Abstract
Objectives
The relationship between the number of teeth and sarcopenia remains poorly investigated. Although nutrition plays an important role in maintaining bone and muscle health, the complex relationship between number of teeth and nutrition in the pathogenesis of sarcopenia remains to be elucidated.
Methods
A large multi-ethnic sample of 4149 participants aged over 50 years old from West China Health and Aging Trend (WCHAT) study was analyzed. We examined the associations between number of teeth with nutritional status and sarcopenia, and the mediating role of nutrition in the association between number of teeth and sarcopenia. Sarcopenia was defined according to the Asian Working Group for Sarcopenia 2019. We assessed nutrition using Mini Nutrition Assessment-Short Form (MNA-SF) scale. Direct relationships between number of teeth, nutrition and sarcopenia were assessed using multiple linear regression. Mediation models and structural equation model (SEM) pathway analysis were used to test the mediating role of nutrition in the relationship between number of teeth and sarcopenia.
Results
Of 4149 participants aged 50 years old or older, the prevalence of sarcopenia was 22.5, 9.0% for moderate sarcopenia, and 13.5% for severe sarcopenia, respectively. Regression analysis indicated a total association between number of teeth (β = − 0.327, 95% CI − 0.471 to − 0.237, p < 0.001) and sarcopenia. After adjusted MNA-SF scores, the association between number of teeth and sarcopenia was still significant (β = − 0.269, 95% CI − 0.364 to − 0.175, p < 0.001), indicating a partial mediation effect of nutrition. Mediation analysis verified nutrition partially mediate the associations between number of teeth and sarcopenia (indirect effect estimate = − 0.0272, bootstrap 95% CI − 0.0324 to − 0.0222; direct effect estimate = − 0.0899, bootstrap 95% CI − 0.1049 to − 0.0738). And this mediation effect was through impacting SMI (indirect effect estimate = − 0.0283, bootstrap 95% CI − 0.0336 to − 0.0232) and grip strength (indirect effect estimate = − 0.0067, bootstrap 95% CI − 0.0094 to − 0.0043). Structural equation model (SEM) framework pathway analysis confirmed the association between number of teeth, nutrition, and sarcopenia.
Conclusions
Our findings indicated that sarcopenia was associated with number of teeth and poorer nutritional status, with nutrition partially mediating the association between number of teeth and sarcopenia. Our findings supported early nutritional assessment and intervention in oral health to mitigate the risk of sarcopenia.
Similar content being viewed by others
Introduction
The world is facing big challenges of an aging population. It was estimated that a large population of 703 million people were over 65 years old in 2019 and that this population would reach 1.5 billion by 2050 globally [1]. China is an aging country right now with a large elderly population. During the process of ageing, sarcopenia is an urgent problem that needs to be solved. Sarcopenia is characterized by the gradual loss of skeletal muscle mass and function with age and was established as a disease in ICD-10-CM (M62.84) [2]. The prevalence of sarcopenia varies widely among studies depending on the assessment method and population, with the prevalence of sarcopenia in the elderly community ranges from 9.9 to 40.4% [3, 4]. The mechanism of sarcopenia is complex and includes hormonal changes, nutritional deficiencies, chronic inflammation, neuromuscular function decline, and decreased physical activity [5].
Recently, oral health is attracting more and more attention. Assessment of oral health includes teeth number, chewing ability, articulatory and oral motor skill, tongue pressure, eating and swallowing ability [6]. These oral health problems could affect the intake of nutritious food and might lead to malnutrition, frailty or sarcopenia finally. Specifically, tooth loss is a common health problem among the elderly. The number of teeth was found be related with handgrip strength, skeletal muscle mass, or sarcopenia in some studies [7,8,9]. Besides, the number of teeth was significantly correlated with chewing function and dietary intake, causing malnutrition [10, 11], leading to muscle loss eventually. This suggested a potential association between number of teeth, nutrition, and sarcopenia. Therefore, in this study, we used mediation analysis to determine if nutrition status mediated the effects of teeth number on sarcopenia.
