Abstract
Background
Aldehyde dehydrogenase 1 (encoded by ALDH1A1) has been shown to protect against Parkinson’s disease (PD) by reducing toxic metabolites of dopamine. We herein revealed an antisense Alu element insertion/deletion polymorphism in intron 4 of ALDH1A1, and hypothesized that it might play a role in PD.
Methods
A Han Chinese cohort comprising 488 PD patients and 515 controls was recruited to validate the Alu insertion/deletion polymorphism following a previous study of tag-single nucleotide polymorphisms, where rs7043217 was shown to be significantly associated with PD. Functional analyses of the Alu element insertion were performed.
Results
The Alu element of ALDH1A1 was identified to be a variant of Yb8 subfamily and termed as Yb8c4. The antisense Yb8c4 insertion/deletion polymorphism (named asYb8c4ins and asYb8c4del, respectively) appeared to be in a complete linkage disequilibrium with rs7043217 and was validated to be significantly associated with PD susceptibility with asYb8c4ins serving as a risk allele (P = 0.030, OR = 1.224, 95% CI = 1.020–1.470). Multiple functional analyses including ALDH1A1 mRNA expression in blood cells of carriers, and reporters of EGFP and luciferase showed that the asYb8c4ins had a suppressive activity on gene transcription. Mechanistic explorations suggested that the asYb8c4ins induced no changes in CpG methylation and mRNA splicing of ALDH1A1 and appeared no binding of transcription factors.
Conclusions
Our results consolidate an involvement of ALDH1 in PD pathogenesis. The asYb8c4 polymorphism may be a functional output of its linkage disequilibrium-linked single nucleotide polymorphisms.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is a common neurodegenerative disorder resulted from the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta. While a few genes such as LRRK2, SNCA and PRKN have been identified to be mutated and causative in familial PD, limited is known in regard with the etiology of sporadic PD. Benefiting from the genome-wide association studies and the following investigations, a number of risk loci towards PD are disclosed in positions such as RIT2, SIPA1L2, and VPS13C [1,2,3]. These genetic susceptibilities, together with environmental insults such as pesticide exposure and prior head injury, are generally believed to lead to the sporadic pathogenesis of PD [4].
Aldehyde dehydrogenase (ALDH), which includes the cytosolic ALDH1 and mitochondrion-located ALDH2, is a family of aldehyde-oxidizing enzyme and catalyzes the conversion of the dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) into 3,4-dihydroxyphenylacetic acid in DA neurons. Accumulation of the DOPAL is toxic to the neurons [5,6,7]. Indeed, pesticide benomyl, which inhibits ALDH activity, leads to increased production of DOPAL and cytotoxicity to rat DA neurons [8, 9]. Inhibition of ALDH activity is also considered to be a mechanism for rotenone-induced neurotoxicity [10]. ALDH1, which is encoded by ALDH1A1, is highly expressed in DA neurons [11], and is noted to be severely reduced in the substantia nigra pars compacta of post-mortem PD brains [12, 13]. The reduction of ALDH1A1 mRNA expression was previously suggested as a potential blood biomarker of PD [14]. ALDH1-positive DA neurons exhibit stronger resistance than the negative ones against α-synuclein-induced cytotoxicity in mice [15]. Double knockout of Aldh1a1 and Aldh2 leads to a significant loss of DA neurons in the substantia nigra of mice [16].
Our previous study of six tag-single nucleotide polymorphisms (SNPs) of ALDH1A1 suggested that rs7043217 is significantly associated with PD risk [17]. During the study, we surprisingly observed a length polymorphism in the intron 4. We hypothesized that this length polymorphism might play a role in PD, and thus performed an association validation and multiple functional analyses.
Methods
Subjects
A total of 1003 Han Chinese were recruited from eastern China, including 488 sporadic PD patients (252 males and 236 females) and 515 controls (286 males and 229 females). The median age of the patients and controls was 66 (interquartile range, 59–72) and 59 (interquartile range, 51–68), respectively. PD patients were diagnosed by two neurologists according to the UK Parkinson's Disease Society Brain Bank Criteria [18]. Patients with a family history of PD, or with secondary and atypical parkinsonism were excluded. Control subjects were free of neurological disorders determined by medical history, physical and laboratory examinations. For quantitation of the ALDH1A1 mRNA levels, another 29 control subjects were recruited, including 15 subjects carrying the insertion genotype of the length polymorphism (7 males and 8 females; median age, 66 years, interquartile range, 62–68) and 14 with the deletion genotype (7 males and 7 females; median age, 67 years, interquartile range, 64–69).
