Abstract
Objective
Sarcopenic obesity is a prevalent geriatric syndrome, characterized by concurrence of sarcopenia and obesity. Sleep duration is linked to both obesity and sarcopenia. However, little was known regarding the association of sleep duration with sarcopenic obesity. In this study, we aimed to examine the association of sleep duration with sarcopenic obesity in multi-ethnic community-dwelling older adults.
Methods
Sarcopenia was defined according to the criteria established by Asian Working Group for Sarcopenia (AWGS) 2019. Obesity was defined as body fat percentage above the 60th percentile specified by sex. Sarcopenic obesity was defined as concurrence of obesity and sarcopenia. Sleep duration was collected by a self-reported questionnaire and was further divided into 5 groups: “<6 h”, “6–7 h”, “7–8 h”, “8–9 h” (reference group) and “≥9 h” (long sleep). Logistic regressions were adopted to examine the association.
Results
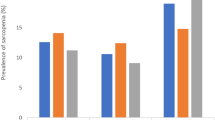
2256 multi-ethnic adults aged 60 and over from the West China Health and Aging Trend (WCHAT) study were involved for present study. Overall, 6.25% of the participants were classified as sarcopenic obesity. In the fully adjusted model, long sleep duration (≥ 9 h) was significantly associated with sarcopenic obesity compared with reference group (OR = 1.81, 95%CI = 1.10–2.98, P = 0.019). However, in subgroup analysis, this association can only be observed in male (OR 1.98, 95% CI = 1.02–3.87, P = 0.043) not in female (OR = 1.83, 95%CI = 0.85–3.94, P = 0.118). Regarding ethnic difference, Han older adults with long sleep duration (≥ 9 h) presented increased risk of sarcopenic obesity while ethnic minorities did not.
Conclusion
This study disclosed that long sleep duration significantly increased the risk of sarcopenic obesity among older adults. And our findings highlight the critical role of assessing sleep duration to identify individuals at risk of sarcopenic obesity.
Similar content being viewed by others
Introduction
Sarcopenic obesity [1] (SO), characterized by concurrence of sarcopenia and obesity, is a health-threatening geriatric syndrome. As we age, fat gain usually occurs in conjunction with muscle loss, which potentiates the development of sarcopenic obesity. Sarcopenic obesity is prevalent in older adults and the prevalence ranges from 2.75–20% [2]. Significant variation exists in sarcopenic obesity prevalence due to different diagnostic criteria. Obesity per se could induce muscle loss and muscle function decline through insulin resistance, chronic inflammation and oxidation stress [3]. Sarcopenia may, in turn, enhance fat accumulation. Both sarcopenia and obesity are related to adverse health outcomes. However, sarcopenic obesity may augment deleterious effect of the either sarcopenia or obesity. It is reported that individuals with sarcopenic obesity had 2-fold increased risks of frailty (odds ratio (OR) = 2.0, 95% confidence interval (95% CI) = 1.42–2.82), nearly 2-fold increased risk of activities of daily living (ADL) disability (OR = 1.58, 95% CI = 1.12–2.24)[4]. Besides, sarcopenic obesity contributes to increased risks of falls (risk ratio (RR) = 1.30, 95% CI = 1.10–1.54) and fractures (incidence rate ratio (IRR) = 1.88, 95% CI = 1.09–3.23) [5]. Moreover, individual with sarcopenic obesity are more prone to cardiometabolic diseases [6] and are associated with higher risk of all-cause mortality(pooled hazards ratio (HR) = 1.21, 95% CI = 1.10–1.32) [7].
To date, specific interventions for sarcopenic obesity are lacking. Yet, potential managements for sarcopenia such as exercise (aerobic or resistance exercise), nutritional supplements (protein, vitamin D, creatine, essential amino acids, multi-nutrients and catechin tea) and testosterone [8, 9], may also be therapeutic strategies for sarcopenic obesity. Besides, a combination of nutritional supplementation and exercise is demonstrated to reduce serum myostatin level[10] and improve hand grip strength [11]. In addition, meta-analysis conducted by Hsu et al. showed that resistance exercise could decrease fat mass as well as improve grip strength. And Low-calorie high-protein diet decreased fat mass but did not affect muscle quality and quantity [12]. Although some novel therapeutic strategies such as testosterone, myostatin inhibitor, selective androgen receptor modulators, anamorelin, neuromuscular activation (whole-body vibration therapy using electric stimuli or tai chi) are under investigation [13], there is still a long way to go. Therefore, identifying risk factors for sarcopenic obesity is a priority.