Method
Study design and data collection
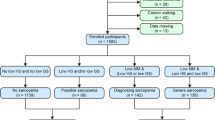
We analyzed the baseline data from the West China Health and Aging Trend (WCHAT) study, which was approved by the Ethical Review Committee of West China Hospital (Committee reference number: 2017(445); Registration number: ChiCTR 1,800,018,895). Previous studies have published details of the questionnaires used to generate this data [12]. Participants aged over 50 years old were enrolled. Before the interview, participants were asked about their willingness to take part in the study and signed the informed consent. Initially, we enrolled 7536 participants. However, only 4500 participants over 50 years old did Bioelectric Impedance Analysis (BIA) analysis. Then 342 participants with missing nutrition information were excluded. Besides, 1 participant was excluded without the information of teeth number. After that, 8 participants were excluded without covariates data. At last, 4149 participants were analyzed in our study (Fig. 1).
Flow chart of study participants. Initially, 7536 participants were enrolled and only 4500 participants did Bioelectric Impedance Analysis (BIA) analysis over 50 years old. Then we kept on excluding 342 subjects without nutrition assessment. Then 1 subject was excluded with missing information of teeth number. After that, 8 subjects were excluded without covariates data. Therefore, 4149 participants were analyzed in our study
We collected demographic characteristics including age, gender, ethnicity, educational levels, and marriage status. Lifestyle characteristics including tea drinking, alcohol drinking, and smoking were collected. Whether living alone and have false teeth were also asked. Participants self-reported medical history of chronic diseases including hypertension, osteoarticular disease, coronary heart disease, lung disease, diabetes, tumors, and others. Cognitive function was assessed using a 10-item Short Portable Mental Status Questionnaire (SPMSQ) [13]. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). And scores > 5 are considered as poor self-reported sleep quality. Depression and anxiety were evaluated using the 15-item Geriatric Depression Scale (GDS-15) [14] and Generalized Anxiety Disorder (GAD-7) [15] questionnaire, separately. And the scores ≥5 were considered as having depression or anxiety in the evaluation. Nutrition status was graded using the Mini Nutrition Assessment-Short Form (MNA-SF) scale. And MNA-SF scores from 0 ~ 7 indicated malnutrition status, 8 ~ 11 indicated malnutrition risk, 12 ~ 14 indicated good nutrition status [16].
Sarcopenia evaluation
Sarcopenia was diagnosed with the Asian Working Group for Sarcopenia (AWGS) 2019 consensus criteria, defined as low relative appendicular skeletal muscle mass index (SMI) in the presence of either low handgrip strength or slow gait speed using updated cutoffs [17]. Bioelectric Impedance Analysis (BIA) was used to test muscle mass by the INbody770 body composition instrument. The cut-off value of the skeletal muscle mass index (SMI) was 7.0 kg/m [2] in male and 5.7 kg/m [2] in female [17]. Grip strength was measured with a grip dynamometer (EH101; Camry, Zhongshan, China). During the test of grip strength, participants was required to held the grip dynamometer with their dominant hand, keep their feet separated (shoulder-width apart), stand upright, with their arms drop naturally. The test should be repeated twice and the largest value was recorded. The cut-off value of grip strength was 26 kg in male and 18 kg in female. Gait speed was tested using an infrared sensor [18]. During the test, participants wore common shoes and was required to walk 6 m. The cut-off value of gait speed was 1.0 m/s.
Statistical analysis
Statistical analysis was performed by using the R software (version 4.0.2). The characteristics of the population were showed as means±standard deviation (SD) or frequencies. Differences of the variables between the sarcopenia and non-sarcopenia group were analyzed. For continuous variables, one-way analysis of variance (ANOVA) was carried out. For categorical variables, chi squared test was carried out. Associations with a p-value of 0.1 or less in the univariate analysis were selected for the multiple regression analysis, which were used to estimate the odds ratio (OR) to identify associations between number of teeth, MNA-SF scores and sarcopenia after adjusting for potential confounders. A multi-categorical mediation model examined the potential mediating role of nutrition, using total MNA-SF score, in the relationship between number of teeth and sarcopenia. And a pathway analysis was shown in the SEM framework using a SEM package in R version 4.0.2 [19].
