Abstract
Background
Insulin resistance, which is closely associated with type 2 diabetes mellitus (T2DM), is a cause of sarcopenia and people with T2DM have a high risk of sarcopenia. Keeping good oral condition by dental care is important for people with T2DM. Keeping good oral condition by dental care is important for people with T2DM. This study has investigated the association between dental care or oral conditions and sarcopenia in people with T2DM.
Methods
Dental care and oral conditions were evaluated based on a self-reported questionnaire. Individuals with both low handgrip strength and low skeletal muscle mass index were diagnosed with sarcopenia.
Results
Among 266 people with T2DM, the proportions of sarcopenia, not having a family dentist, not having a toothbrushing behavior, poor chewing ability, and use of complete dentures were 18.0%, 30.5%, 33.1%, 25.2%, and 14.3%, respectively. The proportions of sarcopenia in people not having a family dentist (27.2% vs. 14.1%, p = 0.017), those with poor chewing ability (26.9% vs. 15.1%, p = 0.047), and use of complete dentures (36.8% vs. 14.9%, p = 0.002) were higher than those in people without. The proportion of sarcopenia in people without toothbrushing behavior tended to be higher than that in people with toothbrushing behavior (25.0% vs. 14.6%, p = 0.057). Not having a family dentist (adjusted odds ratio [OR] 2.48 [95% confidence interval (CI): 1.21–5.09], p = 0.013), poor chewing ability (adjusted OR 2.12 [95% CI: 1.01–4.46], p = 0.048), and use of complete dentures (adjusted OR 2.38 [95% CI: 1.01–5.99], p = 0.046) were related to the prevalence of sarcopenia.
Conclusions
This study revealed that dental care and oral conditions were associated with the prevalence of sarcopenia.
Similar content being viewed by others
Background
The population of people with type 2 diabetes mellitus (T2DM) is increasing worldwide [1]. T2DM is a chronic disease characterized by hyperglycemia because of insulin resistance (IR). In IR states, insulin-stimulated glucose disposal is severely impaired in the skeletal muscle [2]. Therefore, IR induces loss of muscle mass. Sarcopenia, which is defined as muscle strength, mass, and function loss [3] with age, has been associated with cardiovascular disease (CVD) [4] and low quality of life [5]. In addition, sarcopenia is known as a risk factor for mortality [6, 7]. People with T2DM have been reported to have a 1.55-to-3-fold higher risk of sarcopenia than the general population, since people with T2DM recognize IR [8, 9]. Therefore, sarcopenia in people with T2DM requires more attention than that in individuals without diabetes.
People with T2DM had a higher risk of periodontal disease than those without [10]. The severity of periodontal disease was related to glucose tolerance status and the development of glucose intolerance [11] and glycosylated hemoglobin (HbA1c) levels [12, 13]. Furthermore, the severity of periodontal disease affects inflammation and IR [14]. Infection with porphyromonas gingivalis, which causes periodontal disease, is a risk of metabolic syndrome and skeletal muscle metabolic dysfunction via gut microbiome alteration [15]. Furthermore, toothbrushing behavior was associated with smaller increments in the number of teeth with periodontal pocketing [16]. Therefore, it is important for people with diabetes to have a family dentist and regular visits with their dentist.
Chewing is a process that includes predation, crushing, and mixing of food; the formation of a bolus; and delivery of that bolus to the pharynx, which greatly affects food intake [17]. Chewing ability has been shown to be associated with sarcopenia in the general population [18]. In addition, several studies have reported the relationship between chewing ability and muscle strength [19], physical performance [20] and all-cause mortality [21]. Moreover, we also reported that low tongue pressure was related to the presence of sarcopenia [22]. On the other hand, poor chewing ability was associated with the use of removable dentures [23]. The use of complete dentures has been shown to be related to the presence of low handgrip strength [24]. However, previous studies have not researched the relationship between dental care and oral conditions, such as having a family dentist, toothbrushing behavior, chewing ability or use of complete dentures, and the presence of sarcopenia in people with T2DM. Therefore, this cross-sectional study researched the association between dental care and oral conditions, such as having a family dentist, toothbrushing, chewing ability, or use of complete dentures, and sarcopenia in people with T2DM.