A sufficient amount of sleep is crucial for maintaining the health of older adults both physically and psychologically. Too little or too much sleep have been proven to contribute to obesity [14, 15], metabolic syndrome [16], diabetes [17], cardiovascular disease [18] and mortality [19]. In addition, sarcopenia has been associated with sleep duration in several studies [20,21,22]. It is reported that individuals with < 6 h of sleep was associated with 2.76 times increased odds of sarcopenia, while individuals with ≥ 8 h of sleep was related to 1.89 times increased odds of sarcopenia [20] compared with individuals with 6–8 h of sleep. Considering sleep duration is connected to obesity and sarcopenia, it may also be involved in the development of sarcopenic obesity.
To our knowledge, little was known regarding the association of sleep duration with sarcopenic obesity. This study aimed to find out if sleep duration was associated with sarcopenic obesity among multi-ethnic community-dwelling older adults of western China.
Methods
Study population
Analysis was conducted based on data from the West China Health and Aging Trend (WCHAT) study. Details of the WCHAT study have been previously described [23]. The WCHAT study was conducted under the guidance of Declaration of Helsinki and was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445). Besides, this study registered at the Chinese Clinical Trial Registry (number ChiCTR1800018895; date of first registration 16/10/2018). Each participant signed a written informed consent prior to enrollment.
The followings were the inclusion criteria:1) Participants from the WCHAT study who aged 60 years and over; 2) no missing data for appendicular skeletal muscle index, grip strength, gait speed, body fat percentage and sleep duration.
Assessment of sleep duration
Sleep duration was collected via a self-reported questionnaire and was further categorized as < 6 h, 6–7 h, 7–8 h, 8–9 h and ≥ 9 h (long sleep). 7 to 8 h of sleep was recommended for older adults [24] and it was therefore selected as a reference in our analysis.
Assessment of sarcopenia, obesity and sarcopenic obesity
Sarcopenia was defined in accordance with the Asian Working Group for Sarcopenia (AWGS) 2019 [25]. Appendicular skeletal muscle index (SMI) and body fat percentage were measured with a bioimpedance analyzer (InBody 770, Biospace, Korea). The cutoffs for low SMI were 7.0 kg/m2 and 5.7 kg/m2 in male and female, respectively. Dynamometer (EH101; Camry, Zhongshan, China) was used to measure grip strength. For men, the threshold for low grip strength was 28 kg and for women, it was 18 kg. The gait speed (GS) was the primary indicator of physical function and a gait speed of less than 1.0 m/s was considered abnormal. Body fat percentages above the 60th percentile designated by sex were classified as obesity [26]. Concurrence of sarcopenia and obesity was classified as sarcopenic obesity [2].
Covariates
Variables including age, sex, ethnicities (Han/Qiang/Tibetan/Yi/others), education level (illiteracy/primary school/secondary school and above, and marital status (married/single), smoking history, alcohol history and number of chronic diseases (0/1/≥ 2) were collected via in person interview by surveyors. The Mini Nutrition Assessment-Short Form (MNA-SF) scale was adopted to evaluate nutritional status (0 ~ 11 scores as malnutrition risk;12 ~ 14 scores as well nourished) [27]. Physical activity was assessed by using the China Leisure Time Physical Activity Questionnaire (CLTPAQ) [28]. And according to tertiles of the energy consumption, physical activity was divided into three categories: low, moderate, and high.
Statistical analysis
Continuous data and categorical data were presented as means ± standard deviation (SD) and counts (percentages), respectively. Group differences were tested by ANOVA for continuous variables and the chi square test for categorical variables. Logistic regression models were adopted to examine the association of sleep duration with sarcopenic obesity. A multivariable model adjusted for age, sex, education, ethnicities, marital status, smoking history, alcohol history, number of chronic diseases, nutritional status and physical activity was used. Stata software, version 14.0 (Stata Corp, College Station, TX, USA), was used for the analyses. Each statistical test was two-sided and deemed statistically significant at a P < 0.05.
