Abstract
Background
Sarcopenia is a common cause of disability in the aging population, and managing sarcopenia is an important step in building intrinsic capacity and promoting healthy aging. A growing body of evidence suggests that sleep deprivation may be a mediator of the development of sarcopenia. The purpose of this study was to explore the longitudinal association between sleep duration and possible sarcopenia using data from a national sample.
Methods
Two waves of data from the CHARLS database for 2011 and 2015 were used in this study. All possible sarcopenia participants met the Asia Working Group for Sarcopenia 2019 (AWGS 2019) diagnostic criteria. Sleep duration was assessed using a self-report questionnaire, and sleep duration was categorized as short (≤ 6 h), medium (6–8 h), or long (> 8 h) based on previous studies. Longitudinal associations between sleep duration and possible sarcopenia will be calculated by univariate and multifactorial logistic regression analyses and expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
Results
A total of 5654 individuals participated in the follow-up study, with a prevalence of possible sarcopenia of 53.72% (578) in the short sleep duration group, 38.29% (412) in the medium sleep duration group, and 7.99% (86) in the long sleep duration group. According to the crude model of the second-wave follow-up study, short sleep durations were significantly more strongly associated with possible sarcopenia than were medium and long sleep durations (OR: 1.35, 95% CI: 1.17–1.55, P = 0.000). The association between short sleep duration and possible sarcopenia was maintained even after adjustment for covariates such as age, gender, residence, education level, BMI, smoking status, alcohol consumption and comorbidities (OR: 1.18, 95% CI: 1.02–1.36, P = 0.029). In the subgroup analysis, short sleep duration was associated with low grip strength (OR: 1.20, 95% CI: 1.02–1.41, P = 0.031).
Conclusions
Sleep deprivation may be closely associated with the development of possible sarcopenia in middle-aged and elderly people, which provides new insights and ideas for sarcopenia intervention, and further studies are needed to reveal the underlying mechanisms involved.
Similar content being viewed by others
Introduction
The global population is aging rapidly due to increasing life expectancy and declining fertility [1]. The rapid aging of the population has led to a significant increase in the total number of people with care needs in the future [2]. Sarcopenia is a common cause of caregiving burden in the aging population [3], and managing sarcopenia is an important step in building the intrinsic capacity to facilitate a disease-centered to function-centered paradigm shift and achieve healthy aging [4, 5].
Sarcopenia is a complex syndrome of aging characterized by low muscle mass, muscle strength or physical performance [6]0.2018 The European Working Group on Sarcopenia updated the definition of sarcopenia and highlighted muscle strength as the main determinant [7]. Muscle strength mostly peaks in young and early adulthood (18–24 years), is maintained during midlife (25–44 years), and declines from midlife (45 + years) [8]. It is estimated that sarcopenia affects 10–16% of older adults worldwide [9]. Changes in muscle structure associated with increased age may be a key driver of a range of adverse health outcomes in older adults, such as falls, fractures, cognitive decline, and death [9, 10]. To identify those at risk of sarcopenia early and provide timely management, the Asian Working Group for Sarcopenia (AWGS) 2019 consensus introduced the concept of “possible sarcopenia” [11]. Loss of muscle mass or poor physical function that can be assessed with low-cost, easily applied techniques for community screening and clinical practice is referred to as possible sarcopenia [11]. This idea was created to enhance patients’ quality of life and assist in more effectively managing the danger of sarcopenia. Therefore, active exploration of the various risk factors contributing to the burden of sarcopenia should be a priority health matter.
The structure and continuity of sleep in middle-aged and older adults change with age [12, 13]. 50% of older adults complain of sleep disturbances [14]. Inadequate or excessive sleep duration is associated with adverse health outcomes in older adults, such as dementia, diabetes, cardiovascular disease, coronary heart disease, obesity, and death [15,16,17]. Several previous studies have linked short sleep duration and poor sleep quality to multiple dimensions of skeletal muscle damage [18,19,20]. Recent reports have also shown that imbalances in sleep homeostasis can also cause a decrease in growth hormone and testosterone production and may increase cortisol levels and the risk of insulin resistance, which can affect muscle protein synthesis and degradation pathways [21, 22]. In addition, sleep deprivation can cause disturbances in circadian rhythms, leading to imbalances in skeletal muscle metabolism [23].