Methods
Study design, setting, and participants
The KAMOGAWA-DM cohort study, which is a cohort study in progress with diabetes mellitus, was introduced in 2014 to understand the natural disease history of individuals with diabetes mellitus [25]. The KAMOGAWA-DM cohort study included outpatients at the Department of Endocrinology and Metabolism, Kyoto Prefectural University of Medicine Hospital (Kyoto, Japan). The present study was approved by the Research Ethics Committee of Kyoto Prefectural University of Medicine (No. RBMR-E-466-6) and was conducted in accordance with the principles of the Declaration of Helsinki. After obtaining written informed consent, medical data were anonymously collected and compiled into a database. This study included people with T2DM who responded to questionnaires about dental care and oral conditions from March 2015 to April 2021 and agreed to participate in the KAMOGAWA-DM cohort study. The exclusion criteria were as follows: 1) no data on body composition and 2) no data on handgrip strength.
Questionnaire about lifestyle characteristics and chewing ability
Family history of diabetes, duration of diabetes, smoking status, exercise habit, and alcohol consumption habit were assessed using a standardized questionnaire. Based on their responses to the questionnaire, “exercise habit” was defined as carrying out any type of physical activity once or more per week, “smoking habit” was defined as smoking cigarettes or another tobacco product currently, and “alcohol consumption habit” was defined as daily alcohol consumption.
Dental care and oral condition questionnaire
Participants were grouped into two groups: those who had a family dentist or those who did not have a family dentist. The frequency of toothbrushing was how often they brushed their teeth per day: none, sometimes, once, twice, thrice, four times, or five times or more per day. We defined people with toothbrushing behavior if they brushed their teeth ≥ twice per day [16]. Chewing ability was evaluated by the following statements: “I can chew and eat anything,” “There are some food I cannot chew,” “There are many food I cannot chew,” or “I cannot eat with chewing.” In this study, “I can chew and eat anything” was defined as good chewing ability and “There are some food I cannot chew,” “There are many food I cannot chew,” or “I cannot eat with chewing” were defined as having poor chewing ability [26]. Participants were grouped into two groups: those with or without complete denture usage.
Participants’ data
After fasting overnight, venous blood samples were collected to measure the concentrations of fasting plasma glucose, high-density lipoprotein cholesterol, triglycerides, uric acid, and creatinine. Glycosylated hemoglobin (HbA1c) was measured by high-performance liquid chromatography and expressed in the National Glycohemoglobin Standardization Program. The estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was estimated as follows: eGFR = 194 × serum creatinine− 1.094× age− 0.287 (×0.739 for women) [27]. Blood pressure measurements were performed automatically using an automatic blood pressure measurement device (HEM-906; OMRON, Kyoto, Japan) after resting for 5 min in a quiet room. The handgrip strength of each hand was tested by a handgrip dynamometer (Smedley, Takei Scientific Instruments Co., Ltd., Niigata, Japan) twice with each hand, and the maximum value was recorded and used for analysis.
Body composition was assessed using a multifrequency impedance body composition analyzer, InBody 720 (InBody Japan, Tokyo, Japan), which has been shown to have good correlation with dual-energy X-ray absorptiometry [28]. Using this analyzer, body weight (BW, kg) and appendicular muscle mass (kg) were determined, and then, body mass index (BMI, kg/m2) and skeletal muscle mass index (SMI, kg/m2) were calculated, BMI = BW (kg)/height squared (m2) and SMI = appendicular muscle mass (kg)/ height squared (m2), respectively.
Data of medications for diabetes, including glucagon-like peptide-1 agonist, insulin, sodium glucose cotransporter-2 inhibitor, metformin, dipeptidyl peptide 4 inhibitor, sulfonylurea, thiazolidines, glinides, α-glycosidase inhibitors and antihypertensive drugs, were obtained from medical records.
Having hypertension was defined as antihypertensive drugs usage, systolic blood pressure ≥ 140 mmHg, and/or diastolic blood pressure ≥ 90 mmHg.
Definition of sarcopenia
Sarcopenia was defined according to the Asian Working Group for Sarcopenia guidelines, utilizing SMI and handgrip strength [3]. People who had both low muscle strength, indicating handgrip strength < 28 kg for men and < 18 kg for women, and low skeletal muscle mass indicating SMI < 7.0 kg/m2 for men and < 5.7 kg/m2 for women, were diagnosed with sarcopenia [3].
Statistical analyses
Data are presented as frequencies of potential confounding variables or means (standard deviation [SD]). The participants were classified into the following two groups based on having a family dentist, toothbrushing behavior, chewing ability and use of complete dentures. The differences in the continuous variables and categorical variables were evaluated using Student’s t-test and chi-square test, respectively. Logistic regression analyses were run to determine the odds ratio (OR) and 95% confidence interval (CI) for having a family dentist, toothbrushing behavior, chewing ability, or use of complete dentures in the presence of sarcopenia, adjusting for age, sex, smoking habits and exercise habits.
Statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [29], a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Differences were considered statistically significant at p values of < 0.05.
Results
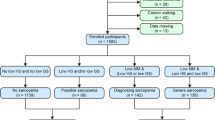
A total of 304 individuals with T2DM were included in the present study. We excluded 38 people: 26 who did not undergo the multifrequency impedance body composition analyzer test, and 12 who did not undergo measurement of handgrip strength; finally, a total of 266 people (162 men and 104 women) were included in this study (shown in Fig. 1).
The clinical characteristics of the study participants are summarized in Table 1. Mean age, BMI, SMI, and handgrip strength were 69.1 ± 8.7 years, 23.6 ± 3.9 kg/m2, 6.9 ± 1.1 kg/m2, and 26.4 ± 8.5 kg, respectively. The proportion of sarcopenia was 18.0% (n = 48), and the proportions of participants not having a family dentist, those without a toothbrushing behavior, those with poor chewing ability, and those with complete dentures usage were 30.5% (n = 81), 33.1% (n = 88), 25.2% (n = 67), and 14.3% (n = 38), respectively. Metformin and dipeptidyl peptide 4 inhibitor were used 43.6% (n = 116) and 35.3% (n = 94).
Table 2 reveals the results of the clinical characteristics of the participants according to dental care and oral condition. The proportion of sarcopenia with people not having a family dentist was higher than those having a family dentist (27.2% vs. 14.1%, p = 0.017). The proportion of sarcopenia with poor chewing ability was higher than those with good chewing ability (26.9% vs. 15.1%, p = 0.047), and those with use of complete denture were higher than those without use of complete denture (36.8% vs. 14.9%, p = 0.002). The proportion of sarcopenia in people without toothbrushing behavior tended to be higher than that in people with toothbrushing behavior, although the difference was not statistically significant (25.0% vs. 14.6%, p = 0.057). The proportion of not having a family dentist (52.6% vs. 26.8%, p = 0.003), no toothbrushing behavior (55.3% vs. 20.2%, p < 0.001), and low chewing ability (55.3% vs. 20.2%, p < 0.001) in people with use of complete dentures was higher than those without.
Furthermore, not having a family dentist (adjusted OR, 2.48 [95% CI: 1.21–5.09], p = 0.013), poor chewing ability (adjusted OR, 2.12 [95% CI: 1.01–4.46], p = 0.048), and use of complete dentures (adjusted OR, 2.38 [95% CI: 1.01–5.99], p = 0.046) were related to the presence of sarcopenia. The absence of toothbrushing behavior was associated with the presence of sarcopenia (unadjusted OR, 1.95 [95% CI: 1.03–3.68], p = 0.040), although it was not statistically significant after adjusting for covariates (adjusted OR, 1.71 [95% CI: 0.81–3.59], p = 0.157) (Table 3).
Discussion
The present study is the first investigation of the relationship between dental care and oral conditions, such as having a family dentist, toothbrushing behavior, chewing ability or usage of complete dentures, and the prevalence of sarcopenia in people with T2DM. The results of the present study showed that not having a family dentist, poor chewing ability, and use of complete dentures were associated with a higher prevalence of sarcopenia.
Possible explanations for the association between dental care or oral condition and a higher prevalence of sarcopenia are as follows:
Periodontal disease severity affects chronic inflammation and IR [14]. Chronic inflammation that occurs in response to many kinds of bacterial community in the subgingival region is features of periodontal disease [30]. Although this chronic inflammatory happens locally in the oral cavity, inflammatory mediators produced by periodontitis, as well as bacteria, can expand from the oral cavity, causing various diseases outside the oral cavity [30]. Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), can trigger IR states [31], and the epidemiological studies also have reported that inflammation is an independent risk of both IR [32] and T2DM [33, 34]. IR has been shown to be a reason for sarcopenia [35, 36]. Furthermore, periodontal disease is recognized as a risk factor for metabolic dysfunction of skeletal muscle [15]. In this study, the proportion of sarcopenia in people who had a family dentist was lower than that in people who did not have a family dentist. This suggests that having a family dentist and maintaining good oral health may reduce IR and prevent sarcopenia, although the presence or absence of periodontal disease was not evaluated. Furthermore, toothbrushing is considered a prerequisite for maintaining good oral health and preventing periodontal disease [16]. In this study, the proportion of sarcopenia in people without toothbrushing behavior was higher than that in those with toothbrushing behavior, although the results of multivariate analysis were not statistically significant. A previous study showed that toothbrushing behavior was related to handgrip strength [37]. Porphyromonas gingivalis, which is periodontitis bacteria, impairs glucose uptake in skeletal muscle associated with altering gut microbiota [15]. In this study, toothbrushing behavior was associated with the presence of low muscle strength. Although further research is needed, toothbrushing may prevent sarcopenia, because toothbrushing protect the development of periodontal disease.
