 2023. MMWR Morb Mortal Wkly Rep 72:651–656
La Rosa G, Brandtner D, Bonanno Ferraro G, Veneri C, Mancini P, Iaconelli M et al (2023) Wastewater surveillance of SARS-CoV-2 variants in October-November 2022 in Italy: detection of XBB.1, BA.2.75 and rapid spread of the BQ.1 lineage. Sci Total Environ 873:162339
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators (2021) Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 47:60–73
Fourati S, Audureau E, Arrestier R, Marot S, Dubois C, Voiriot G et al (2022) SARS-CoV-2 genomic characteristics and clinical impact of SARS-CoV-2 viral diversity in critically ill COVID-19 patients: a prospective multicenter cohort study. Viruses 14:1529
Vieillard-Baron A, Flicoteaux R, Salmona M, Chariot A, De Maupeou D’Ableiges B, Darmon M et al (2022) Omicron variant in the critical care units of Paris metropolitan area the reality research group. Am J Respir Crit Care Med 206:349
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE et al (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584:430–436
Davis-Gardner ME, Lai L, Wali B, Samaha H, Solis D, Lee M et al (2023) Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N Engl J Med 388:183–185
Qu P, Evans JP, Faraone J, Zheng Y-M, Carlin C, Anghelina M et al (2022) Distinct neutralizing antibody escape of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7 and BA.2.75.2 [Internet]. bioRxiv. https://doi.org/10.1101/2022.10.19.512891v1
Cho J, Shin Y, Yang J-S, Kim JW, Kim K-C, Lee J-Y (2023) Evaluation of antiviral drugs against newly emerged SARS-CoV-2 Omicron subvariants. Antiviral Res 214:105609
Acknowledgements
The authors would like to thank all study investigators, Dr Pierre-André Natella, Ms Nolwenn Bombenger for taking care of regulatory aspects, Ms Clélia Chambraud for taking care of data management, Mr Mohamed Ader for clinical data abstraction, the nurses and physicians who took care of the patients, Mr Alexandre Soulier and the laboratory staff who took care of virological samples and the patients and their family for agreeing to participate in the study.
The SEVARVIR investigators include: Henri Mondor, AP-HP, Créteil, Medical ICU: Keyvan Razazi ; Henri Mondor, AP-HP, Créteil, Surgical ICU: Raphaël Bellaïche ; Saint-Louis, AP-HP, Paris: Elie Azoulay; Bichat, Paris, AP-HP: Jean-François Timsit; Tenon, Paris, AP-HP: Matthieu Turpin, Nina de Montmollin; Pitié-Salpétrière, Paris, AP-HP: Julien Mayaux; Louis Mourier, Colombes, AP-HP: Damien Roux; Raymond Poincaré, Garches, AP-HP: Djillali Annane; Nancy: Cédric Hartard: Antoine Kimmoun; Strasbourg: Ferhat Meziani, Louis-Marie Jandeaux, Samira Fafi-Kremer.
Funding
The SEVARVIR study has been funded by the EMERGEN consortium—ANRS Maladies Infectieuses Emergentes (ANRS0153). This study has been labeled as a National Research Priority by the National Orientation Committee for Therapeutic Trials and other researches on Covid-19 (CAPNET). The investigators would like to acknowledge ANRS | Emerging infectious diseases for their scientific support, the French Ministry of Health and Prevention and the French Ministry of Higher Education, Research and Innovation for their funding and support.
Author information
Authors and Affiliations
Consortia
Contributions
NDP, EA, JMP, and SF, designed the study and obtained funding; EA performed statistical analyses; NDP, SP, RF, PB, NH, TP, GV, SJ, SM, DC, AJ, FU, ME, C-EL, FP, SG, LP, AMD, included the patient and were responsible for clinical data collection; AG, EG, AC, LM-J, AP, AH, M-LC, DD, CG-S, SM, FR, SB, CR, and SF were responsible of the management of virological samples; J-MP, and SF were responsible of virological analyses; NDP and SF wrote the first draft of the article; All authors revised and approved the article The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted SF is the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was given by the Comité de Protection des Personnes Sud-Méditerranée I (N° EudraCT/ID-RCB: 2021-A02914-37). Informed consent was obtained from all patients or their relatives.
Consent for publication
Patients received information during their hospital stay that data abstracted from their medical charts could be used for research purposes.
Competing interests
S.F. has served as a speaker for GlaxoSmithKline, Cepheid and Astrazeneca. J.-M.P. has served as an advisor or speaker for Abbvie, Arbutus, Assembly Biosciences, Gilead, GSK, and Merck. E.A. has received fees for lectures from Alexion, Sanofi, Gilead and Pfizer. His hospital has received research grant from Pfizer, MSD and Alexion. D.D. served as an advisor for Gilead-Sciences, ViiV Health care, Janssen-Cilag and MSD. F.P. served as an advisor for Gilead; he also received research grant from Alexion. C.-E.L. received lecture fees from MSD, Aerogen, Advanzpharma, and BioMérieux, outside the submitted work. Other authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of centres participating in the SEVARVIR study. Table S2. Clinical and biological characteristics of the 145 patients with severe SARS-CoV-2 infection at the time of their intensive care unit admission according to the infecting SARS-CoV-2 “sublineage groups” (BA.2 vs BA.4/BA.5 vs BQ.1.1). Table S3. Intensive care management and outcomes of patients with severe SARS-CoV-2 infection (n = 145) during their intensive care unit stay according to the SARS-CoV-2 infecting “sublineage groups” (BA.2 vs BA.4/BA.5 vs BQ.1.1). Table S4. Clinical and biological characteristics of the 145 patients with severe SARS-CoV-2 infection at the time of their intensive care unit admission according to their vital status at day 28.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Prost, N., Audureau, E., Préau, S. et al. Clinical phenotypes and outcomes associated with SARS-CoV-2 Omicron variants BA.2, BA.5 and BQ.1.1 in critically ill patients with COVID-19: a prospective, multicenter cohort study. ICMx 11, 48 (2023). https://doi.org/10.1186/s40635-023-00536-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00536-0