Abstract
Background
Nothing is known about the epidemiology and resistance mechanisms of extended-spectrum ß-lactamase-producing Enterobacteriaceae (ESBL-PE) in Burkina Faso. The objective of this study was to determine ESBL-PE prevalence and to characterize ESBL genes in Burkina Faso.
Methods
During 2 months (June-July 2014), 1602 clinical samples were sent for bacteriologic investigations to the microbiology laboratories of the tree main hospitals of Burkina Faso. Isolates were identified by mass spectrometry using a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) BioTyper. Antibiotic susceptibility was tested using the disk diffusion method on Müller-Hinton agar. The different ESBL genes in potential ESBL-producing isolates were detected by PCR and double stranded DNA sequencing. Escherichia coli phylogenetic groups were determined using a PCR-based method.
Results
ESBL-PE frequency was 58 % (179 strains among the 308 Enterobacteriaceae isolates identified in the collected samples; 45 % in outpatients and 70 % in hospitalized patients). The CTX-M-1 group was dominant (94 %, CTX-M-15 enzyme), followed by the CTX-M-9 group (4 %). ESBL producers were more often found in E. coli (67.5 %) and Klebsiella pneumoniae (26 %) isolates. E. coli isolates (n = 202; 60 % of all Enterobacteriaceae samples) were distributed in eight phylogenetic groups (A = 49, B1 = 15, B2 = 43, C = 22, Clade I = 7, D = 37, F = 13 and 16 unknown); 22 strains belonged to the sequence type ST131. No association between a specific strain and ESBL production was detected.
Conclusions
This report shows the alarming spread of ESBL genes in Burkina Faso. Public health efforts should focus on education (population and healthcare professionals), surveillance and promotion of correct and restricted antibiotic use to limit their dissemination.
Similar content being viewed by others
Background
The emergence and spread of Multidrug Resistant (MDR) bacteria are major public health threats. Particularly, bacteria that produce extended-spectrum ß-lactamases (ESBL) are of great concern because their resistance to penicillins and narrow- and extended-spectrum cephalosporins reduces considerably the treatment options [1]. ESBL genes originally evolved from the ß-lactamase TEM-1, TEM-2 and SHV-1 genes through mutations of the amino acids surrounding the active site and were mainly detected in nosocomial pathogens [2]. However, during the past decade, the rapid and massive spread of CTX-M-type ESBLs has been described worldwide. This has considerably changed their epidemiology because they combine the expansion of mobile genetic elements with specific clonal dissemination [3]. Furthermore, such plasmids typically carry resistance genes also to other drugs, such as aminoglycosides and fluoroquinolones [2]. Recent studies suggest that CTX-M-type ESBL- producing Enterobacteriaceae (ESBL-PE) are endemic in most countries of Europe, Asia and South America, with high rates of CTX-M-type ESBL-producers particularly among Escherichia coli (30 to 90 %) and Klebsiella pneumoniae (10 to 60 %) [4, 5]. Despite these public health concerns, little is known about ESBL diffusion in Africa. ESBL-PE rates between 8.8 and 13.1 % were reported in South Africa [6] and an alarmingly high proportion of ESBL-PE (49.3 %) was found among clinical isolates from Ghana [7]. Conversely, no information is available on the epidemiology of ESBL-producing pathogens in hospital or community settings in Burkina Faso, a low-income country close to Ghana. Therefore, the aim of the present study was to estimate ESBL occurrence in clinical samples from hospitalized and non-hospitalized patients and to characterize the ESBL genes as well as the genetic background of the identified E. coli strains.
Methods
Study setting
During 2 months (June–July 2014), all consecutive clinical samples sent to the microbiology laboratories of the three main hospitals of Burkina were investigated. Specifically:
-
1.
Yalgado Ouedraogo Teaching Hospital (CHU-YO) is the largest medical institution located in Ouagadougou, the capital city with a population of about 2 million inhabitants. This hospital has 716 beds and intensive care units that are used for surgical, medical and trauma emergencies. Annually, more than 20,000 inpatients (children and adults) are admitted among 126,000 consultations.