Genotyping
Genomic DNA was extracted from peripheral blood samples using TIANamp Genomic DNA Kit (Tiangen, Beijing, China) following the manufacturer's instruction. The length polymorphism was analyzed using polymerase chain reaction (PCR) followed by agarose gel electrophoresis. Direct sequencing of the products was performed using an ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA) after processed with BigDye Terminator v3.1 kit (Applied Biosystems, Foster City, CA, USA) at the Beijing Genomics Institute (Beijing, China). The primers, as well as the primers used below, were all detailed in Additional file 1: Table S1.
Methylation analysis
Methylation levels were analyzed by bisulfite sequencing PCR. Bisulfite conversion of genomic DNA was performed using EZ DNA Methylation-Gold kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s protocol. Bisulfite-treated DNA was used for amplification of the CpG island, and the upstream and downstream CpG sites. PCR products were cloned into the plasmid pMD-19 T (Takara, Shiga, Japan) and transformed into E. coli. Ten colonies were randomly selected and sequenced.
Blood cell isolation and real-time PCR
Peripheral blood mononuclear cells (PBMCs) were isolated using a human lymphocyte separation kit (TBD Science, Tianjin, China) according to the manufacturer's protocol. Total RNA was extracted using TriPure Isolation Reagent (Roche, Indianapolis, IN, USA). An amount of 500 ng RNA was used for synthesis of the first-strand cDNA by PrimeScript RT reagent Kit (Takara, Shiga, Japan). Real-time PCR samples were prepared with Faststart Essential DNA Green Master (Roche, Indianapolis, IN, USA) and amplification was performed using the CFX connect real-time system (Bio-Rad Laboratories, Hercules, CA, USA). Value of the cycle threshold was determined by automated threshold analysis using the Opticon Monitor 3.1 software (Bio-Rad Laboratories, Hercules, CA, USA).
Plasmids and luciferase reporter
Target fragments were cloned into pMIR-Report firefly luciferase reporter (Ambion, Austin, TX, USA; between Sac I and Hind III) and pEGFP-C1 (Clontech, Palo Alto, CA, USA; between Xho I and Pst I). The constructs of firefly luciferase reporter were co-transfected with the Renilla luciferase vector pRL-TK (Promega, Madison, WI, USA) into SK-N-SH cells (Cell Bank of Chinese Academy of Sciences, Shanghai, China). Luciferase activity was determined 24 h after transfection by a Dual-Luciferase Reporter Assay kit (Promega, Madison, WI, USA) following the manufacturer’s protocol. Luciferase mRNA levels were determined 24 h after transfection by real-time PCR. Firefly luciferase data were normalized to the activity or mRNA levels of Renilla luciferase. For EGFP expression, fluorescence intensity was observed and quantified 48 h post-transfection of the EGFP constructs in SK-N-SH cells.
DNA pull-down assay
Biotin-labelled double-stranded oligonucleotides were amplified from intron 4 of ALDH1A1 using the primer pair listed in Additional file 1: Table S1 and then purified using MiniBEST Agarose Gel DNA Extraction Kit (Takara, Dalian, China). Nuclear fraction was extracted from SK-N-SH cells (American Type Culture Collection, Manassas, VA, USA) using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China). Five nM of the purified oligonucleotides was then incubated with 500 μg nuclear extracts in a binding buffer (25 mM Tris, 150 mM NaCl and 1 mM PMSF; pH 7.2) for 30 min at room temperature with gentle rotation, followed by 2 h incubation with the addition of 50 μL streptavidin-agarose beads (Sigma, St. Louis, MO, USA). The complexes were washed 4 times with the binding buffer at 4 °C. Proteins were eluted using a lysis buffer (60 mM Tris–HCl at pH 6.8, 5% glycerol, 2% SDS) and subjected to 10% SDS-PAGE as previously described [19]. After visualization using a silver staining kit (Beyotime, Shanghai, China), designated protein bands were cut and subjected to liquid chromatography/tandem mass spectrometry analysis (LC–MS/MS) at Shanghai Applied Protein Technology (Shanghai, China). The LC–MS/MS analysis was performed using an EASY nLC 1000 system coupled to a Q-Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA).
