Abstract
Background
Abdominal obesity (AO) has been regarded as the most dangerous type of obesity. The Conicity-index (C-index) had a high ability to discriminate underlying AO. The purpose of this study was to determine the ability of C-index to predict all-cause mortality among non-cancer Chinese older people.
Methods
The participants were residents of the Wanshou Road community in Beijing, China. Receiver operating curve (ROC) curves were used to determine the sensitivity and specificity of the best cut-off values for different anthropometric measures for predicting all-cause mortality. The area under the curve (AUC) of the ROC curves were calculated to compare the relative ability of various anthropometric measures to correctly identify older people in the community where all-cause mortality occurs. Included subjects were grouped according to C-index tertiles. The association between C-index and all-cause mortality was verified using Kaplan–Meier survival analysis and different Cox regression models.
Results
During a mean follow-up period of 9.87 years, 1821 subjects completed follow-up. The average age was 71.21 years, of which 59.4% were female. The ROC curve results showed that the AUC of the C-index in predicting all-cause mortality was 0.633. Kaplan–Meier survival curves showed a clear dose–response relationship between C-index and all-cause mortality. With the increase of C-index, the survival rate of the study population showed a significant downward trend (P < 0.05). Adjusted for age, gender, hip circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose (FBG), 2-h postprandial blood glucose (2hPG), glycosylated hemoglobin, high-density lipids protein (LDL), triglyceride, serum creatinine, serum uric acid, urine albumin-creatinine ratio (UACR), Mini-Mental State Examination (MMSE), smoking history, and drinking history, COX regression analysis showed that in the model adjusted for all covariates, the risk of all-cause mortality in tertile 3 was 1.505 times that in tertile 1, and the difference was statistically significant.
Conclusions
The C-index is an independent risk factor for all-cause mortality in the non-cancer Chinese older people.
Similar content being viewed by others
Introduction
Obesity is a complex multifactorial disease. Nearly one-third of the world's population is now classified as overweight or obese [1, 2]. Of the more than two billion people worldwide who are overweight or obese, 62% of them live in developing countries [3]. In 2014, China became the country with the largest number of obese people in the world, with a total of 90 million obese individuals, accounting for 14% of the world's obese people [4]. In addition, abdominal obesity has been regarded as the most dangerous type of obesity, and studies have shown that the prevalence of abdominal obesity in China reached 31.5% from 2013 to 2014 [5, 6]. Abdominal obesity has been linked to a number of chronic diseases, such as type 2 diabetes mellitus (T2DM), hypertension, and atherosclerosis [7,8,9]. Studies have also shown that obesity is associated with all-cause mortality [10,11,12,13]. Quantification of visceral adiposity is best determined by imaging studies, such as computed tomography (CT), which is the gold standard method but requires high cost, difficult manipulation, and radiation exposure [14, 15]. On the other hand, anthropometric clinical indicators are readily available and, if accurate, can provide diagnostic possibilities in primary care and follow-up without any inconvenience. However, the most widely used measure of obesity, body mass index (BMI), appears to be rather insensitive to abdominal fat deposition. Because it does not reflect an individual's fat distribution or differentiate between fat mass and muscle mass, BMI has its limitations [16, 17]. Therefore, many special indexes sensitive to abdominal fat have been studied, including waist circumference (WC), abdominal volume index (AVI), body roundness index(BRI), lipid accumulation products (LAP), waist-height ratio (WHtR), and conicity-index (C-index), etc. [18,19,20]. The C-index is based on the assumption that individuals with more fat around the abdomen are biconical, while those with less fat around the midsection are cylindrical [17]. This index involves variables such as weight, height, and WC. C-index was determined as an indicator of body fat distribution, and their values increased with the accumulation of fat in the abdominal region [7]. Numerous studies have pointed to the high ability of C-index in distinguishing underlying abdominal obesity (AO) [14, 21]. Abdominal obesity can be highly discriminated by the anthropometric measure C-index, which is considered to be a major cardiovascular risk factor [22]. C-index has also been found to be associated with hypertriglyceridemia, decreased high-density lipoprotein, and elevated low-density lipoprotein, which may also increase the risk of all-cause mortality [23]. However, there is still a gap in research on whether the C-index can predict all-cause mortality in older community populations. The purpose of this study was to fill a research gap by determining the ability of the C-index to predict all-cause mortality in community-based older people through follow-up.