-
2.
Souro Sanou Teaching Hospital (CHU-SS) is the major healthcare and referral centre for the southern and western regions of Burkina Faso. It has 521 beds distributed in different specialized (medicine, surgery, gynaecology obstetric and paediatric) acute care units. The annual number of hospitalizations ranges from 15,000 to 20,000 patients among 108,000 consultations.
-
3.
Charles de Gaulle Paediatric Teaching Hospital (CHUP-CDG) is the referral paediatric hospital in Ouagadougou with 120 beds. About 6000 children are seen each year and 5000 are hospitalized. The microbiology laboratory also receives samples from adult outpatients.
Specimen collection, identification and antimicrobial susceptibility testing
In June and July 2014 (CHU-SS and CHUP-CDG) and July 2014 (CHU-YO), 1602 clinical samples were sent to the three microbiology laboratories for bacteriologic investigations (CHU-YO: n = 521, CHU-SS: n = 528 and CHUP-CDG: n = 553). Bacterial cultures could be obtained only from 584 of these samples (mainly because of poor pre-analytical sample handling) and they included 308 Enterobacteriaceae isolates. Enterobacteriaceae isolates were recovered from urine (n = 185), pus (n = 56), aspirates from various anatomic sites (n = 38), stool (n = 16), blood (n = 8), vaginal swabs (n = 3) and cerebrospinal fluid samples (n = 2). The remaining 276 isolates included Gram positive cocci (Staphylococcus spp and Streptococcus spp) and Gram negative bacilli (e.g., Pseudomonas aeruginosa and Acinetobacter baumanii). Species identification was performed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Bremen, Germany). Antimicrobial susceptibility was tested with the disk diffusion method on Müller-Hinton agar. The following antibiotics were tested: penicillins (amoxicillin, amoxicillin-clavulanic acid, piperacillin, piperacillin-tazobactam, ticarcillin, ticarcillin-clavulanic acid), monobactam (aztreonam), oxacephem (moxalactam), extended-spectrum cephalosporins (cefepime, cefotaxime, cefpirome, cefpodoxime, cefoxitin, ceftazidime, cephalotin), carbapenems (imipenem), aminoglycosides (amikacin, gentamicin, netilmicin and tobramycin), quinolones (nalidixic acid, ciprofloxacin, levofloxacin, ofloxacin) fosfomycin, chloramphenicol, tetracycline and trimethoprim-sulfamethoxazole. Results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (Version 5.0) (http://www.eucast.org/clinical_breakpoints/). ESBL production was detected by using the combined double-disk synergy method [8]. In case of high-level cephalosporinase production, the combined double-disk synergy test was performed using cloxacillin-supplemented medium. Ertapenem minimal inhibitory concentrations (MIC), determined using the Etest (bioMerieux), were determined for all isolates.
Molecular identification of ESBL genes
DNA was extracted from one single colony for each isolate in a final volume of 100 μL of distilled water by incubation at 95 °C for 10 min followed by a centrifugation step. The presence of blaCTX-M (CTX-M group 1, 2, 8, 9 and 25), blaTEM, blaSHV and blaOXA-like genes was assessed by multiplex PCR according to a previously published method [9]. DNA from reference blaCTX-M, blaTEM, blaSHV and blaOXA-like-positive strains was used as positive control. PCR products were visualized after electrophoresis on 1.5 % agarose gels containing ethidium bromide at 100 V for 80 min. A 100 bp DNA ladder (Promega, USA) was used as a marker size. PCR products were purified using the ExoSAP-IT purification kit (GE Healthcare, Piscataway, NJ, USA) and sequenced bidirectionally on a 3100 ABI Prism Genetic Analyzer (Applied Biosystems). Nucleotide sequence alignment and analyses were performed online using the BLAST program available at the National Center for Biotechnology Information web page http:// www.ncbi.nlm.nih.gov.
