Abstract
Background
Postinduction hypotension is closely related to postoperative complications. Elderly patients with compromised cardiovascular compensatory reserve are more susceptible to hypotension after induction of general anesthesia. This study investigated whether the carotid artery corrected flow time (FTc) and respiratory variation of peak blood flow velocity in the common carotid artery (ΔVpeak) could predict postinduction hypotension in elderly patients.
Methods
This prospective observational study included elderly patients aged 65 to 75 who were scheduled for elective surgery under general anesthesia with ASA physical status class of I-II, without cardiovascular disease, hypertension, diabetes, or obesity. Anesthesia was induced by midazolam, sufentanil, and etomidate and was maintained by sevoflurane. The carotid artery FTc and ΔVpeak were measured by ultrasound before induction of anesthesia. Hemodynamic data were recorded before induction and then during the first 10 min after induction.
Results
Ninety-nine patients were included in the final analysis, of whom 63 developed postinduction hypotension. The area under the receiver operating characteristic curves was 0.87 (0.78 to 0.93) for carotid artery FTc and 0.67 (0.56 to 0.76) for ΔVpeak, respectively. The optimal cutoff value for predicting postinduction hypotension was 379.1 ms for carotid artery FTc, with sensitivity and specificity of 72.2 and 93.7%, respectively. The best cutoff value was 7.5% for ΔVpeak, with sensitivity and specificity of 55.6 and 75.0%, respectively.
Conclusions
The carotid artery FTc is a reliable predictor of postinduction hypotension in elderly patients with ASA status of I or II, without cardiovascular disease, hypertension, diabetes, or obesity. Elderly patients with a carotid artery FTc less than 379.1 ms before anesthesia have a higher risk of postinduction hypotension.
Trial registration
Clinical Trial Registry on August 2nd, 2020 (www.chictr.org.cn; ChiCTR2000035190).
Similar content being viewed by others
Background
Hypotension after induction of general anesthesia, or postinduction hypotension, is quite common in clinical practice, and if severe or prolonged, may cause organ hypoperfusion and ischemia, and may increase the incidence of postoperative adverse outcomes such as myocardial injury, ischemic stroke, acute kidney injury, and even increase 1-year mortality [1,2,3,4,5]. After induction of anesthesia, patients are at particular risk of hypotension mainly due to preexisting hypovolemia, the cardiovascular depressant and vasodilatory effects of induction agents, and a lack of surgical stimulation [6,7,8]. Previous studies have revealed that older age is an independent risk factor for the development of postinduction hypotension [9,10,11]. Elderly patients have a high prevalence of left ventricular diastolic dysfunction, decreased vascular reactivity, and increased sensitivity to anesthetics, and thus, are prone to hemodynamic fluctuation and hypotension. More importantly, elderly patients are less able to tolerate any period of hypotension [5]. Therefore, predicting hypotension after induction of anesthesia in elderly patients is of important clinical value for early intervention and reduction of postoperative complications and mortality.
Recently, ultrasonography for assessing volume status or predicting fluid responsiveness as well as post-induction hypotension has been widely applied in different clinical scenarios [6, 12,13,14,15,16]. However, venous ultrasound indices, represented by inferior vena cava (IVC) diameter and IVC collapsibility index, are less reliable in predicting fluid responsiveness in spontaneous breathing patients, as they are susceptible to the patient’ respiratory effort and patterns [17, 18]. Doppler corrected flow time (FTc) refers to the left ventricular ejection time corrected by heart rate and is known to be proportional to left ventricular preload and cardiac inotropy and inversely proportional to systemic vascular resistance [19, 20]. Previous studies have found that the FTc of carotid artery is unaffected by respiration [21]. Both carotid artery FTc and respiratory variation of peak blood flow velocity in the common carotid artery (ΔVpeak) can be good predictors of fluid responsiveness in spontaneous breathing patients [14, 15]. Furthermore, Maitra et al. [16] demonstrated that the carotid artery FTc is a good predictor of postinduction hypotension in ASA status I and II adult patients, but their study did not include elderly patients.