A growing body of evidence supports the possibility that sleep disorders may be mediators of sarcopenia development. The concept of “possible sarcopenia” is relatively new, and its diagnostic criteria are less stringent than those for sarcopenia. Introducing “possible sarcopenia” to explore its association with sleep duration may help older people prevent and manage the risk of this condition in a more timely manner. However, the role of the association between sleep duration and possible sarcopenia is currently controversial and lacks support from a nationally representative sample, and validating this relationship could help establish good sleep models and provide effective intervention strategies beyond nutritional and exercise interventions. We hypothesize that sleep duration has a U-shaped relationship with possible sarcopenia, and we will verify this hypothesis in a retrospective longitudinal study using the CHARLS database.
Methods
Study population
The China Health and Retirement Longitudinal Study (CHARLS) aims to collect a set of high-quality microdata representing Chinese households and individuals aged 45 and above, to analyze the aging of China’s population, to promote interdisciplinary research on aging and to provide a more scientific basis for the formulation and improvement of relevant policies in China. The CHARLS national baseline survey was conducted in 2011, covering 150 county-level units, 450 village-level units, and 17,000 people in 10,000 households in 28 provinces, and these samples will be followed up every two to three years. The project uses a multistage sampling method proportional to population size (PPS). PPS sampling is also known as probability sampling proportional to size or probability proportional to volume sampling. The sampling principle of PPS is that the unequal probability of phases is exchanged for equal probabilities of the final and overall phases and covers basic personal information, household structure, health status, physical measurements, health service utilization and health insurance, basic community conditions, etc. To date, CHARLS has completed four waves of follow-up data collection, and detailed information about the CHARLS database has been published [24]. The project has also been approved by the Biomedical Ethics Committee of Peking University (IRB00001052-11015; IRB00001052-11014), and the data are available on the CHARLS website (http://charls.pku.edu.cn/index.htm).
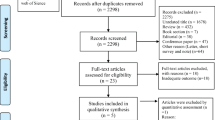
This study proposed using the first wave of 2011 data and the third wave of 2015 data from the CHALRS database as survey respondents. A total of 17,707 individuals participated in the 2011 baseline survey, and we excluded 10,051 individuals because of (1) missing or abnormal data; (2) age < 45 years; or (3) having memory disorders (dementia, Parkinson’s, cerebral atrophy), brain damage/intellectual deficits, a history of stroke, or psychiatric/psychological problems; (4) missing covariates; (5) diagnosis of sarcopenia; a total of 7656 individuals participated in the first wave of the baseline analysis. In the longitudinal study, we excluded individuals who were lost to follow-up or died in wave 3 and lacked information on the diagnosis of possible sarcopenia; ultimately, we screened 5654 eligible participants. The details are shown in the images below (Fig. 1). It is important to note that some data such as height and weight have unit conversion problems and will be corrected after consulting the CHARLS forum. To ensure the authenticity and rigor of the data, data that cannot be corrected will be excluded, and these data will be classified as abnormal. Some abnormal grip strength values (such as 311 kg, 500 kg, etc.) were treated as missing values when comparing the other three groups and other years of measurement data.
Assessment of possible sarcopenia
According to the relevant definition [7, 11], this study proposes to examine possible individuals with sarcopenia according to the AWGS 2019 [11] definition, i.e., meeting the criteria of low muscle strength (M < 28 kg, F < 18 kg) or poor physical performance (five sit-to-stand tests: ≥12 s). Participants were asked to squeeze a mechanical dynamometer (LeapfrogTM WL-1000, Nantong, China) as hard as possible [25]. Participants first stood, held the dynamometer at a right angle, and squeezed the handle for a few seconds. Each hand was tested twice, and the maximum grip force was taken for diagnosis. If, for some reason, it was not possible to measure one hand of the participant or if the grip strength value was abnormal, the maximum value was recorded for the other hand. Physical performance was measured by 5 repetitions of standing up from the chair with the respondent sitting with both arms crossed over the chest on a standard stool equipped with the program. The total time spent standing up straight and sitting down five times as fast as you can, without using your hands, was recorded [23].