PCR detection of Escherichia coli phylogroups and ST131
E. coli phylogenetic grouping was performed using the PCR-based method described by Clermont and al. [10]. For strains assigned to the B2 phylogenetic group, the sequence type (ST) 131 was determined using a PCR method specific for the O25-b serotype with primers that target the pabB and trpA genes, as previously described [11].
Statistical analysis
Statistical analysis was performed with Epi Info, version 3.5.3 [Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA]. Associations between demographic variables (sex, site of infection and age) and infection by ESBL-PEs were analysed by using odds ratio and a multinomial logistic regression model, when appropriate. A value of p < 0.05 was considered to be statistically significant.
Results
Occurrence of ESBL-producing enterobacteriaceae
During the study period, 308 different enterobacterial isolates were recovered from 158 hospitalized and 150 non-hospitalized patients (Table 1). The mean age of these patients was 29.7 ± 24.6 years and the sex ratio 1.4; 118 patients (38 %) were younger than 15. Among these 308 isolates, 179 (58 %) were identified as potential ESBL-PEs by antimicrobial susceptibility testing. PCR analysis confirmed that they all carried ESBL genes (Table 1). Considering the isolate origin, ESBL-PE prevalence was of 65 % (42/65) at CHU-YO, 59 % (84/142) at CHU-SS and 52 % (53/101) at CHUP-CDG. Moreover, ESBL-PEs were found in 45 % of outpatients and 70 % of hospitalized patients (p < 0.001). In hospitalized patients, no demographic factor was significantly associated with ESBL-PE occurrence (p > 0.05) (Table 1). Conversely in outpatients, the ESBL-PE prevalence was significantly higher among patients older than 65 years of age (Odd Ratio [OR] = 6.4, 95 % CI = 0.47–86.34; p < 0.001). ESBL-PE rate was also significantly higher in male than female outpatients (OR = 4.59) and in urinary samples (59 of 119; 50 %) (Table 1). Species identification showed that the 179 ESBL-PEs included 121 (67.5 %) E. coli, 46 (26 %) K. pneumoniae, 7 (4 %) Enterobacter cloacae, 2 (1 %) Providencia stuartii, 1 (0.5 %) Enterobacter aerogenes, 1 (0.5 %) Citrobacter freundi and 1 (0.5 %) Morganella morgannii species (Table 2). The highest proportion of ESBL-PEs was found in blood samples (6/8, 75 %). Moreover, within each species, the fraction of ESBL producers was highest among Morganella morgannii isolates (100 %), followed by K. pneumoniae (66 %) and E. coli (60 %) (Table 2). The 129 non-ESBL-PEs included E. coli (81/2002, 40 %), K. pneumoniae (24/70, 34 %) Enterobacter cloacae (6/13, 46 %), Providencia stuartii (4/6, 66 %) Enterobacter aerogenes (2/3, 77 %) Citrobacter freundi (2/3, 66 %), Salmonella spp (3/3, 100 %), Proteus mirabilis (5/5, 100 %) and Leclercia adecarboxylata (1/1) species.
Antibiotic susceptibility patterns
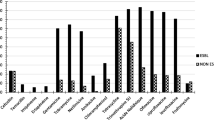
The susceptibility pattern of ESBL producing (n = 179) and non-producing (n = 129) Enterobacteriaceae isolates is shown in Fig. 1. ESBL-PE isolates were more resistant to the other tested antibiotics than non-producers: cotrimoxazole (45 % vs 5 %), gentamicin (89 % vs 27.5 %), tobramycin (86 % vs 9 %), netilmicin (88 % vs 12 %), ciprofloxacin (80 % vs 12 %), ofloxacin (70 % vs 7 %) and levofloxacin (82 % vs 27 %) (p < 0.05). None of the collected Enterobacteriaceae isolates was resistant to imipenem. Four isolates had high ertapenem MCI. Additional investigations showed that these four isolates carried blaOXA181 (47).