Sequence and statistical analysis
Sequence similarity was searched using the BLAST tool at NCBI (http://www.ncbi.nlm.nih.gov/blast). Gene Ontology analysis was performed at http://geneontology.org/. Statistical analyses were performed using the Statistical Package for Social Science Program (SPSS for Windows, version 23.0). Hardy–Weinberg equilibrium in genotype distribution was assessed using χ2 test. Following Kolmogorov–Smirnov test for normality, Mann–Whitney U test was used to evaluate age difference. The χ2 test was also used to assess the differences in gender between the PD cases and controls. The differences in genotype and allele frequencies were analyzed using logistic regression model with gender and age as covariates. Levels of gene expression were expressed as mean ± SE and analyzed by student’s T-test. A two-tailed P value < 0.05 was considered statistically significant.
Results
Identification of an antisense Alu insertion/deletion polymorphism in ALDH1A1
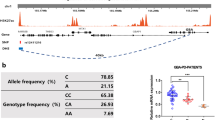
Amplifications using a primer pair targeting intron 4 of ALDH1A1 resulted in an approximate 300 bp difference in the length of PCR products, suggesting a potential length polymorphism in this region (Fig. 1A; uncropped image in Additional file 1: Fig. S1). By sequencing both of the short and long PCR products, an additional segment was identified in the longer form, which was a 290 bp fragment appended with a poly-A tail at the 3'-end and located at 822 bp downstream of exon 4 of ALDH1A1 (Fig. 1B; full sequencing results in Additional file 1: Fig. S2).
Identification of a novel antisense Alu element in intron 4 of ALDH1A1. A Agarose gel electrophoresis of PCR products amplified using the Alu primers. B Sequencing of the short and long PCR products. C Alignment of the additional insertion with Alu Y subfamilies. The identified variant was named Alu Yb8c4. D Schematic illustration of the Alu Yb8c4 insertion in ALDH1A1
Multiple sequence alignments showed that the antisense sequence of this 290 bp fragment was highly homologous to the conserved Alu Y element, and appeared to be a variant of Alu Yb8 subfamily as featured by the GCAGTCCG insertion at position 254–261 (Fig. 1C). Compared to the Yb8 conserved region, this variant contained an extra T at the 1st position, an inserted A at the 225th position and three transition point mutations at the 155th (C > T), 214th (C > T), and 269th (C > T) positions. When the extra T at the 1st position and the poly-A segment were excluded, results of sequence alignment showed that the other four mutations within the Yb8 conserved region were shared by NC_000003.12 and NC_000008.11 (Additional file 1: Fig. S3). According to the standard nomenclature of Alus [20], we named this new Alu element as Alu Yb8c4, which is a new subclass of Yb8. A scheme of identifying the Alu polymorphism was illustrated in Fig. 1D.
Association analyses of the Alu element of ALDH1A1 with PD susceptibility
By using the cohort previously published [17], we found that the insertion/deletion polymorphism of the antisense Yb8c4 (asYb8c4) displayed a complete linkage disequilibrium (r2 = 1) with the PD risk SNP rs7043217. The deletion of asYb8c4 (asYb8c4del) was linked to the C allele of rs7043217, while the insertion of asYb8c4 (asYb8c4ins) was coupled with the T allele. The relative location of the asYb8c4 and rs7043217 was illustrated in Fig. 1D.
We thus collaboratively recruited an independent cohort consisting of 488 PD patients and 515 control subjects to validate the association of the asYb8c4 with PD, before performing further functional analyses. Genotype distributions of the asYb8c4 were in accordance with Hardy–Weinberg equilibrium (P > 0.05). The PD cases and controls were comparable in gender (P > 0.05) but different in age (P < 0.05). Results showed that genotype and allele frequencies of the asYb8c4 were significantly different between the cases and controls (P = 0.045 and P = 0.030, respectively), with the asYb8c4ins serving as a risk allele for PD (OR = 1.224, 95% CI = 1.020–1.470; Table 1). The replication validated the association with PD discovered from the linked SNP, rs7043217 [17]. Further analysis of the asYb8c4ins/del variants in three genetic models (additive, dominant, and recessive) showed that the asYb8c4 was significantly associated with PD in the recessive model (P = 0.016, OR = 1.520, 95% CI = 1.081–2.138) and additive model (P = 0.025, OR = 1.242, 95% CI = 1.028–1.502), again suggesting that the asYb8c4ins was a risk allele for PD (Table 2).