The aim of this study, therefore, was to evaluate the predictive power of carotid artery FTc and ΔVpeak measured preoperatively by bedside ultrasound in predicting hypotension after induction of general anesthesia in elderly patients undergoing elective surgery.
Methods
Study design, setting and participants
This prospective observational study was conducted in the Affiliated Hospital of North Sichuan Medical College from September 2020 to December 2020. Elderly patients who were 65 to 75 years of age with an American Society of Anesthesiologists (ASA) physical status class of I-II and scheduled for elective surgery under general anesthesia were recruited in this study. Patients with a body mass index (BMI) < 18 kg/m2 or > 30 kg/m2, cardiac rhythm other than sinus, a history of hypertension, diabetes, coronary heart disease, cardiac disease including cardiomyopathy and mild to severe valve disease, pulmonary hypertension, peripheral arterial disease or atherosclerosis, preoperative cervical vascular ultrasound abnormalities including plaque, stenosis and anatomic variation, and any previous neck surgery or trauma were excluded. This study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China (2020ER082-1) and registered in the Chinese Clinical Trial Registry in August 2nd, 2020 (www.chictr.org.cn; ChiCTR2000035190). Written informed consent was obtained from all participants.
Study procedure
All patients underwent routine fasting for at least 6 to 8 h and were not allowed to drink any solution or fluid 2 to 4 h prior to surgery. After entering the operating room, the patients were placed in a supine position. The Bene View N15 monitor (Mindray Biomedical Electronics Co., Shenzhen, China) was attached to monitor the electrocardiography, pulse oximetry, heart rate (HR), noninvasive blood pressure (BP), including systolic blood pressure, diastolic blood pressure, and mean arterial pressure (MAP). The bispectral index (BIS; Aspect Medical Systems, Inc., USA) was connected to evaluate the anesthetic depth. Meanwhile, intravenous access was established and crystalloid fluid was infused at a rate of 10 ml/kg/h. Then the patients were kept quietly for at least 10 min before the carotid artery ultrasonography. After the measurement of carotid artery FTc and ΔVpeak, HR and BP were recorded for 3 min at 1-min intervals, and the mean values of HR and MAP were taken as the baseline value. Finally, anesthesia induction and endotracheal intubation were sequentially executed, and the HR and BP were measured every minute for 10 min after the induction of anesthesia. The lowest value of HR and MAP during this period was used to calculate the percentage decrease in HR and MAP of each patient separately: percentage decrease in HR = (baseline HR- lowest HR)/baseline HR × 100%; percentage decrease in MAP = (baseline MAP- lowest MAP)/baseline MAP × 100%. The BIS value at the moment of lowest MAP was recorded.
Carotid artery ultrasonography
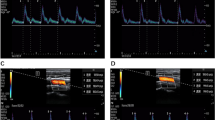
Ultrasound measurements were performed under a vascular setting with a 4–12 MHz linear transducer and ultrasound device (M9Sc-D system; Mindray Inc., China). Carotid artery FTc and ΔVpeak measurements were performed by one trained examiner who was excluded from the study design. The ultrasonic data extraction was conducted by another examiner who was unaware of the patient’s hemodynamic parameters. The right common carotid artery FTc and ΔVpeak were measured in patients with their heads tilted 30° to the left, as previously described by Blehar et al. [22] and Song et al. [23]. Briefly, a long axis view of the right common carotid artery was obtained at the lower border of the thyroid cartilage, and color flow was placed over the blood vessel approximately 2 cm proximal to the carotid artery bifurcation. Then, pulse wave Doppler was selected, and the sampling frame was placed in the area where the carotid artery had its best color flow, with an angle of less than 60°, to obtain the blood flow spectrum. After that, using the caliper function of the machine, cycle time (CT) was measured from the beginning of the systole to the beginning of the following systole, and systolic flow time (ST) was measured from the beginning of the systolic upstroke to the dicrotic notch (Fig. 1). The FTc of the carotid artery was calculated using Bazett’s formula, FTc = ST/\(\surd CT\) [24]. Three carotid artery FTc values were generated from three consecutive cardiac cycles, and the mean of these values was used for analysis. ΔVpeak was measured by tracking the blood flow spectrum with a slowed scanning speed under the same measurement condition as FTc, where the maximum and minimum peak systolic velocities were obtained in a single respiratory cycle, as shown in Fig. 2. The ΔVpeak was calculated as follows: ΔVpeak = (maximum peak velocity – minimum peak velocity) / [(maximum peak velocity + minimum peak velocity)/2] × 100%. Three ΔVpeak values were obtained from three consecutive respiratory cycles, and the average of these values was used for analysis.