Results
Overall, 2256 participants were included with a mean age of 67.6 ± 5.9 years. And 60.2% of them were female. Sarcopenic obesity accounted for 6.25% of the whole participants. Basic characteristics of the participants by sarcopenic obesity status were presented in Table 1. The sarcopenic obesity group differed significantly from the non-sarcopenic obesity group regarding age, sex, ethnicities, education level, smoking history, physical activity, nutritional status as well as sleep duration.
Table 2 presented the results of logistical regression exploring the association of sleep duration with sarcopenic obesity. Our findings showed that sarcopenic obesity was significantly more prevalent among participants who slept for longer time (≥ 9 h) (OR = 1.94, 95%CI = 1.20–3.14, P = 0.006). After controlling for confounders, the association remained significant (OR = 1.81, 95%CI = 1.10–2.98, P = 0.019). Short sleep duration (< 6 h) seemed to exert a protective effect on sarcopenic obesity (OR = 0.39, 95%CI = 0.16–0.96, P = 0.042). However, after adjusting for covariables, the association was not significant.
We further explored the sex and ethnic difference in association of sleep duration with sarcopenic obesity. Results of logistic regression with fully adjusted model were presented in Tables 3 and 4. In male, long sleep duration (≥ 9 h) was significantly correlated with sarcopenic obesity (OR = 1.98, 95% CI = 1.02–3.87, P = 0.043). Nonetheless, no significant association were observed in female (OR = 1.83, 95%CI = 0.85–3.94, P = 0.118). When assessing ethnic difference, we excluded participants in Yi and other minorities because of small sample size. Here, we found that Han participants with long sleep duration (≥ 9 h) presented increased risks of sarcopenic obesity (OR = 2.59, 95%CI = 1.13–5.97, P = 0.024). Whereas, long sleep duration and sarcopenic obesity were not significantly correlated in Qiang (OR = 1.83, 95%CI = 0.70–4.78, P = 0.215) or Tibetan participants (OR = 0.99, 95%CI = 0.39–2.48, P = 0.992).
Discussion
This study aimed to investigate whether sleep duration was related to sarcopenic obesity in multi-ethnic community-dwelling older adults of western China. The findings of our study demonstrated that long sleep duration (≥ 9 h) increased the risk of sarcopenic obesity among older adults. However, this association disappeared in female after stratification by sex. Regarding ethnic difference, the Han older adults with long sleep presented increased risks of sarcopenic obesity while the ethnic minorities did not. Our study provided a new idea for identifying individuals at high-risk of developing sarcopenic obesity.
The results of our study revealed that long sleepers (≥ 9 h) were more prone to developing sarcopenic obesity among older adults, whereas shorter sleepers were not. Presently, association of sleep duration with sarcopenic obesity has not yet been directly studied. But correlation between sleep duration and sarcopenia has already been uncovered. Previous meta-analysis including several cross-sectional analyses disclosed a U-shaped association of sleep duration with sarcopenia [29]. However, a recent longitudinal study [30] demonstrated that only long sleep duration significantly predicted the progression of sarcopenia (OR = 1.66, 95%CI = 1.02–2.69, P = 0.04), which potentially supported our findings. Sleep duration and obesity have also been associated. Meta-analysis of prospective studies conducted by Wu et al. showed that short sleep was correlated to incident obesity while long sleep was not [31]. Yet, in another meta-analysis, long sleep [32] was found to be significantly associated with obesity (OR = 1.08, 95%CI = 1.02–1.15). The inconsistent results may due to difference in measurement methods and adjustments for covariates in individual research. Despite of the discrepancy, these findings implied possible association of long sleep duration with sarcopenic obesity from clinical perspective. And our study further confirmed this association.