Results of the antibiotic susceptibility test for the 179 ESBL-producing (ESBL-PE) and the 129 non-ESBL-producing (NON ESBL-PE) Enterobacteriaceae isolates. The histogram shows the percentage of ESBL-PE and NON ESBL-PE isolates that were susceptible to each tested antibiotic compound. Y axis: Antibiotic susceptibility (%)
Molecular characterization of ESBL and other ß-lactamase genes
Most ESBL-PE isolates (94 %) were identified as CTX-M group 1 producers because all of them carried the blaCTX-M-15 gene. CTX-M group 9 producers represented only 4 % of all ESBL-PEs (blaCTX-M-14 was detected in three isolates and blaCTX-M-27 in five samples (Table 3). The blaSHV-12 gene was detected in two isolates. The ESBL genes were detected alone or in association with one to three other ß-lactamase genes: blaOXA-1, blaSHV-1 and blaTEM-1. While blaCTX-M-15 was found in all the different enterobacterial species, blaCTX-M-14 was detected only in E. coli samples (n = 3), blaCTX-M-27 in E. coli (n = 2), K. pneumoniae (n = 1) and E. cloacae (n = 1) isolates, and blaSHV-12 in one E. coli and one K. pneumoniae sample (Table 3).
Escherichia coli phylogenetic groups and sequence type 131
The phylogenetic group analysis revealed diversity in both ESBL-producing and non-producing E. coli isolates (n = 202). Specifically, E. coli isolates belonged to eight different phylogenetic groups (A = 49, B1 = 15, B2 = 43, C = 22, Clade I = 7, D = 37 F = 13) and 16 could not be classified according to Clermont and al. method [10]. These 16 isolates might represent a new phylogenetic group. Phylogenetic group A was more represented among ESBL-producers (31 of 121; 26 %), followed by group D and B2 (for both: 26 of 121; 21.5 %). Non-ESBL producers belonged mainly to the phylogenetic groups A and B2 (18 and 17 of 81, respectively; 21 %). Moreover, the ST131 sequence type was detected in 16 ESBL-producers and in six non-producers (Table 4).
Discussion
In this study, we investigated the frequency of ESBL production by Enterobacteriaceae isolates from clinical samples sent to the three main hospitals of Burkina Faso in June and July 2014. Overall, 58 % of these isolates were ESBL-PEs. This is much higher than the rates reported in Europe [12, 13] and in other African countries: Algeria (17.7–31.4 %), Egypt (42.9 %) [14] and Ghana (49.4 %) [7]. Lack of antibiotic surveillance may have contributed to increasing the ESBL-PE problem that certainly has been present in Burkina Faso for a long time. Indeed, it has been shown that in countries with limited resources where hygiene is poor and antibiotics are misused, the absence of anti-microbial surveillance programmes increases the risk of multi-resistance development by bacteria in hospitals and in the community [15–17]. We found that blood cultures had the highest proportion of ESBL-PE isolates. This differs from the results of a recent literature review on ESBL-PE prevalence in Africa [18] showing a significantly lower proportion of ESBL-PE in blood cultures than in other specimens. This discrepancy is certainly explained by the small number of enterobacterial strains (eight of which six were ESBL-PEs) recovered from blood samples. Indeed, 107 ESBL-PEs were identified in urine samples (107/185, 58 %), a prevalence similar to what reported in previous studies [7, 19–21]. ESBL producers were more often found in E. coli (67 %) and K. pneumoniae (26 %) isolates, in agreement with previous works showing that these two species are the predominant ESBL-producers worldwide [2, 22]. ESBL-producing E. coli is considered to be responsible for hospital- and community-acquired infections, while ESBL-producing K. pneumoniae is considered mainly a nosocomial pathogen [2, 22]. In agreement, we identified ESBL-producing K. pneumoniae most frequently in samples from hospitalized patients. ESBL-PE prevalence differed considerably between outpatients and inpatients (45 % vs. 70 %: p < 0.001). More than two thirds of enterobacterial infections in hospitalized patients were thus caused by an ESBL-PE. In Burkina Faso, patients are usually hospitalized only in the case of very severe symptoms and after a long and empiric antibiotic therapy. These factors could explain this alarmingly high resistance level in hospitalized patients and also in outpatients (45 % compared with 7.5 % of community-acquired infections in Morocco [23] and 11.7 % in Nigeria) [24]. In outpatients, ESBL-PE frequency was significantly higher in isolates from older patients (more than 65 years of age, [OR] = 6.4, 95 % CI = 0.47–86.34). These results are in agreement with the study by Colodner and al. [3] showing that elderly patients present a higher antibiotic pressure and more underlying diseases, two significant risk factors for infection by ESBL producers [25]. In addition, ESBL-PE rate was significantly higher in male outpatients (OR = 4.59, 95 % CI = 2.14–9.84) and the urinary tract was the most frequent source (59 of 119, 50 %). The possible explanation may be that complicated urinary tract infections are more frequent in elderly men than elderly women [26].