Methods
Subject
This is a cohort study of people over 60 years old on Wanshou Road, a representative urban residential area in Beijing. Sampling and research methods have been reported in previously published articles [24,25,26]. A population-based cross-sectional survey of the participants in the Wanshou Road community, a representative urban residential area in Beijing, was conducted using a two-stage hierarchical cluster sampling method. Between September 2009 and June 2010, a total of 2162 residents aged 60–95 years were selected and invited for screening. Figure 1 shows the recruitment process for the study population. The study protocol was reviewed and approved by the Ethics Committee of the Chinese People's Liberation Army General Hospital. All participants gave informed consent before being recruited. All investigators were trained at the PLA General Hospital (Beijing, China) and passed the test.
Data collection
Men and women completed detailed baseline health and lifestyle questionnaires, and 2162 participated in a health check-up performed by trained nurses using standard procedures [24]. Baseline health data and lifestyle questionnaires were used to obtain some information about the participants, including alcohol and smoking status, history of diabetes, history of hypertension, history of coronary heart disease, National Institutes of Health Stroke Scale (NIHSS), Mini-Mental State Examination (MMSE), and activities of daily living (ADL), etc. [26]. Height is measured with an independent distance measuring device, accurate to 0.1 cm. Weight was calculated with an electronic scale, accurate to 0.1 kg. WC was measured horizontally between the lower edge of the ribs and the iliac crest using a piece of non-elastic tape and hip circumference (HC) was measured at the widest part of the hip and at the top of the iliac crest to an accuracy of 0.1 cm. The participant empties the bladder 30 min before the blood pressure measurement. Rest in a backed chair, allow the whole body to relax and measure the blood pressure with the sphygmomanometer in a quiet environment. The systolic and diastolic blood pressures were measured twice with a time interval of five minutes, and the average of the two was used for analysis. Hypertension is doctor-diagnosed hypertension or systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or received antihypertensive medication. Type 2 diabetes mellitus refers to T2DM diagnosed by a doctor or fasting blood glucose value ≥ 7.0 mmol/L after fasting for more than eight hours, or blood glucose ≥ 11.1 mmol/L after 2 h of oral glucose, or blood glucose ≥ 11.1 mmol/L at any time, or insulin at the time of admission. Fasting blood glucose, 2-h postprandial blood glucose, glycosylated hemoglobin, serum total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides were detected with an automatic biochemical analyzer. All on-site urine samples were taken, the urine albumin concentration (mg/l) and the urine creatinine concentration (g/l) were measured, and the urine albumin-creatinine ratio (mg/g) was calculated [26]. All biochemical analyses were performed in the Department of Biochemistry, Chinese People's Liberation Army General Hospital. 1821 of the subjects agreed to participate in telephone follow-up or signed written informed consent for community field follow-up, which constituted our study population. There were no differences between responders and non-responders in age and gender. This study was ethically approved by the Ethics Committee of the Chinese People's Liberation Army General Hospital.
Anthropometric measurements for identifing obesity
BMI was calculated with the following formula:
C-index was calculated with the following formula:
BRI was calculated with the following formula:
WHtR was calculated with the following formula:
AVI was calculated with the following formula:
LAP was calculated with the following formula:
Grouping and endpoints
The eligible subjects were divided into three groups according to C-index tertiles. Endpoint deaths were defined as indicator events occurring between baseline and follow-up up to December 31, 2020. During follow-up, the primary concern was all-cause mortality. In these analyses, all-cause deaths were defined as deaths from any cause that resulted from phone calls or on-site follow-up provided by family members. For some team members who lost their contact numbers or changed their home addresses, our staff sought help from community workers and police officers in the Population management and Archives Office of Wanshou Road Police Station [26].