Ultrasound measurements of the carotid artery and calculation of FTc. The panel above shows a two-dimensional scan of the right common carotid artery, and the following panel shows the corresponding blood flow spectrum. “1 Time” indicates the systolic flow time (ST), and “2 Time” presents the cycle time (CT)
Ultrasound measurements of the carotid artery and calculation of ΔVpeak. The panel above shows a two-dimensional scan of the right common carotid artery, and the following panel shows the corresponding blood flow spectrum. “1 vel” and “2vel” represent the minimum and maximum values of peak blood flow velocity, respectively, in a single respiratory cycle
Anesthesia protocol
No premedication was given before induction. Anesthesia induction was performed with 0.04 mg/kg midazolam, 0.4 µg/kg sufentanil, and 0.3 mg/kg etomidate, while endotracheal intubation was facilitated with intravenous 0.6 mg/kg rocuronium. After 3 min of mask ventilation, tracheal intubation was performed by an attending anesthesiologist who was blinded to the ultrasound data. There were no episodes of desaturation or difficult intubation. Anesthesia was maintained with inhaled 1–2% sevoflurane to reach a target BIS value of 40–60. Patients were volume-controlled ventilated (tidal volume: 8 ml/kg; respiratory rate: 10–12 breath/min) to maintain an end-tidal carbon dioxide partial pressure between 35 and 40 mmHg. Postinduction hypotension was defined as an absolute MAP of less than 65 mmHg or a more than 20% drop in MAP from the baseline during the recorded period after induction of anesthesia [3]. Postinduction hypotension was treated with intravenous ephedrine in 3 mg bolus doses and repeated when necessary. Significant bradycardia (heart rate < 40 beats/min) was treated with intravenous boluses of atropine (0.3 mg).
Statistical analysis
A pilot study involving 25 subjects revealed that the incidence of postinduction hypotension in elderly patients was 68.0%. Maitra et al. [16] reported that the area under the receiver operating characteristic (ROC) curve was 0.91 for carotid artery FTc to predict postinduction hypotension in adult patients. We assumed that the carotid artery FTc might have a lower predictive validity of 0.70 for postinduction hypotension in elderly patients. As a result, the sample size calculation showed that at least 90 patients were necessary to detect a difference of 0.20 between the ROC curve of the carotid artery FTc (0.70) and the null hypothesis (0.50) [25, 26], with a power of 0.90 and a two-tailed type I error of 0.05. To allow for a possible 20% dropout rate, 108 patients were enrolled.
Normality of the data distribution was assessed with Kolmogorov–Smirnov test. Continuous variables were presented as mean ± standard deviation if normally distributed, or as medians [interquartile ranges] if not. Categorical variables were expressed as absolute numbers (%). Hypotension and non-hypotension groups were compared with an independent t-test for normally distributed data, Mann–Whitney U-test for non-normally distributed data, and χ2 -test or Fisher’s exact test, as appropriate, for categorical variables. The ROC curve was depicted to assess the predictability of carotid artery FTc and ΔVpeak on postinduction hypotension. The best cutoff value was chosen to maximize the Youden index. Multivariate logistic regression analysis was performed to assess the impact of age, BMI, sex, ASA physical status, baseline MAP, carotid artery FTc and ΔVpeak on the occurrence of hypotension after checking for multicollinearity. All statistical analyses were conducted using SPSS (version 25; SPSS Inc., USA) and MedCalc (version 19.6.1; MedCalc, Belgium). Statistical significance was set at P < 0.05.