The biological mechanisms, which link sarcopenic obesity with long sleep duration remains obscure. However, possible mechanisms linking long sleep duration with sarcopenia or obesity may also play a role in the development of sarcopenic obesity. First, long sleep duration is reported to be associated with insulin resistance [33] and chronic inflammation [34], which are also involved in obesity and sarcopenia. Insulin resistance is inversely associated with circulating insulin-like growth factor (IGF1) [35] and it is reported that insulin resistance could inhibit the synthesis of muscle protein by downregulating the IGF-1/phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt) and mammalian Target of Rapamycin (mTOR) activity. Besides, insulin resistance could also promote muscle atrophy by inhibiting IGF1/PI3K/AKT pathways, activating fork head box O transcription factor (FOXO), enhancing atrogin-1 and MuscleRING-Finger-1 effects [36]. And higher level of proinflammatory markers, for example interluckin-6, may promote muscle proteolysis by upregulating ubiquitin-proteasome activity and activating the NF-kB pathways [37]. Moreover, sleep disorders could reduce the secretions of testosterone, which could upregulate the expression of myostatin and Regulated in Development and DNA Damage responses 1 (REDD1), promoting protein proteolysis [36].
On the other hand, long sleep duration is associated with decreased physical activity, a contributing factor for both sarcopenia [38] and obesity [39]. Present studies revealed that self-reported longer sleep (> 9 h) was related to lower daily physical activity. And physical activity decreased by 29 min per additional sleep hour [40,41,42]. Therefore, it may act as a mediator between long sleep and sarcopenic obesity. It is postulated that excessive body fat and low muscle mass/function resulting from low physical activity may in turn disrupt normal sleep pattern via systematic inflammation, insulin resistance and some toxic brain metabolites which could not be timely removed [43], forming a vicious cycle. Moreover, the molecular clock and circadian rhythms are essential for maintaining and adapting skeletal muscle [44] as well as regulating lipid metabolism [45]. Physical activity can modulate the molecular clock in skeletal muscle, affecting phase of circadian rhythms [46]. And exercise has been considered as an effective intervention for sarcopenia [47] and metabolic disease by re-setting circadian clock [48].
Our study firstly identified the association of long sleep duration with sarcopenic obesity in multi-ethnic community-dwelling older adults and explored sex and ethnic difference in their associations. There nevertheless existed some limitations. First, findings of this cross-sectional study failed to reveal a causal link between sarcopenic obesity and sleep duration. And these findings need to be confirmed through longitudinal research. Secondly, data on sleep duration was self-reported, which may introduce bias. Thirdly, we may not able to directly generalize our results to other regions of China. Finally, there were other potential con-founders we failed to address such as dietary preference, detailed diseases and possible drug use.
Conclusion
This study disclosed that long sleep duration significantly increased the risk of sarcopenic obesity among older adults. And our findings highlight the critical role of assessing sleep duration to identify individuals at risk of sarcopenic obesity.
Availability of Data and Materials
The data that support the findings of this study are available from National Clinical Research Center for Geriatrics, West China Hospital but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of National Clinical Research Center for Geriatrics, West China Hospital.
Abbreviations
- SO:
-
Sarcopenic obesity
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ADL:
-
Activities of daily living
- RR:
-
Risk ratio
- IRR:
-
Incidence rate ratio
- HR:
-
Hazards ratio
- AWGS:
-
Asian Working Group for Sarcopenia
- SMI:
-
Appendicular skeletal muscle index
- GS:
-
Gait speed
- MNA-SF:
-
The Mini Nutrition Assessment-Short Form
- CLTPAQ:
-
The China Leisure Time Physical Activity Questionnaire
- SD:
-
Standard deviation
- IGF1:
-
Insulin-like growth factor
- PI3K:
-
Phosphatidylinositol 3-kinase
- Akt:
-
Protein kinase B
- mTOR:
-
Mammalian Target of Rapamycin
- FOXO:
-
Fork head box O transcription factor
- REDD1:
-
Regulated in Development and DNA Damage responses 1
References
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35.
Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, Cruz-Jentoft AJ, Dicker D, Fruhbeck G, Giustina A, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–88.
Guillet C, Masgrau A, Walrand S, Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev. 2012;13(Suppl 2):51–7.
Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing. 2017;46(3):413–20.