Functional impact of the asYb8c4 on ALDH1A1 expression
To determine the functional impact of asYb8c4ins and asYb8c4del, mRNA expression levels of ALDH1A1 were analyzed in PBMCs of 15 asYb8c4ins/ins and 14 asYb8c4del/del carriers with sex and age being matched (P > 0.05). ACTB (encoding β-actin) was used as a reference gene. Results of real-time PCR showed that mRNA expression of ALDH1A1 was significantly (P = 0.02) lower in the asYb8c4ins/ins group compared to that in the asYb8c4del/del group (Fig. 2A). In addition, we analyzed mRNA expression levels of 3 reference genes, including ACTB, GAPDH and HPRT1, in 8 independent PBMC samples. HPRT1 is also a gene commonly used for normalization in PBMCs [21, 22]. A highly similar expression pattern was observed within the three reference genes. The ACTB level showed a strong correlation with the mean level of ACTB, GAPDH and HPRT1 (r2 = 0.9866; Additional file 1: Fig. S4), indicating that ACTB is an appropriate reference gene herein.
Impact of asYb8c4ins and asYb8c4del on gene expression. A mRNA expression of ALDH1A1 in PBMCs of different genotype carriers (n = 14 for asYb8c4del/del; n = 15 for asYb8c4ins/ins). Results were normalized to their respective ACTB levels. B Schematic diagram of the luciferase reporter and EGFP constructs containing asYb8c4ins (-123 to + 406), asYb8c4del (-123 to + 106), and a long fragment with asYb8c4del (L-asYb8c4del; -274 to + 262; length similar to asYb8c4ins). The position + 1 denotes the base immediately before asYb8c4ins. The indicated fragments were amplified from intron 4 of ALDH1A1. C-D Effect of asYb8c4ins, asYb8c4del and L-asYb8c4del on the firefly luciferase activity (C) and mRNA levels (D). Results were normalized to their respective Renilla luciferase activity or mRNA levels (n = 3). E Effect of asYb8c4ins and asYb8c4del on EGFP expression (n = 3). *, P < 0.05. asYb8c4del antisense Yb8c4 deletion, asYb8c4ins antisense Yb8c4 insertion, PBMC peripheral blood mononuclear cell
Luciferase reporter assays were employed to further confirm the role of asYb8c4 in gene expression. Three fragments were generated, including the asYb8c4ins (529 bp), the asYb8c4del (229 bp), and a long fragment of asYb8c4del (named as L-asYb8c4del; 536 bp). The L-asYb8c4del was designed as an additional control to match the asYb8c4ins length to exclude potential effect caused by the length difference between the asYb8c4ins and asYb8c4del. The three fragments were then respectively ligated into the 3’-untranlated region (UTR) of the luciferase gene (Fig. 2B). Results showed that the asYb8c4ins insertion potently suppressed the expression of firefly luciferase as manifested by the activity assay (Fig. 2C) and the mRNA quantification (Fig. 2D), suggesting a transcriptional inhibition by asYb8c4ins. In contrast, the asYb8c4del and L-asYb8c4del insertions did not show a suppressive effect. Similar results were observed when asYb8c4ins and asYb8c4del fragments were incorporated into the 3’-UTR of pEGFP-C1. The EGFP expression was greatly reduced by the asYb8c4ins (Fig. 2E).
Potential mechanisms of the asYb8c4-mediated suppression of ALDH1A1 expression
Sequence analysis showed that the asYb8c4ins introduced a CpG island of 23 CpG dinucleotides into intron 4 of ALDH1A1 (Fig. 3A and Additional file 1: Fig. S5), of which 21 were located in the asYb8c4 element. Besides, there were 3 upstream CpG and 7 downstream CpG sites relative to the location of asYb8c4 (Fig. 3A and Additional file 1: Fig. S5). To determine whether these CpG sites modulated the inhibition of ALDH1A1 expression by asYb8c4ins, cytosine methylation was analyzed in whole blood DNA. Results showed that the introduced CpG islands were comparably hypermethylated in PD cases (96.46%) and controls (95.98%) (Fig. 3B and Additional file 1: Fig. S6A). Both the upstream and downstream CpG sites were also similarly hypermethylated in two sets of control subjects respectively carrying the asYb8c4ins/ins and asYb8c4del/del genotypes (Fig. 3C-D and Additional file 1: Fig. S6B-C).