Results
Of the 108 participants assessed for eligibility, 9 were excluded because of being treated with propofol for hypertension after intubation (n = 6), ineffective images and sonographic measurements (n = 3). Thus, 99 patients completed the study without missing data (Fig. 3). Among them, 4 patients had a history of tuberculosis, 3 had a history of bronchiectasis, 3 had a history of rheumatoid arthritis, and 1 had a history of mild anemia. After induction of anesthesia, hypotension occurred in 63 cases (63.6%), of which 20.67% received a single intravenous injection of ephedrine (3 mg), and 79.37% were treated with two intravenous injections of ephedrine (total dose of 6 mg).
As shown in Table 1, there was no significant difference in age, sex, BMI, percentage of smokers, ASA physical status, baseline MAP, and baseline HR between the hypotension and non-hypotension groups. The type of surgery (thoracic, general, gynecological, otorhinolaryngologic, or orthopedic surgery), the amount of anesthesia drugs (midazolam, sufentanil, or etomidate), and the volume of infusion prior to induction of anesthesia showed no difference between the two groups as well. After induction of anesthesia, the lowest MAP in the hypotension group was significantly lower than that in the non-hypotension group (P < 0.001), and the percentage decrease in MAP was higher than that in the non-hypotension group (P < 0.001), whereas the BIS value at the moment of lowest MAP, the lowest HR, and the percentage decrease in HR were similar in the two groups. Patients who developed hypotension had a shorter carotid artery FTc (355.5 ± 22.9 vs. 386.3 ± 19.0 ms; P < 0.001) and a higher ΔVpeak (8.3 ± 3.8% vs. 6.1 ± 2.4%; P = 0.001) before anesthesia.
The power of carotid artery FTc and ΔVpeak to predict postinduction hypotension is shown in Fig. 4 and Table 2. The area under the ROC curve for carotid artery FTc was 0.87 (95% CI, 0.78 to 0.93; P < 0.001), and the optimal cutoff value was 379.1 ms, with a sensitivity of 72.2% (95% CI, 54.8 to 85.8%) and specificity of 93.7% (95% CI, 84.5 to 98.2%). The gray zone for carotid artery FTc was 362.3–378.1 ms and included 25.3% of the patients. On the contrary, the area under the ROC curve for ΔVpeak was 0.67 (95% CI, 0.56 to 0.76; P = 0.003). The optimal cutoff value of ΔVpeak was 7.5%, with a sensitivity of 55.6% (95% CI, 42.5 to 68.1%) and specificity of 75.0% (95% CI, 57.8 to 87.9%), and the gray zone for ΔVpeak was 3.7–9.1% and included 62.6% of the patients.
Receiver operating characteristic curves showing the predictability of carotid artery FTc (A) and ΔVpeak (B) on hypotension after induction of anesthesia in elderly patients. The circles on the curves indicate the optimal cutoff values determined by maximizing the Youden index. AUC, area under the receiver operating characteristic curve
As indicated in Table 3, multivariate logistic regression analysis identified that carotid artery FTc (Odds ratio = 0.92; 95% CI, 0.89 to 0.96; P < 0.001), but not ΔVpeak (P = 0.159), was the only independent predictor of hypotension after induction of general anesthesia in the elderly. That is, among elderly paitents, every ms decrease in carotid artery FTc increases the risk of postinduction hypotension by 8%.