Statistical analysis of data
All categorical data are presented as percentage (%), continuous data are presented as mean ± standard deviation. The Kolmogorov-Smirnoff test was used to test whether the data had a normal distribution. Baseline characteristics of the study population were analyzed using a one-way analysis of variance (ANOVA) test. For post hoc multiple comparisons, the Tamhane's T2 test is used to assume that the variances are not equal, while the LSD test is used to assume that the variances are equal. Receiver operating characteristic (ROC) curves were used to determine the sensitivity and specificity of optimal cutoff values for obesity measures (BMI, WC, AVR, BRI, LAP, WHtR, and C-index) for predicting all-cause mortality. The area under the curve (AUC) of the ROC curve was calculated to compare the relative ability of various anthropometric measures to correctly identify the occurrence of all-cause mortality in older people in the community.
The chi-square test was used to compare all-cause mortality after ten years in different groups. Cumulative probabilities were compared using the Kaplan–Meier method and the log-rank test. In the Cox proportional hazards model analysis, hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Model 1: All covariates were not adjusted; Model 2: Adjusted for age and gender; Model 3: Adjusted for age, gender, hip circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose, 2-h postprandial blood glucose, glycosylated hemoglobin, high-density lipoprotein, triglyceride, serum creatinine, serum uric acid, urine albumin-creatinine ratio, MMSE, smoking history, and drinking history. A multivariate logistic regression model was established to show the relationship between C-index and all-cause mortality. In the Cox regression model, tolerance (Tol) and variance inflation factors (VIF) are calculated for each covariate to test for multicollinearity, ensuring that there is no prior multicollinearity problem for each covariate. The Omnibus test was used to test the model coefficients, and the results were analyzed by the survival analysis function. In all hypothesis tests, the risk of type 1 error was a priori set at P ≥ 0.05. All statistical tests were two-sided with a significance level of α = 0.05. Statistical analysis was performed using SPSS software (version 26.0).
Results
Baseline characteristics
Among the 1821 study populations, 59.4% were female, grouped by C-index tertiles (Tertile 1: C-index < 1.249; Tertile 2: 1.249 ≤ C-index < 1.312; Tertile 3: C-index ≥ 1.312), and a one-way analysis of variance (ANOVA) test was used to analyze the baseline characteristics of the study population. Table 1 shows the baseline characteristics of the different groups.
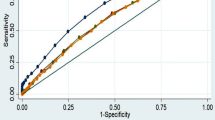
Obesity index and all-cause death
Figure 2 shows the ROC curves and their respective areas under the curve (AUC) for different obesity indices and all-cause mortality for all subjects, male subjects, and female subjects, respectively. Compared with various commonly used clinical obesity indices, C-index showed better ability in predicting all-cause mortality in older people in the community, with a larger area under the curve.
C-index and all-cause death
During a mean follow-up period of 9.87 years (18,029.5 person-years of follow-up), 1821 subjects were followed, of whom 333 died (179 men and 154 women). Overall population mortality, and crude all-cause mortality for men and women were 182.9/1000, 242.2/1000, and 142.3/1000, respectively. Table 2 shows the comparison of all-cause mortality in different groups in the chi-square test.
Figure 3 shows the Kaplan–Meier survival curves under different C-index groups. There was a significant dose–response relationship between C-index and all-cause mortality. With the increase of C-index, the survival rate of the study population showed a significant downward trend, and the P values were all less than 0.001 on the Log-Rank test, Breslow test, and Tarone-Ware test.
To investigate the effect of C-index on all-cause mortality, we fit a Cox proportional hazards model to the data. We built three Cox proportional hazards models. As can be seen from Table 3, in Model 1 without adjusting for any covariates, the risk of all-cause mortality for Tertile 3 was 2.730 times that of Tertile 1, and the risk of all-cause death for Tertile 2 was 1.706 times that of Tertile 1. After adjusting for age and sex, in model 2, the proportion of the risk of all-cause mortality decreased in Tertile 3, which was 1.656 times that of Tertile 1. In Model 3, after adjusting for all relevant covariates that may affect survival, the risk of all-cause mortality in Tertile 3 was 1.505 times that of Tertile 1, and the difference was still statistically significant.
As shown in Fig. 4, in the Cox proportional hazards model, survival analysis curves were made for the three models of C-index. It can also be seen from the survival analysis curves of the three models that there are significant differences in the survival rates of different C-index groups. Groups with higher C-index values had a higher risk of death.