Results
Overall, we enrolled 4149 participants (1498 male and 2651 female) aged 50 years old or older in the study. The mean age of the group was 62.4 ± 8.3 years. Table 1 shows descriptive characteristics of the participants according to sarcopenia assessment. 934 participants were found to be sarcopenia and the prevalence of sarcopenia was 22.5%. Subjects with sarcopenia tended to be older and with aging increasing, the prevalence of sarcopenia increased. It was observed that individuals in the sarcopenia group tended to be male, widowed, has a lower educational level, has smoking history, has cognitive decline and has false teeth (p < 0.001). And subjects with sarcopenia was more likely to live alone (p = 0.0018) and have chronic disease burden (p = 0.013). Besides, the prevalence of sarcopenia was higher in those subjects who were depressed (p = 0.0419) and has sleep disorders (p = 0.0332). Specifically, subjects with sarcopenia has a lower nutrition scores and with the nutrition status decline, the prevalence of sarcopenia was increasing (p < 0.001). Subjects in sarcopenia group has less number of teeth than non-sarcopenia group and with the teeth number decreasing, the higher of the prevalence of sarcopenia (p < 0.001). Specifically, distribution of sarcopenia prevalence with changes in the number of teeth could be seen in Fig. 2, indicated a linear correlation between number of teeth and sarcopenia.
The results of multiple regression analysis in three different models was shown in Table 2. The covariates included gender, age, ethnic group, false teeth status, smoking, educational level, living alone status, marriage status, chronic diseases, depression, sleep disorders and cognitive function were adjusted in all the three models. In model 1, the results showed a significant relationship between number of teeth (β = − 0.327, 95% CI − 0.417 to − 0.237, p < 0.001) and sarcopenia. In Model 2, it showed that after adjusted nutrition scores, the association between number of teeth and sarcopenia was still significant (β = − 0.269, 95% CI − 0.364 to − 0.175, p < 0.001). In model 3, it showed a significant correlation between number of teeth (β = 0.157, 95% CI 0.107 to 0.207, p < 0.001) and nutrition scores.
The relative total, direct and indirect effects for the mediating role of nutrition on the relationship between number of teeth and sarcopenia in mediation models was shown in Table 3. Bootstrapping revealed significant relative indirect effects for sarcopenia (ACME = − 0.0272, 95% CI − 0.0324 to − 0.0222), indicating that nutrition mediated the association between number of teeth and sarcopenia. And after adjusting covariates including gender, age, ethnic group, smoking history, chronic disease status, and cognitive status, the mediation effect was still significant (ACME = − 0.0136, 95% CI − 0.0185 to − 0.0095). And the mediation effect with a proportion of mediation up to 23.24%. The mediation of nutrition in the relationship between number of teeth and the three components of sarcopenia was also analyzed. And this mediation effect was through impacting SMI (indirect effect estimate = − 0.0283, bootstrap 95% CI − 0.0336 to − 0.0232) and grip strength (indirect effect estimate = − 0.0067, bootstrap 95% CI − 0.0094 to − 0.0043). These mediation effects were also shown in Fig. 3. This suggested that nutrition status partially mediated the association between number of teeth and sarcopenia and this mediation effect was through impacting SMI and grip strength.
Mediation effects of nutrition in the relationship between number of teeth with sarcopenia and the three diagnostic components of sarcopenia (gait speed, grip strength, SMI) in an unadjusted model. Nutrition revealed significant relative indirect effects for number of teeth and sarcopenia (ACME = − 0.0272). Nutrition also revealed significant relative indirect effects for SMI (indirect effect estimate = − 0.0283) and grip strength (indirect effect estimate = − 0.0067)
We then performed pathway analysis using the structural equation model (SEM) framework. As shown in Fig. 4, SEM pathway analysis indicated that the association between number of teeth and sarcopenia was negative (SEM co-efficient: − 0.18). And the relationship between number of teeth and MNA-SF scores was positive (SEM co-efficient: 0.11). Besides, the correlation between MNA-SF scores and sarcopenia was negative (SEM coefficient: − 0.28). Moreover, other covariates including age, sex, ethnic groups, smoking history, and cognitive status showed negative estimate coefficients compared to number of teeth and MNA-SF scores. Furthermore, both number of teeth and MNA-SF score showed negative estimate coefficients compared to gait speed, grip strength and SMI. The p value was statistically significant in the entire pathway of the SEM structure model. These results confirmed the relationship between number of teeth, MNA-SF score and sarcopenia.
Path analysis of the nutrition’s mediation effects using the structural equation model (SEM) framework. SEM pathway analysis showed that the correlation between number of teeth and sarcopenia was negative (SEM co-efficient: − 0.18). The correlation between number of teeth and MNA-SF score was positive (SEM co-efficient: 0.11). The correlation between MNA-SF score and sarcopenia was negative (SEM coefficient: − 0.28)
Discussion
As far as we know, this was the first study to evaluate the mediating role of nutrition in the association between number of teeth and sarcopenia. Our study stated that nutrition could partially mediate the effects of teeth loss on sarcopenia in adults over 50 years old and the mediation effect was up to 26.5%. Therefore, better nutrition status could ameliorate negative effects of teeth loss on sarcopenia.