Sleep duration
The duration of nighttime sleep was mainly based on the subjective recollection of the participants, and the questionnaire was used to ask “In the past month, how many hours did you actually fall asleep each night on average? (probably shorter than the time you spent lying in bed)”, and then, based on the participants’ answers, the sleep duration was divided into three categories: short sleep duration (≤ 6 h), medium sleep duration (6–8 h), and long sleep duration (> 8 h) [26].
Covariates
Based on a previous study [27, 28], we considered sociodemographic characteristics and health-related factors in our analysis. Sociodemographic characteristics included age, sex, area of residence (rural or urban), and educational level (< 9 and ≥ 9), and the participants were divided into two groups using junior high school graduation as the cutoff. Health-related factors included body mass index (BMI), ever/current smoking status, ever/current alcohol consumption, and five common comorbidities closely associated with sarcopenia (hypertension, hyperlipidemia, hyperglycemia, respiratory disease, and heart disease) [29, 30]. BMI was defined as weight (unit: kg) divided by the square of the height (unit: m). A BMI < 18.5 kg/m2 is considered underweight, 18.5–23.9 kg/m2 is considered normal weight, and ≥ 24 kg/m2 is considered overweight [31].
Statistical analysis
All analyses will be performed using Stata 17.0 (https://www.stata.com/). This study analyzed wave 1 data from 2011 to determine the baseline characteristics of the different sleep time groups at that time point and to follow up with the same study population after screening for possible sarcopenia, using wave 3 data from 2015 for the same measures. ANOVA and chi-square tests were used to assess the underlying characteristics of the wave 1 data, with categorical variables expressed as frequencies and percentages and quantitative variables expressed as means and standard deviations. Univariate and multivariate logistic regressions were used to analyze the correlation between sleep duration and sarcopenia in the third wave of the data. The sleep duration was subjected to crude and adjusted models as independent variables, where Model 1 included age (≥ 65), sex (female), residence (rural), and education level (< 9 years); Model 2 included Model 1, ever/current smoking status, ever/current alcohol consumption, BMI, hypertension status, hyperlipidemia status, hyperglycemia status, respiratory disease status, and heart disease; and Sarcopenia (absent and probable) was used as the dependent variable. In addition, each subcomponent of the second wave data was submitted separately to the same model as the dependent variable. Following the same screening process, 7776 individuals with low grip strength and 5834 individuals with poor physical performance ultimately participated in the follow-up analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The significance level of the statistical tests was set as p < 0.05 (two tailed).
Results
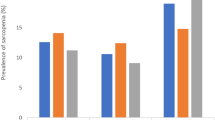
In the first wave of data, a total of 7656 individuals were categorized as having nonsarcopenia. Among them, 3697 (48.29%) individuals had a short sleep duration, 3362 (43.91%) individuals had a medium sleep duration, and 597 (7.80%) individuals had a long sleep duration (Table 1). During follow-up, 2001 individuals were lost or died, as well as 1 individual lacking information for the diagnosis of sarcopenia, leaving 5654 individuals who participated in the first and third waves of the follow-up study. The prevalence of possible sarcopenia was 53.72% (578) in the short sleep duration group, 38.29% (412) in the normal sleep duration group, and 7.99% (86) in the long sleep duration group, for an overall sarcopenia incidence of 19.03% (1076). The prevalence of sarcopenia was relatively greater in the short sleep duration group. (Fig. 2)
According to the univariate logistic regression analysis, compared with moderate and long sleep durations, short sleep durations were more strongly associated with the development of possible sarcopenia (P = 0.000) (Table 2, crude model). After adjustment for Model 1 and Model 2, the association between short sleep duration and possible sarcopenia was maintained (P = 0.029); however, no association was found between long sleep duration and possible sarcopenia. Furthermore, among the covariates, older age, rural residence, low education level, and underweight status were more likely to be associated with possible sarcopenia, and comorbidities in the sarcopenia group were significantly different (P < 0.05), as were hypertension, hyperlipidemia, and hyperglycemia.