Cox survival analysis of C-index and all-cause mortality. Tertile 1: C-index < 1.249; Tertile 2: 1.249 ≤ C-index < 1.312; Tertile 3: C-index ≥ 1.312. A Model 1: All covariates were not adjusted. B Model 2: Adjusted for age and gender. C Model 3: Adjusted for age, gender, hip circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose, 2-h postprandial blood glucose, glycosylated hemoglobin, high-density lipids protein, triglyceride, serum creatinine, serum uric acid, urine albumin-creatinine ratio, Mini-Mental State Examination, smoking history, and drinking history
Hierarchical analysis
A stratified analysis of covariates with a greater effect on all-cause mortality in the COX risk model was performed, stratified by the following variables: age, gender, smoking history, drinking history, history of HT, history of CHD, and history of T2DM. After controlling for all covariates except stratification variables, the association between C-index and risk of all-cause mortality remained in subgroup analyses. Figure 5 shows the forest plot of the stratified analysis, and the results show that the third tertile of the C-index and all-cause mortality were all statistically significant in the population aged ≤ 80 years, male, and with no smoking history. In addition, we stratified separately for the presence or absence of a history of HT, CHD, and T2DM. In the population with T2DM, with Tertile 1 as the reference, the risk of all-cause mortality in the second and third tertiles was 3.660 and 3.780 times that of Tertile 1, and the difference was statistically significant. Among people without diabetes, the risk of all-cause mortality differed only in the third tertile, using Tertile 1 as a reference. In the population with a history of HT and CHD, the results of the stratified analysis were not statistically significant.
The relationship between C-index and all-cause mortality under different stratifications. Tertile 1: C-index < 1.249; Tertile 2: 1.249 ≤ C-index < 1.312; Tertile 3: C-index ≥ 1.312. CHD: Coronary Heart Disease; HT: Hypertension; T2DM: Type 2 diabetes mellitus. Stratified analysis by age, gender, smoking history, drinking history, history of HT, history of CHD, and history of T2DM. Adjusted for age, gender, hip circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose, 2-h postprandial blood glucose, glycosylated hemoglobin, high-density lipids protein, triglyceride, serum creatinine, serum uric acid, urine albumin-creatinine ratio, Mini-Mental State Examination, smoking history, and drinking history
Discussion
The purpose of this study was to determine the ability of the C-index to predict all-cause mortality in Chinese older people. Using an non-cancer older population in a Beijing community, we conducted nearly 10 years of follow-up. We found that C-index was associated with and predicted the risk of all-cause mortality in this population. The Cox regression analysis showed that C-index is an independent risk factor for all-cause death in older people in community. Especially among people with diabetes, older people with a high C-index had a higher risk of all-cause death.
Obesity is critical to the health of the aging population. Excessive obesity, especially abdominal obesity, has long-term negative effects on cardiovascular outcomes and mortality [27]. However, the relationship between obesity and mortality in older people is controversial [28]. On the other hand, in some recent reports defined as the "obesity paradox", older people who are overweight or mildly obese may be more favorable than underweight [29, 30]. Therefore, it is particularly important to further clarify the relationship between obesity and the long-term survival of older people. In this study of older people in the Chinese community, the C-index was more predictive of all-cause mortality than other obesity indicators, with an AUC of 0.633. Second, the AUC of C-index was greater than that of women in male community elders. By Cox proportional risk model analysis, we analyzed the ability of C-index to predict all-cause mortality among older people in the community. Our results suggest that C-index can be used as an independent risk factor for all-cause mortality. Previous studies on C-index mainly focused on the risk of death from coronary heart disease (CHD) [31], and there was no research on the relationship between C-index and all-cause death. In 2014, Tonding et al. found in a study of obesity markers and coronary heart disease risk in diabetic patients that C-index was the body obesity marker most associated with a higher risk of fatal CHD in these diabetic patients [32]. However, other studies have had mixed results [33]. The Framingham Heart Study by Kim et al. showed that C-index was not associated with increased CHD morbidity or mortality [34]. BMI is a better predictor of CHD morbidity and mortality than C-index. In contrast to our study, the Framingham cohort included non-institutionalised white men and women aged between 30 and 62 years. During 24 years of follow-up, 248 men and 150 women died from coronary heart disease-related causes. Factors such as age, race and gender may all have had a differential impact on the results. To further demonstrate the association between C-index and all-cause death, we performed a stratified analysis, stratified by age, gender, smoking history, drinking history, history of HT, history of CHD, and history of T2DM. After controlling for all covariates except stratified variables, the association between C-index and risk of all-cause mortality persisted in the subgroup analysis. The association between the third tertile of the c index and all-cause mortality was statistically significant in subjects aged ≤ 80 years, or in subjects with no history of smoking, or in male subjects. In addition, we stratified separately for the presence or absence of diabetes. Furthermore, we stratified the presence or absence of diabetes separately. Among diabetic patients, the second and third tertiles had a statistically significant 3.660 and 3.780 times higher risk of all-cause mortality than Tertile 1, respectively. In people without diabetes, using Tertile 1 as a reference, the difference in risk of all-cause mortality was only statistically significant in the third tertile. It can be seen that in the diabetic population, patients with abdominal obesity have a high risk of death.