Discussion
In this study, we found that preoperative carotid ultrasound measurements were predictive of hypotension after induction of anesthesia in ASA I-II elderly patients undergoing elective surgery who did not have cardiovascular disease, hypertension, diabetes, or obesity. The carotid artery FTc, but not ΔVpeak, showed a good predictive ability. The cutoff value of carotid artery FTc was 379.1 ms, with a sensitivity of 72.2%, specificity of 93.7%, and gray zones between 362.3 and 378.1 ms including 25.3% of patients.
In our study, we chose an absolute MAP less than 65 mmHg or a decrease in MAP more than 20% from baseline as the definition of hypotension, which has been demonstrated to be associated with both progressive kidney and myocardial injury [3]. Hemodynamic changes were recorded during the first 10 min after induction, during which no surgery or intense external stimulation was exerted. This time period is also the common waiting time from induction to the start of surgery at most medical centers [6, 27, 28]. In order to detect the predictive effect of carotid artery FTc and ΔVpeak on postinduction hypotension in the elderly, some previously reported risk factors including ASA III-V, a history of hypertension or diabetes, emergency surgery, use of propofol for induction, and high dosage of fentanyl were excluded [9,10,11]. Since preoperative hypovolemia is a major cause of hypotension after induction of general anesthesia [6], fluid infusion was started once the patients entered the operating room, and there was no difference in the amount of infusion between the hypotension and non-hypotension groups. Besides, the BIS values of the hypotension group at the lowest time of MAP were 53 ± 2, suggesting that these elderly patients were not likely to be under deep anesthesia at that moment. In this case, postinduction hypotension occurred in approximately 63.6% of the elderly patients, presumably caused by an underlying preoperative hypovolemic state and the synergistic inhibitory effect of anesthetics like midazolam [29] and etomidate [30] on the cardiovascular system.
Since the carotid artery is a superficial blood vessel located in the neck, ultrasound carotid measurements are technically easy for anesthesiologists to obtain. Furthermore, based on the rationale that both carotid artery FTc and ΔVpeak can predict fluid responsiveness in spontaneously breathing patients [14, 15] and that preoperative hypovolemia contributes to postinduction hypotension [6], carotid ultrasound measurements can serve as a useful tool for anesthesiologists to predict postinduction hypotension. Carotid artery FTc and ΔVpeak were first confirmed by Maitra and colleagues [16] to be able to predict the occurrence of hypotension after induction of general anesthesia in surgical patients. Maitra et al. [16] reported that the carotid artery FTc predicted postinduction hypotension with an area under the ROC curve of 0.91 and the best cutoff value of 330.2 ms in ASA I or II adult patients (18—65 years) undergoing elective surgery. In our current study, a similar area under the ROC curve of carotid artery FTc (0.87) was achieved, but the cutoff value (379.1 ms) was longer than that of Maitra et al. That is, elderly patient aged 65–75 years with a carotid artery FTc less than 379.1 ms at rest is more likely to develop hypotension after the induction of general anesthesia. The reason why the optimal cutoff value of carotid artery FTc in elderly patients in our study was longer than that of adult patients in Maitra et al.’s study is probably that a slower heart rate of elderly people leads to a relatively prolonged left ventricular systolic duration, which is consistent with the findings that aortic FTc is positively correlated with age [31]. On the other hand, approximately 68% of patients in Maitra et al.’s trial had hypertension, which might increase left ventricular afterload and thus shorten their carotid artery FTc [19].
Consistent with Maitra et al., we revealed a poor predictive role of ΔVpeak on postinduction hypotension in elderly patients, due to its low area under the ROC curve (only 0.67), large gray zone (including 62% of patients), and failing to achieve statistical significance in multivariate logistic regression analysis. As a dynamic index that changes with respiration, the predictive effect of ΔVpeak on postinduction hypotension is mainly based on the cardiopulmonary interaction. We can speculate that either the irregular changes in tidal volume between breaths or the insufficient intrathoracic pressure alteration to trigger an effective cardiopulmonary interaction could weaken the predictive power of ΔVpeak.