There may be changes in sleep duration at 4-year intervals, so we divided sleep duration into stable, slightly stable (spanning 1 group), and unstable (spanning 2 groups) according to whether there was an increase or decrease in sleep duration across the groups. Eventually, there were 3273, 2098 and 283 people in the three groups mentioned above, and after adjusting for confounders, neither the slightly stable group (OR: 1.10, 95% CI: 0.95–1.28, P = 0.194) nor the unstable group (OR: 1.25, 95% CI: 0.91–1.71, P = 0.165) was associated with sarcopenia; i.e., changes in sleep duration in this study did not affect the results. To further validate this conclusion, we reran the analysis after excluding the change group, and the results showed that short sleep duration was still strongly associated with sarcopenia even after adjusting for confounders (OR: 1.25, 95% CI: 1.02–1.52, P = 0.031). This result strongly supports the reliability of the findings of this study.
In the subgroup analysis, a total of 7776 participants were followed up in the grip strength group, and 5834 participants were followed up in the 5 chair stand test group. According to the crude model, short sleep duration was significantly associated with both low grip strength and poor physical performance (OR: 1.41, 95% CI: 1.21–1.65, P = 0.000 and OR: 1.31, 95% CI: 1.12–1.55, P = 0.001), while long sleep duration was only associated with low grip strength (OR: 1.41, 95% CI: 1.09–1.81, P = 0.009). However, in the logistic regression model, short sleep duration was only associated with low grip strength (P = 0.031) and not with poor physical performance. A long sleep duration was not associated with any of the components (Table 3). We also examined the effect of sleep duration on grip strength and performance in the 5 chair stand test cohort during follow-up, revealing that neither the slightly stable group (OR: 1.00, 95% CI: 0.86–1.17, P = 0.962; OR: 1.00, 95% CI: 0.84–1.17, P = 0.953) nor the unstable group (OR: 1.36, 95% CI: 0.99–1.86, P = 0.056; OR: 1.40, 95% CI: 1.00–1.98, P = 0.052) had a significant impact on the results.
Discussion
Our results suggest that short sleep duration is associated with possible sarcopenia in middle-aged and older adults. This relationship was maintained even after adjustment for sociodemographic characteristics and health-related factors. In addition, we found that short sleep duration was significantly associated with low grip strength.
The results of the present study suggest that short sleep duration is associated with sarcopenia in middle-aged and older adults, which is inconsistent with the findings of Korean and Japanese studies [28, 32], which concluded that long sleep duration was associated with sarcopenia, but the ages of the subjects in these two studies were not the same—20 years or older and 65 years or older—and the range of classifications for sleep duration differed. However, several previous cross-sectional studies have shown a U-shaped relationship between sleep duration and sarcopenia, i.e., either short or long sleep duration may be a risk factor for sarcopenia [33, 34]. Therefore, there is an inconsistent association between sleep duration and sarcopenia observed in the current study. Although the present study revealed a strong association between only short sleep duration and sarcopenia, a meta-analysis showed that the prevalence of sarcopenia was greater in those who were sleep deprived [20] and that older women with insomnia, low sleep quality and short sleep duration were more likely to be frail [35]. Moreover, there is evidence that short sleep duration is associated with impairment in multiple dimensions of sarcopenia, such as low muscle mass and muscle strength [18, 19, 36], and these findings were confirmed in our study.
One-third of one’s life is spent in sleep, and sleep is essential for maintaining a normal and healthy life. The changing lifestyles of the 21st century have made “sleep deprivation” an epidemic, with sleep deprivation weakening productivity levels, increasing the risk of death, and taking a heavy toll on modern economies [37]. The mechanisms associated with sleep deprivation and sarcopenia are not fully understood. Sleep deprivation may lead to circadian rhythm disorders [23], increased insulin resistance [38], increased plasma cortisol levels, decreased testosterone levels, and even reduced myogenic fibronectin synthesis, muscle weight and muscle fiber cross-sectional area [21, 22, 39], thereby affecting muscle protein homeostasis. Recent evidence suggests that shorter sleep also leads to increased gut proinflammatory bacteria [40] and induces chronic systemic hypoinflammation [41], followed by a decrease in insulin sensitivity via the gut-muscle axis, causing reduced skeletal muscle insulin sensitivity, activation of ubiquitin proteases and the autophagic lysosomal system [42], ultimately leading to decreased muscle mass and loss of muscle function. In addition, individuals who are short sleepers are unlikely to meet the normal activity recommendations [43]. Conversely, exercise may be a practical and effective means to mitigate sleep deprivation-induced mitochondrial dysfunction, insulin resistance, sarcoplasmic protein synthesis, and circadian rhythm changes [44]. Thus, exercise and sleep complement each other, and the combination of good sleep patterns and exercise may be a new and effective intervention strategy for improving sarcopenia.