In addition, maintaining good oral health prevents oral frailty. Oral frailty, which is now recognized as the accumulation of a poor oral function and condition, is reported to be associated with risk of incident mortality, malnutrition, dysphagia, physical frailty, and need for long-term care, and oral frailty causes poor chewing ability [38]. Previous studies have reported the relationship between chewing ability and handgrip strength [39] or general function [20]. Furthermore, chewing ability has been found to be related to sarcopenia in the general population [18]. Poor chewing ability has been known to be a risk factor for malnutrition [40]. In this study, the presence of sarcopenia in people with poor chewing ability was higher than those with good chewing ability. Therefore, maintaining good chewing ability may prevent sarcopenia.
A previous study showed that the use of complete dentures is associated with the presence of low handgrip strength [24]. In this study, the use of complete dentures was related to the presence of sarcopenia. People who use complete dentures often have denture stomatitis, which is a common inflammatory disease that affects the mucosa under complete dentures, and the progression of denture stomatitis without treatment may cause systemic infection [41]. Oral infections increase the levels of interleukin-6 and TNF-α receptors [42], which are associated with inflammation.
However, there were certain limitations in this study. First, the data of dental care and oral health status were based on self-reporting, and some concerns were raised the accuracy of the data. Second, the presence or absence of periodontal disease and denture stomatitis were not evaluated. Finally, the design of this study was cross-sectional in nature. Thus, the causal relationship between dental care and oral condition, such as having a family dentist, toothbrushing behavior, chewing ability, or use of complete dentures, and the prevalence of sarcopenia is unclear. Moreover, having a family dentist, toothbrushing behavior, chewing ability, and use of complete dentures may affect each other.
Conclusions
This study identified that not having a family dentist, poor chewing ability, and use of complete dentures were related to a higher prevalence of sarcopenia in people with T2DM. Clinicians should pay attention to the dental care and oral conditions of individuals with T2DM to prevent sarcopenia.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BDHQ:
-
Brief-type self-administered diet history questionnaire
- BMI:
-
Body mass index
- BW:
-
Body weight
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated glomerular filtration rate
- GLP-1:
-
Glucagon-like peptide-1
- HbA1c:
-
Glycosylated hemoglobin
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- SGLT2:
-
Sodium-glucose cotransporter-2
- SMI:
-
Skeletal muscle mass index
- T2DM:
-
Type 2 diabetes mellitus
References
Bradley D. Type 2 Diabetes in the elderly: challenges in a unique patient population. J Geriatr Med Gerontol. 2016;2:14.
Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307e2.
Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013;8:e60119.
Beaudart C, Locquet M, Reginster JY, Delandsheere L, Petermans J, Bruyère O. Quality of life in sarcopenia measured with the SarQoL®: impact of the use of different diagnosis definitions. Aging Clin Exp Res. 2018;30:307–13.
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12:1–16.
Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;October 2015:290–8.
Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes Mellitus is Associated with increased risk of Sarcopenia: a systematic review and Meta-analysis. Calcif Tissue Int. 2020;107:453–63.
Feng L, Gao Q, Hu K, Wu M, Wang Z, Chen F, et al. Prevalence and risk factors of Sarcopenia in patients with diabetes: a Meta-analysis. J Clin Endocrinol Metab. 2022;107:1470–83.
Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–40.
Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama Study. J Dent Res. 2004;83:485–90.
Demmer RT, Desvarieux M, Holtfreter B, Jacobs DR, Wallaschofski H, Nauck M, et al. Periodontal status and A1C change: longitudinal results from the study of Health in Pomerania (SHIP). Diabetes Care. 2010;33:1037–43.
Hayashida H, Kawasaki K, Yoshimura A, Kitamura M, Furugen R, Nakazato M, et al. Relationship between periodontal status and HbA1c in nondiabetics. J Public Health Dent. 2009;69:204–6.
Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, et al. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2012;35:2235–42.
