Abstract
Esophageal stricture is a common complication after endoscopic submucosal dissection (ESD) for superficial esophageal cancer and precancerous lesions, we intend to investigate the independent risk factors of esophageal stricture after ESD by adding the data of included living habits, established a nomogram model to predict the risk of esophageal stricture, and verified it by external data. The clinical data and living habits of patients with early esophageal cancer and precancerous lesions who underwent ESD in the Affiliated Hospital of North Sichuan Medical College and Langzhong People's Hospital from March 2017 to August 2021 were retrospectively collected. The data collected from the two hospitals were used as the development group (n = 256) and the validation group (n = 105), respectively. Univariate and multivariate logistic regression analyses were used to determine independent risk factors for esophageal stricture after ESD and establish a nomogram model for the development group. The prediction performance of the nomogram model is internally and externally verified by calculating C-Index and plotting the receiver operating characteristic curve (ROC) and calibration curve, respectively. The results showed that Age, drinking water temperature, neutrophil–lymphocyte ratio, the extent of esophageal mucosal defect, longitudinal diameter of resected mucosa, and depth of tissue invasion (P < 0.05) were independent risk factors for esophageal stricture after ESD. The C-Index of the development group and validation group was 0.925 and 0.861, respectively. The ROC curve and area under the curve (AUC) of the two groups suggested that the discrimination and prediction performance of the model were good. The two groups of calibration curves are consistent and almost overlap with the ideal calibration curve, indicating that the predicted results of this model are in good agreement with the actual observed results. In conclusion, this nomogram model has a high accuracy for predicting the risk of esophageal stricture after ESD, providing a theoretical basis for reducing or avoiding esophageal stricture and guiding clinical practice.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is the seventh most common cancer and the sixth most common cause of cancer death in the world, and new cases and deaths in China account for 53.7% and 55.7% of the global total, respectively [1]. Esophageal squamous cell carcinoma (ESCC) is the main histological type, the 5-year overall survival rate for patients with advanced ESCC is less than 15%, while in patients diagnosed early in the disease, this may reach 85% [2]. Therefore, early diagnosis and treatment of esophageal cancer can significantly reduce its mortality and is the key to improving patient survival and prognosis.
At present, with the development of endoscopic techniques, endoscopic submucosal dissection (ESD) has gradually become the treatment of choice for early esophageal cancer and precancerous lesions [3]. Esophageal stricture, as one of the major complications after ESD, is characterized by varying degrees of dysphagia [4]. Although it has been shown that lesion size, the extent of mucosal defect, and depth of tissue invasion may be risk factors for esophageal stricture after ESD [5,6,7,8,9]. However, the risk factors of esophageal stricture after ESD are not fully clear, and studies on the effects of living habits on esophageal stricture after ESD have not been reported. If the risk factors of esophageal stricture after ESD can be identified, it will help clinicians to take targeted preventive measures in advance, which is very important to reduce or avoid the occurrence of esophageal stricture.
The nomogram model plays an important role in predicting the individualized assessment of patients, the occurrence of diseases and survival prognosis [10,11,12]. However, there are few reports on constructing nomogram models to predict esophageal stricture after ESD. Therefore, this paper retrospectively analyzed the relevant data of patients after ESD to identify the independent risk factors of esophageal stenosis after ESD, and then constructed a nomogram model, and assessed the predictive value of this model by means of internal and external validation, providing a theoretical basis for guiding clinical practice.
Materials and methods
Patients
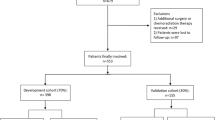
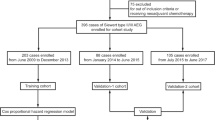
In this paper, we retrospectively collected patients with superficial esophageal squamous cell carcinoma and precancerous lesions who underwent esophageal ESD in two hospitals: North Affiliated Hospital of Sichuan Medical College and Langzhong People's Hospital from March 2017 to August 2021, and collected the clinical data and living habits of these patients. The date of first patient data access for this study was January 20, 2022. After strict inclusion criteria and exclusion criteria, 256 patients in the Affiliated Hospital of North Sichuan Medical College and 105 patients in Langzhong People's Hospital were included, and relevant data were collected as the development dataset and validation dataset, respectively. In this study, we routinely used oral steroids in patients with more than 3/4 of the circumferential percentage of mucosal defects, who started oral prednisone acetate at a dose of 30 mg/day on the third postoperative day, which was reduced by 5 mg every 2 weeks. When the dose was reduced to 20 mg/day, 5 mg was reduced every week. Steroids were administered for a total of 8 weeks. In the development group, 67 patients received steroids; in the validation group, 23 patients received steroids. Postoperative re-examinations at endoscopes were performed every 3 months during the first year after ESD to determine whether esophageal strictures developed. This study is a retrospective design and we applied to the ethics committee for a waiver of informed consent, which waived the need for participant consent. The ethical study was performed at The Affiliated Hospital of North Sichuan Medical College under the Declaration of Helsinki and the protocol was approved by the ethics committee of the Affiliated Hospital of North Sichuan Medical College (batch number: 2022ER018-1).
The inclusion criteria included: (1) preoperative comprehensive imaging examination to rule out peripheral lymph nodes and distant metastasis; (2) preoperative endoscopic biopsy findings suggestive of early ESCC or precancerous lesions; (3) endoscopic ultrasonography was performed to rule out cases with tumor invasion depth exceeding the mucosal layer; (4) none of the patients had serious underlying diseases.
Exclusion criteria included: (1) incomplete, blurred, or lost patient-relevant data; (2) Postoperative pathology after ESD revealed esophageal adenocarcinoma, papilloma, spindle cell tumor, etc., with the depth of tissue invasion exceeding 200 μm in the submucosa; (3) loss to follow-up or patient death; (4) patient with a history of other tumors.