Aging not only promotes body fat gain but also changes its distribution. This fat distribution is characterized by a decrease in subcutaneous fat in the gluteal femoral region, which reduces the ability of subcutaneous adipocytes to store body fat. Thus, circulating free fatty acids from ectopic fat deposits in older people are increased. Increased visceral, intrahepatic, and intramuscular fat can lead to insulin resistance and metabolic changes [35]. Abdominal fat distribution is associated with increased insulin resistance and risk of T2DM and cardiovascular disease [36]. Fat accumulation is associated with pro-inflammatory cytokines, oxidative stress, and insulin resistance, and also contributes to muscle fiber atrophy and mitochondrial dysfunction, potentially contributing to the development and progression of sarcopenia [23]. Insulin resistance and hyperinsulinemia due to abdominal obesity were also associated with increased aortic stiffness [37, 38]. These studies have proved that abdominal obesity is significantly harmful to older people, which also promotes the occurrence of all-cause mortality in older people. However, the mechanism of various diseases in older people with abdominal obesity is still not well studied, and more basic research on abdominal obesity should be paid attention to.
Limitations and strengths of the study
C-index is a parameter that takes into account WC, weight, and height. In European and American populations, it is considered to be a health indicator comparable to the waist-to-hip ratio (WHR) [39]. However, there are few studies on the application of C-index in China. The C-index has a theoretical range that includes built-in adjustments for height and weight, allows direct comparison of abdominal adiposity between individuals and even between populations, and does not require hip circumference to assess fat distribution. Another strength of our study is that we have a mean follow-up of 9.87 years, and the results obtained with longer follow-up are more reliable. In addition, our research population is older people in community. In today's aging world, deepening the research on older people in community is of great significance to the health management and disease prevention of older people. Our study also has some limitations. First, there is a lack of accurate measurements of body composition determined by dual energy X-ray absorptiometry (DXA) or magnetic resonance imaging, which are capable of distinguishing abdominal visceral fat from subcutaneous fat. Second, 263 people were lost to follow-up during our study. Although the lost population only accounted for 12.2% of the study population, it may also have a certain impact on the results.
Conclusions
In conclusion, our study shows that C-index is an independent risk factor for all-cause mortality among non-cancer older people in the community, especially in the diabetic population, patients with abdominal obesity had a high risk of all-cause death.
Availability of data and materials
The research data used to support the findings of this study are available from the corresponding authors upon request.
Abbreviations
- AO:
-
Abdominal obesity
- T2DM:
-
Type 2 diabetes mellitus
- C-index:
-
Conicity-index
- ROC:
-
Receiver operating characteristic
- CT:
-
Computed tomography
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- AVI:
-
Abdominal volume index
- BRI:
-
Body roundness index
- LAP:
-
Lipid accumulation products
- WHtR:
-
Waist-height ratio
- FBG:
-
Fasting blood glucose
- 2hPG:
-
2-Hour postprandial blood glucose
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- NIHSS:
-
National Institutes of Health Stroke Scale
- MMSE:
-
Mini-Mental State Examination
- ADL:
-
Activity of Daily Living
- HC:
-
Hip circumference
- ANOVA:
-
A one-way analysis of variance
- AUC:
-
Area under the curve
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- CHD:
-
Coronary heart disease
- WHR:
-
Waist-to-hip ratio
- DXA:
-
Dual energy X-ray absorptiometry
References
Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506–14.