The current study has some limitations. First, we included ASA status I or II elderly patients with undetectable carotid artery plaque or stenosis under ultrasound; therefore, our findings cannot be extrapolated in patients with higher ASA grades or patients with hypertension, diabetes, obesity, and cardiac disease. Second, the induction drug regimen in our study includes midazolam, sufentanil, and etomidate. Our findings may not be applicable to elderly patients who received propofol for induction, becasue propofol may trigger a higher incidence of postinduction hypotension [32]. Third, this study was a single-center small sample study. Fourth, patients in our study did not receive any breathing training, which could lead to underestimating of the actual predictive power of ΔVpeak.
Conclusions
In conclusion, the carotid artery FTc, but not ΔVpeak, is a reliable predictor of postinduction hypotension (induced by midazolam, sufentanil, and etomidate and maintained by sevoflurane) in ASA I or II elderly patients without metabolic or cardiovascular diseases. Elderly patients were likely to develop postinduction hypotension when the carotid artery FTc prior to anesthesia was less than 379.1 ms.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologist
- BIS:
-
Bispectral index
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- CT:
-
Cycle time
- FTc:
-
Corrected flow time
- IVC:
-
Inferior vena cava
- MAP:
-
Mean arterial pressure
- ROC:
-
Receiver operating characteristic
- ST:
-
Systolic flow time
- ΔVpeak:
-
Respiratory variation of peak blood flow velocity in the common carotid artery
References
Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–64.
Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–15.
Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65.
Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307–19.
Bijker JB, van Klei WA, Vergouwe Y, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111(6):1217–26.
Zhang J, Critchley LA. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124(3):580–9.
Juri T, Suehiro K, Tsujimoto S, et al. Pre-anesthetic stroke volume variation can predict cardiac output decrease and hypotension during induction of general anesthesia. J Clin Monit Comput. 2018;32(3):415–22.
Lin FQ, Li C, Zhang LJ, et al. Effect of rapid plasma volume expansion during anesthesia induction on haemodynamics and oxygen balance in patients undergoing gastrointestinal surgery. Int J Med Sci. 2013;10(4):355–61.
Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64.
Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622–8.
Jor O, Maca J, Koutna J, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth. 2018;32(5):673–80.
Okamura K, Nomura T, Mizuno Y, Miyashita T, Goto T. Pre-anesthetic ultrasonographic assessment of the internal jugular vein for prediction of hypotension during the induction of general anesthesia. J Anesth. 2019;33(5):612–9.
Choi MH, Chae JS, Lee HJ, Woo JH. Pre-anaesthesia ultrasonography of the subclavian/infraclavicular axillary vein for predicting hypotension after inducing general anaesthesia: a prospective observational study. Eur J Anaesthesiol. 2020;37(6):474–81.
Kim DH, Shin S, Kim N, Choi T, Choi SH, Choi YS. Carotid ultrasound measurements for assessing fluid responsiveness in spontaneously breathing patients: corrected flow time and respirophasic variation in blood flow peak velocity. Br J Anaesth. 2018;121(3):541–9.
Xu L, Dai S, Shen J, Lv C, Tang Y, Chen X. The predictive ability of carotid artery corrected flow time and respirophasic variation in blood flow peak velocity measured by ultrasonography for fluid responsiveness in parturients for cesarean delivery. Minerva Anestesiol. 2020;86(10):1039–46.
Maitra S, Baidya DK, Anand RK, Subramanium R, Bhattacharjee S. Carotid artery corrected flow time and respiratory variations of peak blood flow velocity for prediction of hypotension after induction of general anesthesia in adult patients undergoing elective surgery: a prospective observational study. J Ultrasound Med. 2020;39(4):721–30.
Airapetian N, Maizel J, Alyamani O, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.
Long E, Oakley E, Duke T, Babl F, Paediatric Research in Emergency Departments International Collaborative (PREDICT). Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47(5):550–9.