In this study most ESBL-PEs were resistant to multiple drugs, especially to fluoroquinolones, aminoglycosides, cotrimoxazole and tetracycline, as described in previous studies [27–29]. This level of multi-resistance could lead to potential therapeutic impasses. Indeed, more than three quarters of ESBL-PE isolates were resistant to fluoroquinolones and aminoglycosides (but for amikacin), thus compromising the choice of antibiotic treatment, especially for outpatients with urinary tract infections. Moreover alternative antimicrobial agents, such as amikacin, fosfomycin and imipenem, are very expensive and difficult to obtain in Burkina Faso. These alarming results should act as an impetus for the establishment of antibiotic control policies. Indeed, currently, there is no restriction in the use of antibiotics in Burkina Faso. Antibiotics can be purchased over the counter without medical prescription. Patients may buy only a few tablets of an antibiotic because of limited money availability. Moreover, patients may begin an antimicrobial regimen and stop it when they feel better, before the end of the treatment, to save the remaining tablets for another time.
Finally, we found that 94 % of ESBL-PEs carried the blaCTX-M-15 gene. In the last decade, CTX-M enzymes, particularly CTX-M-15, have emerged worldwide and are the most prevalent in Europe, America and Asia [30–36]. Moreover, eight strains were CTX-M group 9 producers (blaCTX-M-27 in five and blaCTX-M-14 in three). These genes have been previously detected in E. coli isolates in Kenya [37] and in Egypt [38]. Nevertheless, the blaCTX-M-15 gene remains dominant in the African continent: 59 % of ESBL-PE in South Africa [34], 83 % in Mali [39], 91 % in Tunisia [40] and 96 % in Cameroon [41]. The blaSHV-12 gene (detected in one E. coli and one K. pneumoniae sample) has emerged in recent years and has been also detected in different Enterobacteriaceae isolates in the previously quoted studies in African countries [34, 38–41].
The phylogenetic group assignment of the 202 E. coli isolated showed a high diversity in both populations (out-patients and in-patients) without any association between a specific strain and ESBL production. This indicates that the high frequency of ESBL carriage is not caused by the epidemic spread of a single resistant clone. This contrasts with previous studies in which the dissemination of CTX-M-15-producing isolates was associated with the spread of the ST131 E. coli strain belonging to phylogenetic group B2 [42–44]. Indeed, in the present study, most isolates were assigned to the commensal groups A (49/202, 24 %) and B2 (43/202, 21 %). Only 13 % (16/121) of ESBL-producers and 7 % (6/81) of non ESBL-producers belonged to the ST131 clone. Moreover, some ESBL-producing E. coli isolated from urine, pus and blood samples belonged to three phylogenetic groups associated with CTX-M-15 dissemination: the virulent extra-intestinal group D (26/121) [45] and groups C (11/121) and F (10/121), usually detected in urinary tract infections [46]. This important genetic diversity among isolates suggests that the high rate of ESBL production and associated resistance are more likely caused by the diffusion of plasmids carrying antibiotic resistance genes than to cross-transmission between patients. The maintenance of these plasmids was probably favoured by antibiotic pressure. Further investigations, including multilocus sequence typing and plasmid characterization, are needed to complete this study.