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96.
Mu L, Liu J, Zhou G, Wu C, Chen B, Lu Y, et al. Obesity prevalence and risks among chinese adults: findings from the China PEACE million persons project, 2014–2018. Circ Cardiovasc Qual Outcomes. 2021;14(6): e007292.
Zhang X, Zhang M, Zhao Z, Huang Z, Deng Q, Li Y, et al. Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 national chronic disease and risk factor surveillance. Ann Intern Med. 2020;172(4):291–3.
Després JP, Arsenault BJ, Côté M, Cartier A, Lemieux I. Abdominal obesity: the cholesterol of the 21st century? Can J Cardiol. 2008;24 Suppl D(Suppl D):7d–12d.
Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44(9):955–6.
Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–9.
Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, et al. Obesity: pathophysiology and intervention. Nutrients. 2014;6(11):5153–83.
Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67.
McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes Care. 2012;35(2):296–8.
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82.
David CN, Mello RB, Bruscato NM, Moriguchi EH. Overweight and abdominal obesity association with all-cause and cardiovascular mortality in the elderly aged 80 and over: a cohort study. J Nutr Health Aging. 2017;21(5):597–603.
Roriz AK, Passos LC, de Oliveira CC, Eickemberg M, Moreira Pde A, Sampaio LR. Evaluation of the accuracy of anthropometric clinical indicators of visceral fat in adults and elderly. PLoS ONE. 2014;9(7): e103499.
Kong M, Xu M, Zhou Y, Geng N, Lin N, Song W, et al. Assessing Visceral Obesity and Abdominal Adipose Tissue Distribution in Healthy Populations Based on Computed Tomography: A Large Multicenter Cross-Sectional Study. Front Nutr. 2022;9:871697.
Garrow JS. Three limitations of body mass index. Am J Clin Nutr. 1988;47(3):553.
Nkwana MR, Monyeki KD, Lebelo SL. Body Roundness Index, A Body Shape Index, Conicity Index, and Their Association with Nutritional Status and Cardiovascular Risk Factors in South African Rural Young Adults. Int J Environ Res Public Health. 2021;18(1):281.
Chang Y, Guo X, Li T, Li S, Guo J, Sun Y. A body shape index and body roundness index: two new body indices to identify left ventricular hypertrophy among rural populations in northeast China. Heart Lung Circ. 2016;25(4):358–64.
Jabłonowska-Lietz B, Wrzosek M, Włodarczyk M, Nowicka G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol Pol. 2017;75(11):1185–91.
Guo X, Ding Q, Liang M. Evaluation of eight anthropometric indices for identification of metabolic syndrome in adults with diabetes. Diabetes Metab Syndr. 2021;14:1431–43.
Eickemberg M, Amorim L, Almeida M, Pitanga FJG, Aquino EML, Fonseca M, et al. Abdominal obesity in ELSA-Brasil (Brazil’s Longitudinal Study of Adult Health): construction of a latent gold standard and evaluation of the accuracy of diagnostic indicators. Ciencia & saude coletiva. 2020;25(8):2985–98.
Milagres LC, Martinho KO, Milagres DC, Franco FS, Ribeiro AQ, Novaes JF. Waist-to-height ratio and the conicity index are associated to cardiometabolic risk factors in the elderly population. Ciencia & saude coletiva. 2019;24(4):1451–61.
Chung W, Park JH, Chung HS, Yu JM, Kim DS, Moon S. Utility of the Z-score of log-transformed A Body Shape Index (LBSIZ) in the assessment for sarcopenic obesity and cardiovascular disease risk in the United States. Sci Rep. 2019;9(1):9292.
He Y, Jiang B, Wang J, Feng K, Chang Q, Fan L, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47(8):1588–94.
Xu X, He J, Wang S, Zhu P, Chen Q, Zhang X, et al. Ankle-brachial index and brachial-ankle pulse wave velocity are associated with albuminuria in community-based Han Chinese. Clin Exp Hypertens (New York, NY : 1993). 2016;38(7):618–23.