This study calculated the prevalence of sarcopenia using AWGS 2019 diagnostic criteria. The results demonstrated that the prevalence of sarcopenia was 22.5% (moderate sarcopenia: 9.0%; severe sarcopenia: 13.5%), a little higher than other studies. Ye C et al. analyzed data from China Health and Retirement Longitudinal Study which including 14,130 participants aged over 50 years old, showing the prevalence of sarcopenia was 19.8% (moderate sarcopenia: 11.9%; severe sarcopenia: 7.9%) [20]. Another meta-analysis summarized 9 studies and discovered that the prevalence of sarcopenia was 14% among 7656 participants aged over 50 years old living in community [21]. Considering that the participants in our study were recruited from rural areas, most of them had no formal education(n = 1253, 30.2%), which may have been the main reason for the high prevalence of sarcopenia [22]. Consisting with other studies, sarcopenia prevalence in our study was age-related (50-59ys, 12.1%; 60-69ys, 21.7%; 70-79ys, 40.3%; ≥80ys, 68.8%), indicating a dosage effect.
In our study, we found a significant negative association between number of teeth and sarcopenia, after adjusting false teeth status, gender, age, ethnic group, life styles (smoking), educational level, living alone status, marriage status, chronic diseases status, depression, sleep disorders and cognitive status. This was consistent with other studies. One study found elderly patients with less than 20 number of teeth were more likely to have diagnosed sarcopenia (OR 5.79, 95%CI 3.90–8.59) or severe sarcopenia (OR 7.32, 95%CI 4.28–12.52) in adjusted model compared with patients having more than 20 number of teeth [8]. Another study also found that number of teeth was associated with low handgrip strength (OR = 0.961; 95% CI, 0.932–0.992) and possible sarcopenia (OR = 0.949; 95% CI, 0.907–0.992) [7]. Besides, the remaining 0–9 teeth was found to be associated with low grip strength compared to high grip strength in men (OR 1.39, 95% CI 1.03–1.88) [23]. The underling mechanism was as following. Firstly, less number of teeth led to poor chewing ability and dietary intake, thus leading to malnutrition which was a risk factor in sarcopenia [10]. Secondly, elderly who have less number of teeth would less likely to eat meat, nut and other hard or raw food. And this kind of food contain protein, calcium and vitamins, playing an important role in maintaining muscle mass [24]. Thirdly, the number of missing teeth could reflect the cumulative oral inflammatory status which was also a factor in the mechanism of sarcopenia [25]. This meant that number of teeth was an important factor related with sarcopenia with a dosage effect. And sarcopenia prevalence in our study ranging from 15.3% in those individuals with 24–32 teeth to 42.9% in those individuals with 0–8 teeth.
Specifically, we found a partial mediation effect of nutrition in the relationship between number of teeth and sarcopenia. The mediation model and SEM framework pathway analyses showed that the mediation effect of nutrition was high up to 26.5% in adjusted model. There are several plausible explanations for our findings. Firstly, there is a close relationship between number of teeth, nutrition status, and sarcopenia. A recent study has confirmed that improving nutrition and oral health may be an effective way to reduce or delay the occurrence of sarcopenia [8]. Sarcopenia can also impact swallowing related muscle. Secondly, individuals who had less teeth increased the risk of being malnutrition [26]. Elderly who has few or no natural teeth would select a diet that they can chew in comfort. Such diets are low in fruits and vegetables intake with associated reduction in dietary nutrition intake [27]. Conversely, malnourished older adults are likely to have poorer oral function, leading to more teeth loss [28]. Thirdly, oral health can be associated with balance and exercise through neuronal mechanism. And some research had found that dental occlusion influenced postural stability [29]. Nutrition therapy is also essential to improve muscle mass and function in patients with sarcopenia [30]. Therefore, malnutrition promotes teeth loss, which further increases the risk of sarcopenia in the elderly. Use false teeth is important to promote early intervention in nutrition and sarcopenia.