Conclusions
In summary, this first survey shows an alarmingly high frequency of multi-resistant ESBL-PEs among clinical isolates in Burkina Faso. The analysis of the resistance genes highlighted an important dissemination of blaCTX-M-15 without clonal dissemination, suggesting a strong antibiotic selection pressure in hospital and community settings. Public health efforts should focus on educating the population and healthcare professionals about the proper use of antibiotics to halt/limit the spread of multi-resistant bacteria.
Abbreviations
CHUP-CDG, Charles de Gaulle Paediatric Teaching Hospital; CHU-SS, Souro Sanou Teaching Hospital; CHU-YO, Yalgado Ouedraogo Teaching Hospital; ESBL, extended-spectrum ß-lactamases; ESBL-PE, extended-spectrum ß-lactamase-producing Enterobacteriaceae; MALDI-TOF, matrix-assisted laser desorption ionization-time of flight; MDR, multidrug resistant; OR, odd ratio
References
Rahal JJ. Extended-spectrum beta-lactamases: how big is the problem? Clin Microbiol Infect. 2000;6 Suppl 2:2–6.
Newire EA, Ahmed SF, House B, Valiente E, Pimentel G. Detection of new SHV-12, SHV-5 and SHV-2a variants of extended spectrum beta-lactamase in klebsiella pneumoniae in Egypt. Ann Clin Microbiol Antimicrob. 2013;12:16.
Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, et al. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;23(3):163–7.
Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–75.
Rossolini GM, D’Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008;14 Suppl 1:33–41.
Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–86.
Obeng-Nkrumah N, Twum-Danso K, Krogfelt KA, Newman MJ. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg. 2013;89(5):960–4.
Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10(4):867–78.
Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–5.
Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58–65.
Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, Boyd D, Mulvey MR, Nordmann P, Ruppe E, Sarthou JL et al. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J Antimicrob Chemother. 2009;64(2):274–7.
Canton R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, Coque TM. Prevalence and spread of extended-spectrum beta-lactamase-producing enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14 Suppl 1:144–53.
Coque TM, Baquero F, Canton R: Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 2008;13(47):195–200.
Storberg V. ESBL-producing Enterobacteriaceae in Africa - a non-systematic literature review of research published 2008–2012. Infect Ecol Epidemiol 2014;4:50–55.
Jaggi N, Sissodia P, Sharma L. Control of multidrug resistant bacteria in a tertiary care hospital in India. Antimicrob Resist Infect Control. 2012;1(1):23:2047–2994.
Kanafani ZA, Mehio-Sibai A, Araj GF, Kanaan M, Kanj SS. Epidemiology and risk factors for extended-spectrum beta-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am J Infect Control. 2005;33(6):326–32.
Marcel JP, Alfa M, Baquero F, Etienne J, Goossens H, Harbarth S, Hryniewicz W, Jarvis W, Kaku M, Leclercq R et al. Healthcare-associated infections: think globally, act locally. Clin Microbiol Infect. 2008;14(10):895–907.
Sangare SA, Maiga AI, Guindo I, Maiga A, Camara N, Savadogo S, Diallo S, Bougoudogo F, Armand-Lefevre L, Andremont A et al. Prevalence of extended-spectrum beta-lactamase-producing enterobacteriaceae isolated from blood cultures in Africa. Med Mal Infect. 2015;45(9):374–82.
Cardwell SM, Crandon JL, Nicolau DP, McClure MH, Nailor MD. Epidemiology and economics of adult patients hospitalized with urinary tract infections. Hosp Pract. 2015;44(1):33–40.
Kumar M, Dutta R, Saxena S, Singhal S. Risk factor analysis in clinical isolates of ESBL and MBL (including NDM-1) producing Escherichia coli and klebsiella species in a tertiary care hospital. J Clin Diagn Res. 2015;9(11):DC08–13.