Gandham A, Mesinovic J, Jansons P, Zengin A, Bonham MP, Ebeling PR, Scott D. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: A systematic review and meta-analysis. Obes Rev. 2021;22(5):e13187.
Evans K, Abdelhafiz D, Abdelhafiz AH. Sarcopenic obesity as a determinant of cardiovascular disease risk in older people: a systematic review. Postgrad Med. 2021;133(8):831–42.
Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, Deng R, Cheng ASK: Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr 2019, 19(1):183.
Agostini F, Bernetti A, Di Giacomo G, Viva MG, Paoloni M, Mangone M, Santilli V, Masiero S. Rehabilitative Good Practices in the Treatment of Sarcopenia: A Narrative Review. Am J Phys Med Rehabil. 2021;100(3):280–7.
de Sire A, Ferrillo M, Lippi L, Agostini F, de Sire R, Ferrara PE, Raguso G, Riso S, Roccuzzo A, Ronconi G, et al: Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14(5).
de Sire A, Baricich A, Reno F, Cisari C, Fusco N, Invernizzi M. Myostatin as a potential biomarker to monitor sarcopenia in hip fracture patients undergoing a multidisciplinary rehabilitation and nutritional treatment: a preliminary study. Aging Clin Exp Res. 2020;32(5):959–62.
Avola M, Mangano GRA, Testa G, Mangano S, Vescio A, Pavone V, Vecchio M. Rehabilitation Strategies for Patients with Femoral Neck Fractures in Sarcopenia: A Narrative Review. J Clin Med 2020, 9(10).
Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11(9).
Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr Obes Rep. 2019;8(4):458–71.
Fan Y, Zhang L, Wang Y, Li C, Zhang B, He J, Guo P, Qi X, Zhang M, Guo C, et al. Gender differences in the association between sleep duration and body mass index, percentage of body fat and visceral fat area among chinese adults: a cross-sectional study. BMC Endocr Disord. 2021;21(1):247.
Li Q: The association between sleep duration and excess body weight of the American adult population: a cross-sectional study of the national health and nutrition examination survey 2015–2016. BMC public health 2021, 21(1):335.
Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes. 2013;3:e65.
Liu H, Chen G, Wen J, Wang A, Mu Y, Dou J, Gu W, Zang L, Zhang S, Lyu Z. Association between sleep duration and incidence of type 2 diabetes in China: the REACTION study. Chin Med J (Engl) 2022.
Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92.
Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92.
Chien MY, Wang LY, Chen HC. The Relationship of Sleep Duration with Obesity and Sarcopenia in Community-Dwelling Older Adults. Gerontology. 2015;61(5):399–406.
Kwon YJ, Jang SY, Park EC, Cho AR, Shim JY, Linton JA. Long Sleep Duration is Associated With Sarcopenia in Korean Adults Based on Data from the 2008–2011 KNHANES. J Clin Sleep Med. 2017;13(9):1097–104.
Kim RH, Kim KI, Kim JH, Park YS. Association between Sleep Duration and Body Composition Measures in Korean Adults: The Korea National Health and Nutrition Examination Survey 2010. Korean J Fam Med. 2018;39(4):219–24.
Hou L, Liu X, Zhang Y, Zhao W, Xia X, Chen X, Lin X, Yue J, Ge N, Dong B. Cohort Profile: West China Health and Aging Trend (WCHAT). J Nutr Health Aging. 2021;25(3):302–10.
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–7 e302.
Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89(6):1895–900.
Zhang L, Wang C, Sha SY, Kwauk S, Miller AR, Xie MS, Dong YQ, Kong QQ, Wu LJ, Zhang FZ, et al. Mini-nutrition assessment, malnutrition, and postoperative complications in elderly Chinese patients with lung cancer. J BUON. 2012;17(2):323–6.
Conway JM, Irwin ML, Ainsworth BE. Estimating energy expenditure from the Minnesota Leisure Time Physical Activity and Tecumseh Occupational Activity questionnaires - a doubly labeled water validation. J Clin Epidemiol. 2002;55(4):392–9.
Pourmotabbed A, Ghaedi E, Babaei A, Mohammadi H, Khazaie H, Jalili C, Symonds ME, Moradi S, Miraghajani M: Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep Breath 2020, 24(4):1267–1278.