Among the 3 components of sarcopenia (speed, grip strength and SMI), we found the mediation role of nutrition was through impacting grip strength and SMI, not gait speed. This was consistent with previous studies. Kim et al. found that the participants with NRT ≥ 20 had more SMI than those with NRT < 20 in both sexes. And SMI was correlated with number of teeth in men (r = 0.018, p < 0.001) and in women (r = − 0.007, p < 0.001) [31]. Another study found that hopeless teeth and less posterior occlusion was related to a greater risk of low handgrip strength [32]. Furthermore, lack of posterior occlusal support at baseline was found to be independently predicted the incidence of declined gait speed over 3 years [33]. In a word, a longitudinal study was required to confirm our findings.
There are some limitations in this study. Firstly, most participants enrolled in our study were relatively younger and healthy. Secondly, we only analyzed baseline data of the WCHAT study. Thirdly, we did not assess oral health including chewing ability, articulatory oral motor skill, tongue pressure, and swallowing. All of these were potential source of bias. A longitudinal study was required to establish the relationship between number of teeth, nutrition and sarcopenia.
Conclusions
In conclusion, our study demonstrated that number of teeth is negatively associated with sarcopenia and the relationship was partially mediated by nutrition. Our results supported the need for early identification and interventions for malnutrition in at-risk older adults with teeth loss to prevent the downstream cascade of sarcopenia.
Availability of data and materials
The data-set generated and analyzed during the current study will be available from the corresponding author on a reasonable request.
Abbreviations
- WCHAT:
-
West China Health and Aging Trend study
- GDS-15:
-
15-item Geriatric Depression Scale
- SPMSQ:
-
Short Portable Mental Status Questionnaire
- PSQI:
-
Pittsburgh sleep quality index
- SEM:
-
Structural equation model
- MNA-SF:
-
Mini Nutrition Assessment-Short Form scale
- ACME:
-
Average causal mediation effects (indirect effect)
- ADE:
-
Average direct effects
References
Man W, Wang S, Yang H. Exploring the spatial-temporal distribution and evolution of population aging and social-economic indicators in China. BMC Public Health. 2021;21(1):966.
Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–4.
Mayhew AJ, Amog K, Phillips S, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J Nutrition, Health Aging. 2020;24(1):83–90.
Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29(1):11–7.
Tanaka T, Takahashi K, Hirano H, et al. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J Gerontol A Biol Sci Med Sci. 2018;73(12):1661–7.
Abe T, Tominaga K, Ando Y, et al. Number of teeth and masticatory function are associated with sarcopenia and diabetes mellitus status among community-dwelling older adults. A Shimane CoHRE study. 2021;16(6):e0252625.
Cao W, Zhu A, Chu S, et al. Correlation between nutrition, oral health, and different sarcopenia groups among elderly outpatients of community hospitals: a cross-sectional study of 1505 participants in China. BMC Geriatr. 2022;22(1):332.
Lee JH, Lee SY. Relationship between oral health behaviour and handgrip strength: a cross-sectional study with 7589 Korean adults. 2020;78(6):438–44.
Iwasaki M, Taylor GW, Manz MC, et al. Oral health status: relationship to nutrient and food intake among 80-year-old Japanese adults. Comm Dent Oral Epidemiol. 2014;42(5):441–50.
Inomata C, Ikebe K, Kagawa R, et al. Significance of occlusal force for dietary fibre and vitamin intakes in independently living 70-year-old Japanese: from SONIC study. J Dent. 2014;42(5):556–64.
Hou L, Liu X, Zhang Y, et al. Cohort profile: West China health and aging trend (WCHAT). J Nutr Health Aging. 2020.
Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–41.
Wang H, Hai S, Liu Y, et al. [prevalence of sarcopenia and associated factors in community-dwelling elderly populations in Chengdu China]. Sichuan da xue xue bao. Yi xue ban. 2019;50(2):224–8.
Swinson RP. The GAD-7 scale was accurate for diagnosing generalised anxiety disorder. Evidence-based medicine. 2006;11(6):184.
Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol Series A, Biol Sci Med Sci. 2001;56(6):M366–72.
Kim M, Won CW. Sarcopenia in Korean community-dwelling adults aged 70 years and older: application of screening and diagnostic tools from the Asian working Group for Sarcopenia 2019 update. J Am Med Dir Assoc. 2020;21(6):752–8.