The strengths of this study are the high quality of a longitudinal study based on data from a national Chinese sample to explain the causal relationship between sleep duration and sarcopenia and the representative sample of the CHARLS project using the PPS random sampling method. In addition, this study considered the effect of changes in sleep duration during the follow-up period on the results. This study also has several limitations. First, because the CHARLS database lacks a measure of muscle mass, this study utilizes a possible definition of sarcopenia from the 2019 AWGS, proposed for early identification of sarcopenia in primary care settings and communities, so the prevalence of sarcopenia in the sample may be greater than that identified for sarcopenia [45]. In addition, the CHARLS collects information on their spouse and other family members, and questionnaire burden may also affect the quality of responses. Second, we assessed sleep duration using a self-administered questionnaire from the CHARLS database. This has been reported to cause inconsistencies in objectively recorded sleep duration, leading to the possibility of false negatives and false positives [46]. Recall bias is also inevitable. Third, we did not consider the effect of sleep quality or daily variation in sleep duration on the results. Finally, we failed to address other covariates of sleep duration associated with sarcopenia, such as physical activity, medications, depression, and other disorders. We recommend using tools such as smart bracelets to measure objective sleep duration and DXA/BIA body composition analyzers to assess subjects with identified sarcopenia to reduce error.
Conclusion
In conclusion, our longitudinal study concluded that short sleep duration was associated with possible sarcopenia in middle-aged and older adults, and more prospective studies with larger sample sizes are needed to confirm and further explore the underlying biological mechanisms involved. We recommend considering the effect of sleep parameters when performing sarcopenia prevention and treatment.
Data availability
The datasets generated and/or analyzed during the current study are available in the [CHARLS] repository. The URL is http://charls.pku.edu.cn/index.htm. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1160–203. https://doi.org/10.1016/S0140-6736(20)30977-6.
Gong J, Wang G, Wang Y et al. Nowcasting and forecasting the care needs of the older population in China: analysis of data from the China Health and Retirement Longitudinal Study (CHARLS) [published correction appears in Lancet Public Health. 2023;8(2):e98]. Lancet Public Health. 2022;7(12):e1005-e1013. https://doi.org/10.1016/S2468-2667(22)00203-1.
Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol Biol Sci Med Sci. 2013;68(11):1379–86. https://doi.org/10.1093/gerona/glt089.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia [published correction appears in Lancet. 2019;393(10191):2590]. Lancet. 2019;393(10191):2636–2646. https://doi.org/10.1016/S0140-6736(19)31138-9.
Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol Biol Sci Med Sci. 2018;73(12):1653–60. https://doi.org/10.1093/gerona/gly011.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older people. Age Ageing. 2010;39(4):412–23. https://doi.org/10.1093/ageing/afq034.
Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis [published correction appears in Age Ageing. 2019;48(4):601]. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169.
Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE. 2014;9(12):e113637. https://doi.org/10.1371/journal.pone.0113637. Published 2014 Dec 4.
Yuan S, Larsson SC. Epidemiology of Sarcopenia: prevalence, risk factors, and consequences [published online ahead of print, 2023 Mar 11]. Metabolism. 2023;155533. https://doi.org/10.1016/j.metabol.2023.155533.
Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;9(21):7964–78. https://doi.org/10.1002/cam4.3428.
Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. https://doi.org/10.1016/j.jamda.2011.01.003.
Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–73. https://doi.org/10.1093/sleep/27.7.1255.
Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15(4):401–9. https://doi.org/10.1016/j.sleep.2013.11.791.
Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56(2):181–9. https://doi.org/10.1159/000236900.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. https://doi.org/10.1016/j.smrv.2017.06.011.
Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. https://doi.org/10.1016/j.sleep.2016.08.006.
Li M, Wang N, Dupre ME. Association between the self-reported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord. 2022;304:20–7. https://doi.org/10.1016/j.jad.2022.02.039.