Potential mechanisms of the asYb8c4ins-mediated suppression of ALDH1A1 expression. A Distribution schematic of the CpG island and sites in intron 4 of ALDH1A1. B Methylation levels of the asYb8c4ins-introduced CpG island in peripheral blood of PD cases and controls (n = 5). C-D Methylation levels of upstream (C) and downstream (D) CpG sites in peripheral blood of subjects carrying the asYb8c4ins/ins or asYb8c4del/del genotype (n = 5). E Reverse-transcribed PCR products amplified between ALDH1A1 exons 3 and 5 from peripheral blood of subjects carrying the asYb8c4ins/ins or asYb8c4del/del genotype. F Silver staining gel of the DNA pull-down products from nuclear extract of SK-N-SH cells. Arrows indicate the protein bands used for mass-spectrometry identification. asYb8c4del antisense Yb8c4 deletion, asYb8c4ins antisense Yb8c4 insertion, PD Parkinson’s disease
We then investigated whether the asYb8c4 insertion or deletion altered the RNA splicing pattern of ALDH1A1. Results of reverse-transcribed PCR between exons 3 and 5 showed a single band of the same size in subjects carrying the asYb8c4ins/ins or asYb8c4del/del genotype (Fig. 3E; uncropped image in Additional file 1: Fig. S1), excluding RNA splicing as a mechanism of such ALDH1A1 under-expression.
A DNA pull-down assay combined with mass spectrometry was performed to identify potential transcription factors binding to the asYb8c4 insertion/deletion loci. Three unique bands eluted from the baits of asYb8c4del (band 1 and 2) and asYb8c4ins (band 3) were excised for proteomic analysis (Fig. 3F; uncropped image in Additional file 1: Fig. S1). Nucleus-localized proteins with molecular weights in the approximate range were as follows: 40S ribosomal protein S5, cilia- and flagella-associated protein 20, peroxiredoxin-1 and 60S ribosomal protein L9 from band 1 (20–23 kDa), serine/arginine-rich splicing factor 1 and 40S ribosomal protein S3 from band 2 (25–28 kDa), and pre-mRNA-processing factor 40 homolog A, DNA mismatch repair protein Msh2 and elongin-A from band 3 (85–115 kDa). Amongst, Msh2 and serine/arginine-rich splicing factor 1 were with higher unique peptide count. However, results of Gene Ontology suggested that no protein from the asYb8c4del bait possessed molecular function of transcription activation, and none from the asYb8c4ins bait possessed function of transcription repression (Additional file 1: Table S2).
Discussion
A linkage between ALDH1 activity and PD pathogenesis is supported by evidence from animal studies and human results of brain expression [12, 13, 15]. In the present study, we disclose a novel antisense Alu element termed asYb8c4 in intron 4 of ALDH1A1. Its insertion/deletion polymorphism is genetically associated with susceptibility of PD in a Han Chinese population. The asYb8c4 appears to be a functional variant modulating ALDH1A1 gene expression.
The association of asYb8c4 with PD in an independent cohort validates our previous finding that rs7043217 is a PD risk tag-SNP [17], since asYb8c4 is in complete linkage disequilibrium with rs7043217. The inheritance of rs7043217 was shown to be dominant and additive in the previous cohort, while the Yb8c4 was recessive and additive in the current one. The independent replication suggests that the inheritance of rs7043217 and Yb8c4 is most likely additive toward PD. As previously noted, we also found a genetic interaction between variants of ALDH1A1 and ALDH2 [17]. Indeed, the risk factors for PD are multifaceted [4]. Individual variants, genetic interactions in between, and functional impact on gene expressions, may collectively lead the way to PD pathogenesis. In addition, rs3764435 of ALDH1A1 was reported to be associated with PD in Mexicans [23]. However, this SNP is not in linkage disequilibrium with rs7043217 in either Mexicans or Han Chinese as shown in 1000 Genomes Project.
Alu elements are the most abundant mobile elements and belong to the short interspersed elements with over 1 million copies in human genome [24]. The human Alu family is composed of a series of distinct subfamilies of different genetic ages that are characterized by some specific mutations, of which the Ya5 and Yb8 mainly represent the young subfamilies in humans [25]. Some of these young Alu elements can accumulate new mutations and generate new subfamilies. Subclasses of Yb elements, such as Yb8a1, Yb10 and Yb11, have been recently characterized suggesting that Alu elements are still evolving [26]. In this study, we find the three mutations of base substitution in Yb8c4 are all C > T transitions, which otherwise comprise CpG sites in Yb8. Indeed, cytosine methylation could lead to a high frequency of C to T transition mutation [27], indicating that the Yb8c4 is a younger subclass originated from Yb8. When searching Yb8c4 against the genome aggregation database (https://gnomad.broadinstitute.org; gnomAD), a multiallelic variant (13 segment insertion alleles, length 4–48 bases) is suggested at the same locus (chr9-72,928,069). No additional information is available yet regarding its frequency and presence in ethnicities. It remains to be determined whether these two polymorphisms are the same.