Singer M, Allen MJ, Webb AR, Bennett ED. Effects of alterations in left ventricular filling, contractility, and systemic vascular resistance on the ascending aortic blood velocity waveform of normal subjects. Crit Care Med. 1991;19(9):1138–45.
Wodey E, Carre F, Beneux X, Schaffuser A, Ecoffey C. Limits of corrected flow time to monitor hemodynamic status in children. J Clin Monit Comput. 2000;16(3):223–8.
Doctor M, Siadecki SD, Cooper D Jr, et al. Reliability, laterality and the effect of respiration on the measured corrected flow time of the carotid arteries. J Emerg Med. 2017;53(1):91–7.
Blehar DJ, Glazier S, Gaspari RJ. Correlation of corrected flow time in the carotid artery with changes in intravascular volume status. J Crit Care. 2014;29(4):486–8.
Song Y, Kwak YL, Song JW, Song JW, Kim YJ, Shim JK. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br J Anaesth. 2014;113(1):61–6.
Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2(2):177–94.
Obuchowski NA, McClish DK. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med. 1997;16(13):1529–42.
Negida A, Fahim NK, Negida Y. Sample size calculation guide - Part 4: How to calculate the sample size for a diagnostic test accuracy study based on sensitivity, specificity, and the area under the ROC curve. Adv J Emerg Med. 2019;3(3): e33.
Kendale S, Kulkarni P, Rosenberg AD, Wang J. Supervised machine-learning predictive analytics for prediction of postinduction hypotension. Anesthesiology. 2018;129(4):675–88.
Padley JR, Ben-Menachem E. Low pre-operative heart rate variability and complexity are associated with hypotension after anesthesia induction in major abdominal surgery. J Clin Monit Comput. 2018;32(2):245–52.
Silva-Jr JM, Katayama HT, Nogueira FAM, Moura TB, Alves TL, de Oliveira BW. Comparison of dexmedetomidine and benzodiazepine for intraoperative sedation in elderly patients: a randomized clinical trial. Reg Anesth Pain Med. 2019;44(3):319–24.
Abou Arab O, Fischer MO, Carpentier A, et al. Etomidate-induced hypotension: a pathophysiological approach using arterial elastance. Anaesth Crit Care Pain Med. 2019;38(4):347–52.
Schober P, Loer SA, Schwarte LA. Perioperative hemodynamic monitoring with transesophageal doppler technology. Anesth Analg. 2009;109(2):340–53.
Lee JM, Min G, Lee JM, et al. Efficacy and safety of etomidate-midazolam for screening colonoscopy in the elderly: a prospective double-blinded randomized controlled study. Medicine (Baltimore). 2018;97(20): e10635.
Acknowledgements
We would like to thank all the doctors, nurses, technicians, and patients involved in this study for their cooperation.
Funding
This work was supported by grants from the Scientific Research Fund of Health Commission of Sichuan Province, China [grant number 20PJ178], the North Sichuan Medical College [grant number CBY-QA07], and the Science & Technology Department of Sichuan Province, China [grant number 2022NSFSC0700].
Author information
Authors and Affiliations
Contributions
Conception and design: Ji Wang, Yulan Li, Hang Su, Juan Zhao, and Faping Tu; Data collection: Yulan Li and Hang Su; Data analysis: Ji Wang and Juan Zhao; Drafting the manuscript: Yulan Li; Revision of the manuscript after critical review: Ji Wang and Faping Tu; Final edit: Ji Wang, Yulan Li, Hang Su, Juan Zhao, and Faping Tu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For the recruited participants, informed consent forms in writing duly were collected before their examination. This study was conducted in compliance with the tenets of the Helsinki Declaration, and with the approval of the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Li, Y., Su, H. et al. Carotid artery corrected flow time and respiratory variations of peak blood flow velocity for prediction of hypotension after induction of general anesthesia in elderly patients. BMC Geriatr 22, 882 (2022). https://doi.org/10.1186/s12877-022-03619-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03619-x