Zhang A, Li M, Qiu J, Sun J, Su Y, Cai S, et al. The relationship between urinary albumin to creatinine ratio and all-cause mortality in the elderly population in the Chinese community: a 10-year follow-up study. BMC Nephrol. 2022;23(1):16.
Bahat G, Tufan A, Aydin Y, Tufan F, Bahat Z, Akpinar TS, et al. The relationship of body mass index and the functional status of community-dwelling female older people admitting to a geriatric outpatient clinic. Aging Clin Exp Res. 2015;27(3):303–8.
Bahat G, Ilhan B, Catikkas NM, Tufan A, Ozturk S, Dogan H, Karan MA. Associations between obesity, selfreported weakness and their combinations with mortality in nursing home residents. Acta Clin Belg. 2022:1–10.
Bahat G, Muratlı S, İlhan B, Tufan A, Tufan F, Aydin Y, et al. Body mass index and functional status in community dwelling older Turkish males. Aging Male. 2015;18(4):228–32.
Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol. 2015;11(1):55–62.
Motamed N, Perumal D, Zamani F, Ashrafi H, Haghjoo M, Saeedian FS, et al. Conicity index and waist-to-hip ratio are superior obesity indices in predicting 10-year cardiovascular risk among men and women. Clin Cardiol. 2015;38(9):527–34.
Tonding SF, Silva FM, Antonio JP, Azevedo MJ, Canani LH, Almeida JC. Adiposity markers and risk of coronary heart disease in patients with type 2 diabetes mellitus. Nutr J. 2014;13(1):124.
Sakr Y, Madl C, Filipescu D, Moreno R, Groeneveld J, Artigas A, et al. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34(11):1999–2009.
Kim KS, Owen WL, Williams D, Adams-Campbell LL. A comparison between BMI and conicity index on predicting coronary heart disease: the Framingham heart study. Ann Epidemiol. 2000;10(7):424–31.
Rezende FA, Ribeiro AQ, Mingoti SA, Pereira PF, Marins JC, Priore SE, et al. Anthropometric patterns of adiposity, hypertension and diabetes mellitus in older adults of Viçosa, Brazil: a population-based study. Geriatr Gerontol Int. 2018;18(4):584–91.
Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Investig. 2015;125(5):1790–2.
Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52(1):172–9.
Wohlfahrt P, Somers VK, Cifkova R, Filipovsky J, Seidlerova J, Krajcoviechova A, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235(2):625–31.
Zhang J, Zhu W, Qiu L, Huang L, Fang L. Sex- and age-specific optimal anthropometric indices as screening tools for metabolic syndrome in chinese adults. Int J Endocrinol. 2018;2018:1067603.
Acknowledgements
We would like to acknowledge all the participants in this study, including the professionals who collected the data.
Funding
This study was supported by the “National Key R&D Program of China” (Funding No.2020YFC2008900), the National Defense Science and Technology Innovation Special Zone Project (19–163-15-ZD-009–001-10), the Military Medical Youth Growth Project of PLA General Hospital (Funding No. QNC19005), and the Logistics Scientific Research Project of the Chinese PLA (Funding No. 19BJZ30).
Author information
Authors and Affiliations
Contributions
Anhang Zhang, Yingnan Li, and Shouyuan Ma proposed the design of the study and wrote the main manuscript text. Qiligeer Bao, Jin Sun, Yongkang Su, Shuang Cai, and Man Li organize data and analyze data. Bokai Cheng, Jing Dong, and Yan Zhang prepared figures and tables. Shuxia Wang and Ping Zhu corrected the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was consistent with the ethical principles of the Helsinki Declaration and was approved by the Chinese PLA General Hospital. The study protocol was reviewed and approved by the Ethics Committee of the Chinese People's Liberation Army General Hospital. All participants were asked for permission to use their medical data for a non-commercial study, and written informed consent was obtained from them.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anhang Zhang, Yingnan Li and Shouyuan Ma contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, A., Li, Y., Ma, S. et al. Conicity-index predicts all-cause mortality in Chinese older people: a 10-year community follow-up. BMC Geriatr 22, 971 (2022). https://doi.org/10.1186/s12877-022-03664-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03664-6