Alu element polymorphisms or mutations have been functionally linked to the risks of multiple diseases, including neurodegenerative disorders, haemophilia, breast cancer and heritable pulmonary arterial hypertension [28,29,30]. Amongst, Yb8 is one of the most active Alu subfamilies [31]. The current study showed that the asYb8c4 polymorphism in ALDH1A1 is associated with the risk of PD, and the asYb8c4ins may be a functional variant inhibiting gene expression at transcriptional level as manifested by our PBMC, EGFP and luciferase reporter results. These results suggest that rs7043217 and its represented SNPs [17] may functionally attribute their risk effect to the asYb8c4 polymorphism. As a comparison, little effect of inhibition was observed when an antisense Alu Sp element was ligated into the 3’-UTR of EGFP in a previous study using the same EGFP expression system [32]. These data suggest that Yb8c4 may be one of the functional subclasses of Alu elements.
Previous studies have shown that Alu elements can modulate gene transcription by introduction of transcription factor binding sites, change of methylation in their genomic vicinity, and alternation of RNA splicing and editing [33,34,35]. Unfortunately, no altered methylation status is detected in the Yb8c4-introduced CpG island between PD patients and controls, nor in its nearby CpG sites between asYb8c4ins/ins and asYb8c4del/del carriers. RNA splicing of ALDH1A1 is also not changed by the insertion or deletion of asYb8c4. But we should also note that the expression, methylation, and splicing analyses were all performed on peripheral blood, which may differ from those in dopaminergic neurons. A further validation is desired if neuronal tissues are available. Among the nucleus-localized proteins acquired from the DNA pull-down assay, 40S ribosomal protein S5, 60S ribosomal protein L9 and 40S ribosomal protein S3 are component of the ribosome involved in translation process; Cilia- and flagella-associated protein 20 is involved in post-translational modification of the tubulin subunits of microtubules [36]; Peroxiredoxin-1 is an antioxidant enzyme [37]. Serine/arginine-rich splicing factor 1 and pre-mRNA-processing factor 40 homolog A are involved in pre-mRNA splicing [38, 39]; DNA mismatch repair protein Msh2 is involved in DNA repair [40]; Elongin-A is a transcription elongation factor increasing the overall rate of mRNA chain elongation by RNA polymerase II [41]. Since the asYb8c4 inhibits gene expression, the proper binding factor presumably should be transcription repressor for asYb8c4ins allele and transcription activator for asYb8c4del allele. However, no such protein factors are identified. Nonetheless, it is possible that the abundance of proper transcription factors may be under detection limit by this method. The underlying mechanism of asYb8c4 inhibiting gene transcription thus warrants further investigation. Of note, DNA mismatch repair protein Msh2 is of high possibility to bind asYb8c4ins oligonucleotide, suggesting that this antisense Alu element is prone to DNA mismatch.
Conclusions
The current study reveals an asYb8c4ins/del polymorphism in intron 4 of ALDH1A1 and demonstrates its positive association with PD susceptibility in a Han Chinese population. The asYb8c4ins serves as a risk allele towards PD and may transcriptionally downregulate ALDH1A1 gene expression. The asYb8c4 polymorphism may be a functional output of its linkage disequilibrium-linked SNPs. These findings genetically consolidate an involvement of ALDH1 in PD pathogenesis.
Availability of data and materials
The sequence of Alu Yb8c4 has been deposited in GenBank under the accession number MW922677. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALDH:
-
Aldehyde dehydrogenase
- asYb8c4del :
-
Antisense Yb8c4 deletion
- asYb8c4ins :
-
Antisense Yb8c4 insertion
- CI:
-
Confidence interval
- DA:
-
Dopaminergic
- DOPAL:
-
3,4-Dihydroxyphenylacetaldehyde
- LC–MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- PBMC:
-
Peripheral blood mononuclear cell
- PCR:
-
Polymerase chain reaction
- PD:
-
Parkinson’s disease
- SNP:
-
Single nucleotide polymorphism
References
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–102.
Wang JY, Gong MY, Ye YL, Ye JM, Lin GL, Zhuang QQ, et al. The RIT2 and STX1B polymorphisms are associated with Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:300–2.
Zou M, Li R, Wang JY, Wang K, Wang YN, Li Y, et al. Association analyses of variants of SIPA1L2, MIR4697, GCH1, VPS13C, and DDRGK1 with Parkinson’s disease in East Asians. Neurobiol Aging. 2018;68:159e157-159e114.