Jung HW, Roh HC, Kim SW, Kim S, Kim M, Won CW. Cross-Comparisons of Gait Speeds by Automatic Sensors and a Stopwatch to Provide Converting Formula Between Measuring Modalities. 2019;23(2):71–6.
Cheung MW. metaSEM: an R package for meta-analysis using structural equation modeling. Front Psychol. 2014;5:1521.
Ye C, Zheng X, Aihemaitijiang S, et al. Sarcopenia and catastrophic health expenditure by socio-economic groups in China: an analysis of household-based panel data. 2022.
Almohaisen N, Gittins M, Todd C. Prevalence of Undernutrition, Frailty and Sarcopenia in Community-Dwelling People Aged 50 Years and Above. Systematic Review and Meta-Analysis. 2022;14(8).
Liu X, Hao Q, Hou L, et al. Ethnic groups differences in the prevalence of sarcopenia using the AWGS criteria. J Nutr Health Aging. 2020;24(6):665–71.
Yun J, Lee Y. Association between oral health status and handgrip strength in older Korean adults. Eur Geriatr Med. 2020;11(3):459–64.
Stenman U, Ahlqwist M, Björkelund C, Hakeberg M. Oral health-related quality of life--associations with oral health and conditions in Swedish 70-year-old individuals. Gerodontology. 2012;29(2):e440–6.
Meisel P, Wilke P, Biffar R, Holtfreter B, Wallaschofski H, Kocher T. Total tooth loss and systemic correlates of inflammation: role of obesity. Obesity (Silver Spring, Md). 2012;20(3):644–50.
Zelig R, Goldstein S, Touger-Decker R, et al. Tooth loss and nutritional status in older adults: a systematic review and Meta-analysis. JDR Clin Transl Res. 2022;7(1):4–15.
Walls AW, Steele JG. The relationship between oral health and nutrition in older people. Mech Ageing Dev. 2004;125(12):853–7.
Iwasaki M, Hirano H, Ohara Y, Motokawa K. The association of oral function with dietary intake and nutritional status among older adults: latest evidence from epidemiological studies. Japanese Dental Sci Rev. 2021;57:128–37.
Gangloff P, Louis JP, Perrin PP. Dental occlusion modifies gaze and posture stabilization in human subjects. Neurosci Lett. 2000;293(3):203–6.
Nakahara S, Takasaki M, Abe S, et al. Aggressive nutrition therapy in malnutrition and sarcopenia. Nutrition (Burbank, Los Angeles County, Calif). 2021;84:111109.
Kim S, Min JY, Lee HS, Kwon KR, Yoo J, Won CW. The Association Between the Number of Natural Remaining Teeth and Appendicular Skeletal Muscle Mass in Korean Older Adults. 2018;22(4):194–9.
Kim SH, Che X, Park HJ. Hopeless tooth and less posterior occlusion is related to a greater risk of low handgrip strength: A population-based cross-sectional study. 2021;16(12):e0260927.
Hatta K, Ikebe K, Mihara Y. Lack of posterior occlusal support predicts the reduction in walking speed in 80-year-old Japanese adults: a 3-year prospective cohort study with propensity score analysis by the SONIC study group. 2019;36(2):156–62.
Acknowledgements
We thank all the volunteers for the participation and personnel for their contribution in the WCHAT study.
Funding
This work was funded by National Key R&D Program of China (2018YFC2002401), National Key R&D Program of China (2020YFC2008604), the National Natural Science Foundation of China (No.82101653), Post-doc Coronavirus Epidemic Prevention and Control Fund (0040204153349), West China Hospital Postdoctoral Fund (2020HXBH011).
Author information
Authors and Affiliations
Contributions
XX contributed to conceptualization, data collection, data curation, formal analysis, writing the original draft, and review and editing of the paper. FH contributed to data collection, data curation, and review and editing of the paper. ZX contributed to data collection, data curation. LH contributed to data collection, data curation. GZ contributed to data collection, data curation. XL contributed to study conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and review and editing of the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Subjects (or their guardians) have given their written informed consent for study participation. The current research was approved by the Ethical Review Committee of West China Hospital with the committee’s reference number 2017(445) and the registration number is ChiCTR 1800018895. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not Applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xia, X., Xu, Z., Hu, F. et al. Nutrition mediates the relationship between number of teeth and sarcopenia: a pathway analysis. BMC Geriatr 22, 649 (2022). https://doi.org/10.1186/s12877-022-03350-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03350-7