Oh MM, Chae JY, Kim JW, Kim JW, Yoon CY, Park MG, et al. Positive culture for extended-spectrum beta-lactamase during acute prostatitis after prostate biopsy is a risk factor for progression to chronic prostatitis. Urology. 2013;81(6):1209–12.
Nakayama T, Ueda S, Huong BT, Tuyen Le D, Komalamisra C, Kusolsuk T, et al. Wide dissemination of extended-spectrum beta-lactamase-producing Escherichia coli in community residents in the Indochinese peninsula. Infect Drug Resist. 2015;8:1–5.
Barguigua A, El Otmani F, Talmi M, Reguig A, Jamali L, Zerouali K, Timinouni M. Prevalence and genotypic analysis of plasmid-mediated beta-lactamases among urinary klebsiella pneumoniae isolates in Moroccan community. J Antibiot (Tokyo). 2013;66(1):11–6.
Afunwa RA, Odimegwu DC, Iroha RI, Esimone CO. Antimicrobial resistance status and prevalence rates of extended spectrum beta-lactamase (ESBL) producers isolated from a mixed human population. Bosn J Basic Med Sci. 2011;11(2):91–6.
Rodriguez-Bano J, Navarro MD. Extended-spectrum beta-lactamases in ambulatory care: a clinical perspective. Clin Microbiol Infect. 2008;14 Suppl 1:104–10.
Raz R, Okev N, Kennes Y, Gilboa A, Lavi I, Bisharat N. Demographic characteristics of patients with community-acquired bacteriuria and susceptibility of urinary pathogens to antimicrobials in northern Israel. Isr Med Assoc J. 2000;2(6):426–9.
Andriatahina T, Randrianirina F, Hariniana ER, Talarmin A, Raobijaona H, Buisson Y, Buisson Y, Richard V. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis. 2010;10:204.
Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, et al. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis. 2006;42(7):925–34.
Gangoue-Pieboji J, Koulla-Shiro S, Ngassam P, Adiogo D, Ndumbe P. Antimicrobial activity against gram negative bacilli from yaounde central hospital. Cameroon Afr Health Sci. 2006;6(4):232–5.
Drieux L, Brossier F, Duquesnoy O, Aubry A, Robert J, Sougakoff W, Lecso-Bornet M, Jarlier V. Increase in hospital-acquired bloodstream infections caused by extended spectrum beta-lactamase-producing Escherichia coli in a large French teaching hospital. Eur J Clin Microbiol Infect Dis. 2009;28(5):491–8.
Hara T, Sato T, Horiyama T, Kanazawa S, Yamaguchi T, Maki H. Prevalence and molecular characterization of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli from 2000 to 2010 in Japan. Jpn J Antibiot. 2015;68(2):75–84.
Ho PL, Poon WW, Loke SL, Leung MS, Chow KH, Wong RC, Yip KS, Lai EL, Tsang KW. Community emergence of CTX-M type extended-spectrum beta-lactamases among urinary Escherichia coli from women. J Antimicrob Chemother. 2007;60(1):140–4.
Machado E, Coque TM, Canton R, Novais A, Sousa JC, Baquero F, Peixe L. High diversity of extended-spectrum beta-lactamases among clinical isolates of enterobacteriaceae from Portugal. J Antimicrob Chemother. 2007;60(6):1370–4.
Peirano G, van Greune CH, Pitout JD. Characteristics of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli from community hospitals in south Africa. Diagn Microbiol Infect Dis. 2011;69(4):449–53.
Silva J, Aguilar C, Becerra Z, Lopez-Antunano F, Garcia R. Extended-spectrum beta-lactamases in clinical isolates of enterobacteria in Mexico. Microb Drug Resist. 1999;5(3):189–93.
Zhang H, Zhou Y, Guo S, Chang W. High prevalence and risk factors of fecal carriage of CTX-M type extended-spectrum beta-lactamase-producing enterobacteriaceae from healthy rural residents of taian. China Front Microbiol. 2015;6:239.