Fu L, Yu X, Zhang W, et al. The Relationship between Sleep Duration, Falls, and muscle Mass: a Cohort Study in an Elderly Chinese Population. Rejuvenation Res. 2019;22(5):390–8. https://doi.org/10.1089/rej.2018.2102.
Pana A, Sourtzi P, Kalokairinou A, Pastroudis A, Chatzopoulos ST, Velonaki VS. Association between muscle strength and sleep quality and duration among middle-aged and older adults: a systematic review. Eur Geriatr Med. 2021;12(1):27–44. https://doi.org/10.1007/s41999-020-00399-8.
Rubio-Arias JÁ, Rodríguez-Fernández R, Andreu L, Martínez-Aranda LM, Martínez-Rodriguez A, Ramos-Campo DJ. Effect of Sleep Quality on the prevalence of Sarcopenia in older adults: a systematic review with Meta-analysis. J Clin Med. 2019;8(12):2156. https://doi.org/10.3390/jcm8122156. Published 2019 Dec 6.
Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related Sarcopenia: possible connections and clinical implications. Ageing Res Rev. 2015;23(Pt B):210–20. https://doi.org/10.1016/j.arr.2015.07.003.
Dattilo M, Antunes HK, Medeiros A, et al. Paradoxical sleep deprivation induces muscle atrophy. Muscle Nerve. 2012;45(3):431–3. https://doi.org/10.1002/mus.22322.
Morrison M, Halson SL, Weakley J, Hawley JA. Sleep, circadian biology and skeletal muscle interactions: implications for metabolic health. Sleep Med Rev. 2022;66:101700. https://doi.org/10.1016/j.smrv.2022.101700.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. https://doi.org/10.1093/ije/dys203.
Zhang F, Yu Y, Wang H, et al. Association between handgrip strength and depression among Chinese older adults: a cross-sectional study from the China Health and Retirement Longitudinal Study. BMC Geriatr. 2023;23(1):299. https://doi.org/10.1186/s12877-023-04034-6.
Hu K, Li W, Zhang Y, et al. Association between outdoor artificial light at night and sleep duration among older adults in China: a cross-sectional study. Environ Res. 2022;212(Pt B):113343. https://doi.org/10.1016/j.envres.2022.113343.
Smith L, Shin JI, Veronese N, et al. Sleep duration and sarcopenia in adults aged ≥ 65 years from low and middle-income countries. Aging Clin Exp Res. 2022;34(7):1573–81. https://doi.org/10.1007/s40520-022-02074-3.
Nakakubo S, Doi T, Tsutsumimoto K, Kurita S, Ishii H, Shimada H. Sleep duration and progression to Sarcopenia in Japanese community-dwelling older adults: a 4 year longitudinal study. J Cachexia Sarcopenia Muscle. 2021;12(4):1034–41. https://doi.org/10.1002/jcsm.12735.
Gao Q, Hu K, Yan C, et al. Associated factors of Sarcopenia in Community-Dwelling older adults: a systematic review and Meta-analysis. Nutrients. 2021;13(12):4291. https://doi.org/10.3390/nu13124291. Published 2021 Nov 27.
Li CW, Yu K, Shyh-Chang N, et al. Pathogenesis of Sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781–94. https://doi.org/10.1002/jcsm.12901.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Kwon YJ, Jang SY, Park EC, Cho AR, Shim JY, Linton JA. Long sleep duration is Associated with Sarcopenia in Korean adults based on data from the 2008–2011 KNHANES. J Clin Sleep Med. 2017;13(9):1097–104. https://doi.org/10.5664/jcsm.6732. Published 2017 Sep 15.
Chien MY, Wang LY, Chen HC. The relationship of Sleep duration with obesity and Sarcopenia in Community-Dwelling older adults. Gerontology. 2015;61(5):399–406. https://doi.org/10.1159/000371847.
Pourmotabbed A, Ghaedi E, Babaei A, et al. Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep Breath. 2020;24(4):1267–78. https://doi.org/10.1007/s11325-019-01965-6.