Nakakubo S, Doi T, Tsutsumimoto K, Kurita S, Ishii H, Shimada H. Sleep duration and progression to sarcopenia in Japanese community-dwelling older adults: a 4 year longitudinal study. J Cachexia Sarcopenia Muscle. 2021;12(4):1034–41.
Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med. 2014;15(12):1456–62.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36.
Brady EM, Bodicoat DH, Hall AP, Khunti K, Yates T, Edwardson C, Davies MJ. Sleep duration, obesity and insulin resistance in a multi-ethnic UK population at high risk of diabetes. Diabetes Res Clin Pract. 2018;139:195–202.
Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4.
Lam CS, Chen MH, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, Safa R, Smith HM, Peng X, Sawyer DB, et al. Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. 2010;30(7):1479–84.
Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res Rev. 2015;23(Pt B):210–20.
Wang T. Searching for the link between inflammaging and sarcopenia. Ageing Res Rev. 2022;77:101611.
Chiba I, Lee S, Bae S, Makino K, Shinkai Y, Katayama O, Harada K, Takayanagi N, Shimada H. Difference in sarcopenia characteristics associated with physical activity and disability incidences in older adults. J Cachexia Sarcopenia Muscle. 2021;12(6):1983–94.
Fox KR, Hillsdon M. Physical activity and obesity. Obes Rev. 2007;8(Suppl 1):115–21.
Bellavia A, Akerstedt T, Bottai M, Wolk A, Orsini N. Sleep duration and survival percentiles across categories of physical activity. Am J Epidemiol. 2014;179(4):484–91.
Savin KL, Patel SR, Clark TL, Bravin JI, Roesch SC, Sotres-Alvarez D, Mossavar-Rahmani Y, Evenson KR, Daviglus M, Ramos AR, et al. Relationships of Sleep Duration, Midpoint, and Variability with Physical Activity in the HCHS/SOL Sueno Ancillary Study. Behav Sleep Med. 2021;19(5):577–88.
Pettee Gabriel K, Sternfeld B, Shiroma EJ, Perez A, Cheung J, Lee IM. Bidirectional associations of accelerometer-determined sedentary behavior and physical activity with reported time in bed: Women’s Health Study. Sleep Health. 2017;3(1):49–55.
Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med Rev. 2018;40:127–34.
Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms. 2015;30(2):84–94.
Li Y, Ma J, Yao K, Su W, Tan B, Wu X, Huang X, Li T, Yin Y, Tosini G, et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J Pineal Res. 2020;69(3):e12682.
Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012;44(9):1663–70.
Choi Y, Cho J, No MH, Heo JW, Cho EJ, Chang E, Park DH, Kang JH, Kwak HB. Re-Setting the Circadian Clock Using Exercise against Sarcopenia. Int J Mol Sci 2020, 21(9).
Gabriel BM, Zierath JR. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019;15(4):197–206.
Acknowledgements
We thank all the participants for their contribution in the WCHAT study.
Funding
This work was founded by National Key R&D Program of China (2020YFC2005600, 2020YFC2005602 and 2017YFC0840101); “Chengdu Science and Technology Bureau Major Science and Technology Application Demonstration Project” (2019YF0900083SN); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University Z2021JC003.
Author information
Authors and Affiliations
Contributions
MY formulated the research question, designed the study, analyzed the data, and drafted the paper. MY, YZ, WZ, MG, SJ, XS designed the study, analyzed the data, and revised the paper. BD assisted with formulating the research question, interpretation of data, supervising the quality of the paper. All authors reviewed, provided feedback to, and confirmed the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The WCHAT study was approved by the Ethics Committee of West China Hospital, Sichuan University (reference: 2017–445) and was conducted under the guidance of Declaration of Helsinki. All participants gave a written informed consent before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, M., Zhang, Y., Zhao, Wy. et al. Association of sleep duration with sarcopenic obesity in multi-ethnic older adults: findings from the WCHAT Study. BMC Geriatr 22, 899 (2022). https://doi.org/10.1186/s12877-022-03543-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03543-0