Watanabe K, Katagiri S, Takahashi H, Sasaki N, Maekawa S, Komazaki R, et al. Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J. 2020;May 2020:1–16.
Joshi S, Suominen AL, Knuuttila M, Bernabé E. Toothbrushing behaviour and periodontal pocketing: an 11-year longitudinal study. J Clin Periodontol. 2018;45:196–203.
Krall E, Hayes C, Garcia R. How dentition status and masticatory function affect nutrient intake. J Am Dent Assoc. 1998;129:1261–9.
Murakami M, Hirano H, Watanabe Y, Sakai K, Kim H, Katakura A. Relationship between chewing ability and sarcopenia in japanese community-dwelling older adults. Geriatr Gerontol Int. 2015;15:1007–12.
Moriya S, Notani K, Murata A, Inoue N, Miura H. Analysis of moment structures for assessing relationships among perceived chewing ability, dentition status, muscle strength, and balance in community-dwelling older adults. Gerodontology. 2014;31:281–7.
Takata Y, Ansai T, Awano S, Hamasaki T, Yoshitake Y, Kimura Y, et al. Relationship of physical fitness to chewing in an 80-year-old population. Oral Dis. 2004;10:44–9.
Iinuma T, Arai Y, Takayama M, Abe Y, Ito T, Kondo Y, et al. Association between maximum occlusal force and 3-year all-cause mortality in community-dwelling elderly people. BMC Oral Health. 2016;16:4–11.
Kaji A, Hashimoto Y, Kobayashi Y, Sakai R, Okamura T, Miki A, et al. Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: a cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr Gerontol Int. 2019;19:153–8.
Liang YH, Chou C, Chen YJ, Chou YF, Lin CY, Chou C, et al. Impact of periodontal disease and chewing ability on the quality of life of the elderly in an affluent community. J Formos Med Assoc. 2020;119:1693–701.
Yun J, Lee Y. Association between oral health status and handgrip strength in older korean adults. Eur Geriatr Med. 2020;11:459–64.
Sakai R, Hashimoto Y, Ushigome E, Miki A, Okamura T, Matsugasumi M, et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: the KAMOGAWA-DM cohort study. Endocr J. 2018;65:395–402.
Statistical survey of actual status for salary in the private sector in Japan. 2018. https://www.mhlw.go.jp/stf/houdou/0000177189_00001.html. Accessed 7 May 2021.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int. 2015;15:1013–22.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic inflammation as a link between Periodontitis and Carcinogenesis. Mediators Inflamm. 2019;2019:1029857.
Ling PR, Bistrian BR, Mendez B, Nawfal W. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism.1994;43:279–84.
Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Med Assoc. 2001;286:327–34.
Hu FB, Meigs JB, Li TY, Rifai N, Manson JAE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700.
Kim TN, Choi KM. Sarcopenia. Definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10.
Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, Sarcopenia and Diabetes. J Am Med Dir Assoc. 2014;15:853–9.
Lee JH, Lee SY, Han K, Han JS. Relationship between oral health behaviour and handgrip strength: a cross-sectional study with 7589 korean adults. Acta Odontol Scand. 2020;78:438–44.
Shiraishi A, Wakabayashi H, Yoshimura Y. Oral management in Rehabilitation Medicine: oral Frailty, oral Sarcopenia, and Hospital-Associated oral problems. J Nutr Health Aging. 2020;24:1094–9.
Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, et al. A new classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Invest. 2015;6:242–6.
Samnieng P, Ueno M, Shinada K, Zaitsu T, Wright FAC, Kawaguchi Y. Oral health status and chewing ability is related to mini-nutritional assessment results in an older adult population in Thailand. J Nutr Gerontol Geriatr. 2011;30:291–304.
Yarborough A, Cooper L, Duqum I, Mendonça G, McGraw K, Stoner L. Evidence regarding the treatment of denture stomatitis. J Prosthodont. 2016;25:288–301.
Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:326–32.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FT design of the work, analysis and interpretation of data and written the manuscript. YH conception and design the work, acquisition, analysis and interpretation of data and revising the manuscript. AK and RS conception and design the work, acquisition data and contributed Discussion. YK (Yuka Kawate), TO, YK (Yuriko Kondo), EU, SM, TS, and NN acquisition data and contributed Discussion. HO conception and design of the work, acquisition data, and contributed Discussion. MH design of the work, acquisition data and contributed Discussion. MA and MY acquisition data and contributed Discussion. MF conception and design the work, acquisition and interpretation of data and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Kyoto Prefectural University of Medicine (No. RBMR-E-466-6) and was conducted in accordance with the principles of the Declaration of Helsinki. All participants submitted written informed consent.