Kiiru J, Kariuki S, Goddeeris BM, Butaye P. Analysis of beta-lactamase phenotypes and carriage of selected beta-lactamase genes among Escherichia coli strains obtained from Kenyan patients during an 18-year period. BMC Microbiol. 2012;12:155.
Mohamed Al-Agamy MH, El-Din Ashour MS, Wiegand I. First description of CTX-M beta-lactamase-producing clinical Escherichia coli isolates from Egypt. Int J Antimicrob Agents. 2006;27(6):545–8.
Duval V, Maiga I, Maiga A, Guillard T, Brasme L, Forte D, Madoux J, Vernet-Garnier V, De Champs C. High prevalence of CTX-M-type beta-lactamases among clinical isolates of enterobacteriaceae in Bamako, Mali. Antimicrob Agents Chemother. 2009;53(11):4957–8.
Dahmen S, Bettaieb D, Mansour W, Boujaafar N, Bouallegue O, Arlet G. Characterization and molecular epidemiology of extended-spectrum beta-lactamases in clinical isolates of enterobacteriaceae in a Tunisian university hospital. Microb Drug Resist. 2010;16(2):163–70.
Lonchel CM, Melin P, Gangoue-Pieboji J, Assoumou MC, Boreux R, De Mol P. Extended-spectrum beta-lactamase-producing enterobacteriaceae in Cameroonian hospitals. Eur J Clin Microbiol Infect Dis. 2013;32(1):79–87.
Coque TM, Novais A, Carattoli A, Poirel L, Pitout J, Peixe L, Baquero F, Canton R, Nordmann P. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg Infect Dis. 2008;14(2):195–200.
Moubareck C, Daoud Z, Hakime NI, Hamze M, Mangeney N, Matta H, Mokhbat JE, Rohban R, Sarkis DK, Doucet-Populaire F. Countrywide spread of community- and hospital-acquired extended-spectrum beta-lactamase (CTX-M-15)-producing enterobacteriaceae in Lebanon. J Clin Microbiol. 2005;43(7):3309–13.
Rossi F, Baquero F, Hsueh PR, Paterson DL, Bochicchio GV, Snyder TA, Satishchandran V, McCarroll K, DiNubile MJ, Chow JW. In vitro susceptibilities of aerobic and facultatively anaerobic gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (study for monitoring antimicrobial resistance trends). J Antimicrob Chemother. 2006;58(1):205–10.
Janvier F, Delacour H, Tesse S, Larreche S, Sanmartin N, Ollat D, Rapp C, Merens A. Faecal carriage of extended-spectrum beta-lactamase-producing enterobacteria among soldiers at admission in a French military hospital after aeromedical evacuation from overseas. Eur J Clin Microbiol Infect Dis. 2014;33(10):1719–23.
Iranpour D, Hassanpour M, Ansari H, Tajbakhsh S, Khamisipour G, Najafi A. Phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. Biomed Res Int. 2015;2015:846219.
Acknowledgements
We would like to thank INSERM, IRD, CHU. We thank Elisabetta Andermarcher for assistance in preparing and editing the manuscript.
Funding
None.
Authors’ contributions
Conceived and designed the experiments: OAS, CC, JPH, GS, SI, NB, SL and OR. Performed the experiments: OAS, KF and PA, AS, BN. Contributed reagents/materials/analysis tools: OAS, KF and PA, AS, BN. Contributed to the writing of the manuscript: OAS, CC, JPH, GS, DD. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Arbitrary numbers were assigned to the isolates recovered from patient specimens. The study was approved by the ethics authorities of each hospital: MS/SG/CHUSS/DG/DL 2014–171 July 2, 2014. This allowed us to conduct our study. Informed written consent was obtained from subjects and at least one parent of each child before enrollment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ouedraogo, AS., Sanou, M., Kissou, A. et al. High prevalence of extended-spectrum ß-lactamase producing enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect Dis 16, 326 (2016). https://doi.org/10.1186/s12879-016-1655-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-1655-3