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Masato A, Plotegher N, Boassa D, Bubacco L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener. 2019;14:35.
Doorn JA, Florang VR, Schamp JH, Vanle BC. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism Relat Disord. 2014;20(Suppl 1):S73-75.
Michel TM, Kasbauer L, Gsell W, Jecel J, Sheldrick AJ, Cortese M, et al. Aldehyde dehydrogenase 2 in sporadic Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(Suppl 1):S68-72.
Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:636–41.
Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem Res Toxicol. 2014;27:1359–61.
Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson’s disease. J Neurochem. 2015;133:14–25.
Cai H, Liu G, Sun L, Ding J. Aldehyde Dehydrogenase 1 making molecular inroads into the differential vulnerability of nigrostriatal dopaminergic neuron subtypes in Parkinson’s disease. Transl Neurodegener. 2014;3:27.
Galter D, Buervenich S, Carmine A, Anvret M, Olson L. ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson’s disease and in the ventral tegmental area in schizophrenia. Neurobiol Dis. 2003;14:637–47.
Werner CJ, Haussen RHV, Mall G, Wolf S. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteome Science. 2008;6:8.
Molochnikov L, Rabey JM, Dobronevsky E, Bonucelli U, Ceravolo R, Frosini D, et al. A molecular signature in blood identifies early Parkinson’s disease. Mol Neurodegener. 2012;7:26.
Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, et al. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest. 2014;124:3032–46.
Wey MCY, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and Motor Dysfunction in Mice Lacking Cytosolic and Mitochondrial Aldehyde Dehydrogenases: Implications for Parkinson’s Disease. PLoS One. 2012;7:e31522.
Fan H-H, Guo Q, Zheng J, Lian Y-Z, Huang S-S, Sun Y, et al. ALDH1A1 genetic variations may modulate risk of Parkinson’s disease in Han Chinese population. Front Neurosci. 2021;15:193.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Zhu JH, Chen CL, Flavahan S, Harr J, Su B, Flavahan NA. Cyclic stretch stimulates vascular smooth muscle cell alignment by redox-dependent activation of Notch3. Am J Physiol Heart Circ Physiol. 2011;300:H1770-1780.
Batzer MA, Deininger PL, Hellmann-Blumberg U, Jurka J, Labuda D, Rubin CM, et al. Standardized nomenclature for Alu repeats. J Mol Evol. 1996;42:3–6.
Capomaccio S, Cappelli K, Spinsanti G, Mencarelli M, Muscettola M, Felicetti M, et al. Athletic humans and horses: comparative analysis of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) expression in peripheral blood mononuclear cells in trained and untrained subjects at rest. BMC Physiol. 2011;11:3.
Martinez-Fierro ML, Garza-Veloz I, Carrillo-Sanchez K, Martinez-Gaytan V, Cortes-Flores R, Ochoa-Torres MA, et al. Expression levels of seven candidate genes in human peripheral blood mononuclear cells and their association with preeclampsia. Hypertens Pregnancy. 2014;33:191–203.
Salas-Leal AC, Sandoval-Carrillo A, Romero-Gutierrez E, Castellanos-Juarez FX, Mendez-Hernandez EM, La Llave-Leon O, et al. rs3764435 Associated With Parkinson’s Disease in Mexican Mestizos: Case-Control Study Reveals Protective Effects Against Disease Development and Cognitive Impairment. Front Neurol. 2019;10:1066.
Deininger P. Alu elements: know the SINEs. Genome Biol. 2011;12:236.
Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002;3:370–9.
Ahmed M, Li W, Liang P. Identification of three new Alu Yb subfamilies by source tracking of recently integrated Alu Yb elements. Mob DNA. 2013;4:25.
Coulondre C, Miller JH, Farabaugh PJ, Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–80.
Jourdy Y, Janin A, Fretigny M, Lienhart A, Negrier C, Bozon D, et al. Reccurrent F8 Intronic Deletion Found in Mild Hemophilia A Causes Alu Exonization. Am J Hum Genet. 2018;102:199–206.
Payer LM, Steranka JP, Yang WR, Kryatova M, Medabalimi S, Ardeljan D, et al. Structural variants caused by Alu insertions are associated with risks for many human diseases. Proc Natl Acad Sci U S A. 2017;114:E3984–92.
Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab. 1999;67:183–93.
Konkel MK, Walker JA, Hotard AB, Ranck MC, Fontenot CC, Storer J, et al. Sequence Analysis and Characterization of Active Human Alu Subfamilies Based on the 1000 Genomes Pilot Project. Genome Biol Evol. 2015;7:2608–22.
Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. EMBO J. 2008;27:1694–705.
Kuehnen P, Mischke M, Wiegand S, Sers C, Horsthemke B, Lau S, et al. An Alu Element-Associated Hypermethylation Variant of the POMC Gene Is Associated with Childhood Obesity. PLoS Genet. 2012;8:e1002543.
Polak P, Domany E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genomics. 2006;7:133.
Payer LM, Steranka JP, Ardeljan D, Walker J, Fitzgerald KC, Calabresi PA, et al. Alu insertion variants alter mRNA splicing. Nucleic Acids Res. 2019;47:421–31.
Nazarian A, Yashin AI, Kulminski AM. Summary-Based Methylome-Wide Association Analyses Suggest Potential Genetically Driven Epigenetic Heterogeneity of Alzheimer’s Disease. J Clin Med. 2020;9(5):1489.
Lovatt M, Adnan K, Kocaba V, Dirisamer M, Peh GSL, Mehta JS. Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol. 2020;30:101417.
Yu T, Cazares O, Tang AD, Kim HY, Wald T, Verma A, et al. SRSF1 governs progenitor-specific alternative splicing to maintain adult epithelial tissue homeostasis and renewal. Dev Cell. 2022;57(624–637):e624.
Choudhary B, Marx O, Norris AD. Spliceosomal component PRP-40 is a central regulator of microexon splicing. Cell Rep. 2021;36:109464.
Graham WJT, Putnam CD, Kolodner RD. The properties of Msh2-Msh6 ATP binding mutants suggest a signal amplification mechanism in DNA mismatch repair. J Biol Chem. 2018;293:18055–70.
Ardehali MB, Damle M, Perea-Resa C, Blower MD, Kingston RE. Elongin A associates with actively transcribed genes and modulates enhancer RNA levels with limited impact on transcription elongation rate in vivo. J Biol Chem. 2021;296:100202.
Acknowledgements
The authors are thankful to their colleagues for clinical data collection and management of patients. The authors appreciate all of the subjects participating in this study.
Funding
This work was supported by the National Natural Science Foundation of China (31701080, 81771380, 81771510 and 82071585), Zhejiang Provincial Natural Science Foundation (LD19H090001 and LZ19H090002), Science and Technology Department of Zhejiang Province (2018C37111), and Wenzhou Municipal Science and Technology Bureau (Y20190074 and C20170003).
Author information
Authors and Affiliations
Contributions
JHZ and XZ designed the research; HHF, JZ, LC and HJD conducted the experiments; HHF and KYW analyzed the data; XZ, XYH and ZW contributed samples; HHF and JHZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Second Affiliated Hospital and Yuying Children’s hospital, Wenzhou Medical University (No. 2017–31). All of the subjects participating in the study signed written informed consents. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Primers and restriction enzymes used in this study. Table S2. Mass-spectrometry results of DNA pulled-down proteinsa. Figure S1. Uncropped gel images for Fig. 1A, Fig. 3E and Fig. 3F. The red boxes represent the cropped area. Figure S2. Sequencing results of the short product (A) and the long product (B). The insertion ended with poly-A is highlighted in yellow; The inserted position is after the T which is highlighted in green. Figure S3. Similarity search results of the Yb8c4 sequence excluding the T at the 1st position and poly-A segment. Checked box indicates 100% matched. Figure S4. Reference gene expressions in PBMC samples. (A) Ct values of ACTB, GAPDH and HPRT1 in individual samples. (B) Correlation analysis of gene expression between ACTB and mean of ACTB, GAPDH and HPRT1. n = 8. Ct, cycle threshold; PBMC, peripheral blood mononuclear cell. Figure S5. Distribution of CpG sites in intron 4 of ALDH1A1. The asYb8c4 element is indicated in red. The analyzed CpG sites are highlighted in yellow. Figure S6. Individual methylation plots of the asYb8c4ins-introduced CpG island (A), the upstream (B) and downstream (C) CpG sites. Closed cycle indicates methylated CpG, open circle indicates unmethylated CpG. asYb8c4del, antisense Yb8c4 deletion; asYb8c4ins, antisense Yb8c4 insertion; Ctrl, control; PD, Parkinson’s disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, HH., Zheng, J., Huang, XY. et al. An antisense Alu transposon insertion/deletion polymorphism of ALDH1A1 may functionally associate with Parkinson’s disease. BMC Geriatr 22, 427 (2022). https://doi.org/10.1186/s12877-022-03132-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03132-1