Consent for publication
Not applicable.
Competing interests
Hashimoto Y received personal fees from Novo Nordisk Pharma Ltd., Mitsubishi Tanabe Pharma Corp., Kowa Company Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd., outside of the submitted work. Okada H received grant support from the Japan Society for the Promotion of Science, and personal fees from Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., MSD K.K., Kyowa Hakko Kirin Company Ltd., Kowa Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Sanofi K.K., and Mitsubishi Tanabe Pharma Corporation. Nakanishi N received grant support from Japan Society for the Promotion of Science (JSPS KAKENHI grant numbers: 19K23999 and 20K16158) and The Japan Food Chemical Research Foundation, and personal fees from Novo Nordisk Pharma Ltd., and Kowa Pharmaceutical Co., Ltd. Osaka T received grants from Combi Corporation, and personal fees from Toa Eiyo Corp., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., MSD K.K., Takeda Pharmaceutical Co., Ltd., Kowa Pharma Co., LTD., Eli Lilly Japan K.K., and AstraZeneca K.K., outside of the submitted work. Senmaru T received personal fees from Kyowa Hakko Kirin Co., Ltd., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Co., Kowa Pharma Co., Ltd., Sanofi K.K., Taisho Toyama Pharma Co., Ltd., Kissei Pharma Co., Ltd., MSD K.K., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., and Takeda Pharma Co., Ltd., outside of the submitted work. Ushigome E received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure, Astellas Foundation for Research on Metabolic Disorders (grant number: 4024), Japan Society for the Promotion of Science, Mishima Kaiun Memorial Foundation, and personal fees from Sumitomo Dainippon Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Sanofi K.K., Kowa Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., AstraZeneca K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Ltd., and MSD K.K., outside of the submitted work. Donated Fund Laboratory of Diabetes therapeutics is an endowment department, supported with an unrestricted grant from Taiyo Kagaku Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. Hamaguchi M received grants from Yamada Bee Farm, Oishi Kenko Inc., Nippon Boehringer Ingelheim Co., Ltd., AstraZeneca K.K., and Ono Pharma Co., Ltd., and personal fees from Eli Lilly, Japan, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp., AstraZeneca K.K., Ono Pharma Co., Ltd., and Kowa Pharma Co., Ltd., outside of the submitted work. Asano M received personal fees from Takeda Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Abbott Japan Co., Ltd., Novo Nordisk Pharma Ltd., Chugai Pharmaceutical Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd., outside of the submitted work. Yamazaki M received personal fees from Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Kyowa Hakko Kirin Co., Ltd., Kowa Company, Limited, Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca PLC, and MSD K.K., outside of the submitted work. Fukui M received grants from Eli Lilly, Japan, K.K., Nippon Boehringer Ingelheim Co., Ltd., Sanwa Kagagu Kenkyusho Co., Ltd., Oishi Kenko Inc., MSD K.K., Kowa Pharma Co., Ltd., Kissei Pharma Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corp., Abbott Japan Co., Ltd., Daiichi Sankyo Co., Ltd., Johnson & Johnson K.K. Medical Co., Astellas Pharma Inc., Kyowa Kirin Co., Ltd., Novo Nordisk Pharma Ltd., Yamada Bee Farm, Taisho Pharma Co., Ltd., Terumo Corp., Takeda Pharma Co., Ltd., Tejin Pharma Ltd., Sanofi K.K., Nippon Chemiphar Co., Ltd., and TERUMO CORPORATION, and personal fees from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., MSD K.K., Mochida Pharma Co., Ltd., Eli Lilly Japan K.K., Kissei Pharma Co., Ltd., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corp., TERUMO CORPORATION, Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., Takeda Pharma Co., Ltd., Teijin Pharma Ltd., Ono Pharma Co., Ltd., Taisho Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Abbott Japan Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Arkray Inc., Medtronic Japan Co., Ltd., Novo Nordisk Pharma Ltd., Kowa Pharma Co., Ltd., Nipro Corp., and Sanofi K.K., outside of the submitted work. The other authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takahashi, F., Hashimoto, Y., Okada, H. et al. Dental care and oral conditions are associated with the prevalence of sarcopenia in people with type 2 diabetes: a cross-sectional study. BMC Endocr Disord 23, 76 (2023). https://doi.org/10.1186/s12902-023-01331-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01331-4