Moreno-Tamayo K, Manrique-Espinoza B, Ortiz-Barrios LB, Cárdenas-Bahena Á, Ramírez-García E, Sánchez-García S. Insomnia, low sleep quality, and sleeping little are associated with frailty in Mexican women. Maturitas. 2020;136:7–12. https://doi.org/10.1016/j.maturitas.2020.03.005.
Denison HJ, Jameson KA, Sayer AA, et al. Poor sleep quality and physical performance in older adults. Sleep Health. 2021;7(2):205–11. https://doi.org/10.1016/j.sleh.2020.10.002.
Hafner M, Stepanek M, Taylor J, Troxel WM, van Stolk C. Why sleep matters-the economic costs of Insufficient Sleep: a Cross-country comparative analysis. Rand Health Q. 2017;6(4):11. Published 2017 Jan 1.
VanHelder T, Radomski MW. Sleep deprivation and the effect on exercise performance. Sports Med. 1989;7(4):235–47. https://doi.org/10.2165/00007256-198907040-00002.
Saner NJ, Lee MJ, Pitchford NW, et al. The effect of sleep restriction, with or without high-intensity interval exercise, on myofibrillar protein synthesis in healthy young men. J Physiol. 2020;598(8):1523–36. https://doi.org/10.1113/JP278828.
Morwani-Mangnani J, Giannos P, Belzer C, Beekman M, Eline Slagboom P, Prokopidis K. Gut microbiome changes due to sleep disruption in older and younger individuals: a case for Sarcopenia? Sleep. 2022;45(12):zsac239. https://doi.org/10.1093/sleep/zsac239.
Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin–proteasome and autophagy–lysosome pathways. FASEB J. 2011;25(1):99–110. https://doi.org/10.1096/fj.10-164152.
Štefan L, Vrgoč G, Rupčić T, Sporiš G, Sekulić D. Sleep duration and Sleep Quality are Associated with Physical Activity in Elderly people living in nursing homes. Int J Environ Res Public Health. 2018;15(11):2512. https://doi.org/10.3390/ijerph15112512. Published 2018 Nov 9.
Saner NJ, Lee MJ, Kuang J, et al. Exercise mitigates sleep-loss-induced changes in glucose tolerance, mitochondrial function, sarcoplasmic protein synthesis, and diurnal rhythms. Mol Metab. 2021;43:101110. https://doi.org/10.1016/j.molmet.2020.101110.
Shin HE, Kim M, Won CW. Differences in characteristics between older adults meeting criteria for Sarcopenia and possible Sarcopenia: from research to primary care. Int J Environ Res Public Health. 2022;19(7):4312. https://doi.org/10.3390/ijerph19074312. Published 2022 Apr 4.
Miner B, Stone KL, Zeitzer JM, et al. Self-reported and actigraphic short sleep duration in older adults. J Clin Sleep Med. 2022;18(2):403–13. https://doi.org/10.5664/jcsm.9584.
Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–7. https://doi.org/10.1016/j.bbi.2009.06.001.
Funding
This research was supported by the Dongguan Social Science and Technology Development Project (No: 20211800904952), the Key Project of Guangzhou Science and Technology Program, China (No: 202206010142), the Science and Technology Planning Project of Guangdong Province, China (No: 2023B1212060018) and the Guangdong Basic and Applied Basic Research Foundation (No: 2021B1515140026).
Author information
Authors and Affiliations
Contributions
Linfeng Chen: Formal analysis, Writing - original draft, Writing - review & editing. Qingyun Li: Validation, Writing - review & editing. Xiaoyun Huang: Conceptualization, Formal analysis, Supervision, Writing - original draft, Writing - review & editing, Funding acquisition. Zhong Li: Conceptualization, Formal analysis, Supervision, Writing - original draft, Writing - review & editing, Funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study did not include any animal or human experiments. This study was approved by the Biomedical Ethics Council of Beijing University. The IRB approval number for the main household survey, including anthropometrics, is IRB00001052-11015; the IRB approval number for biomarker collection is IRB00001052-11014.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Li, Q., Huang, X. et al. Association between sleep duration and possible sarcopenia in middle-aged and elderly Chinese individuals: evidence from the China health and retirement longitudinal study. BMC Geriatr 24, 594 (2024). https://doi.org/10.1186/s12877-024-05168-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05168-x