Pathological diagnostic criteria: According to the criteria of the World Health Organization [13], pathological diagnosis includes low-grade intraepithelial neoplasia (LGIN), high-grade intraepithelial neoplasia (HGIN), and esophageal squamous cell carcinoma (ESCC).
Data collection
In both the development and validation groups, we collected data on the clinical and lifestyle habits of patients with early esophageal cancer and precancerous lesions treated with ESD.
-
(1) clinical data: Age, gender, preoperative high neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lympho-monocyte ratio (LMR), the longitudinal length of the resected mucosa, lesion location, en bloc resection, percentage of the circumferential mucosal defect, postoperative pathological type, positive margin, and depth of Invasion.
-
(2) living habits data: Long-term smoking, long-term drinking, high protein diet, fruit and vegetable intake, eating speed, pickled food, fried food, and high-temperature water intake.
Variables and Definitions:
-
(1)
NLR, PLR, and LMR: Preoperative blood routine was collected from patients, including neutrophil, lymphocyte, and monocyte counts. The neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and Lympho-monocyte ratio (LMR) were calculated for each patient, and then the median of the above parameters was found. Those above the median were in the high-value group, and those below the median were in the low-value group.
-
(2)
Sites of esophageal lesions: The sites of esophageal lesions were classified according to the latest segmented criteria [14]: 1) cervical esophagus: 15—20 cm from the upper incisors; 2) upper thoracic esophagus: 20—25 cm from the upper incisors; 3) middle thoracic esophagus: 25—30 cm from the upper incisors; 4) lower thoracic esophagus: 30—40 cm from the upper incisors.
-
(3)
Depth of tissue invasion: All of them were based on the Paris classification criteria for gastrointestinal tumors in 2002 [15]. The depth of invasion of the diseased tissue was divided into M (confined to the mucosal layer) and SM (infiltrating into the submucosa). Among them, M is divided into M1 (involving the mucosal epithelial layer), M2 (involving the lamina propria), and M3 (involving the Muscularis mucosae); SM is divided into SM1 (depth of invasion no more than 200 μm into the submucosa), SM2 (depth of invasion more than 200 μm into the submucosa), and SM3 (depth of invasion reaching the muscular propria).
-
(4)
Assessment of living habits: Referring to the questionnaire survey on living habits by Liu et al. [16]. If the patient usually eats/drinks (e.g., high protein diet, fruit and vegetable intake, pickled food, fried food, high-temperature water intake) more frequently than 3 times a week, it is defined as frequent, and vice versa. Limit the length of time the patient is eating to between 20 and 40 min, defined as fast or slow if the time is below or above this threshold, respectively, and normal otherwise. If the patient's drinking water temperature reaches the threshold of self-perceived warmth, it is defined as high-temperature water intake. Patients who smoked more than 20 cigarettes per day for more than 10 years were defined as long-term smoking; patients who drank more than 100 g per day for more than 10 years were defined as long-term drinking.
-
(5)
Esophageal stricture: Esophageal stricture was defined if the patient had symptoms of dysphagia or if standard endoscopy (9.8 mm in diameter) could not pass through the esophagus [17].
Statistical analysis
Data were statistically analyzed using SPSS 25.0 software and R 4.2.0 software. Enumeration data were compared by chi-square test, measurement data were expressed as mean ± standard deviation \(\left(\overline{\mathrm{x}} \pm \mathrm{s }\right)\), and an independent sample t-test was used for comparison. In this study, P-value, odds ratio (OR), and 95% confidence interval (CI) were used to describe the independent risk factors of esophageal stricture after ESD.
SPSS 25.0 software was used for univariate analysis of variables in the developmental group, and multivariate logistic stepwise regression analysis was performed for statistically significant variables (P < 0.05) to obtain independent risk factors for esophageal stricture after ESD. Based on the results of multivariate logistic stepwise regression analysis, a nomogram model for predicting esophageal stricture was successfully developed using R 4.2.0 software, and the model fit was assessed by the Hosmer–Lemeshow test.
In this paper, we use development group data for internal validation and validation group data for external validation to test the predictive performance of this nomogram model separately. We use the data of the development group and the validation group to draw the ROC curve and calculate the C-Index, respectively, to evaluate the predictive accuracy of this model, and then use the Bootstrap method to draw the calibration curve of these two groups, and further evaluate whether the prediction probability of this model is consistent with the observation probability. Flowchart for the methods is shown in Fig. 1.
Results
Participant characteristics
A total of 361 patients after esophageal ESD were included in this study, consisting of a development group (n = 256) and a validation group (n = 105), with a ratio of 7:3. In the development group, there were 147 males and 109 females, with an average age of 63.01 ± 8.397 (years); in the validation group, there were 53 males and 52 females, with an average age of 63.10 ± 7.252 (years). All patients were followed up, and postoperative endoscopic reexamination was performed every 3 months in the first year, if there were no significant abnormalities, subsequent reexamination was performed once a year. In the development group, 38 patients developed postoperative stenosis, with an incidence rate of esophageal stenosis of 14.8%; in the validation group, 20 patients developed postoperative stenosis, with an incidence rate of esophageal stenosis of 19.05%. The basic characteristics and living habits of the development group and validation group are shown in Table 1, respectively. There were statistically significant differences between the two groups in terms of BMI, longitudinal diameter of the resected mucosa, en bloc resection, extent of dissected mucosa, long-term smoking, high-protein diet, pickled food, fruit, and vegetable intake, and there was no significant difference in the other variables.
Univariate and multivariate logistic regression analysis
Table 2 summarizes the results of developing group univariate and multivariate logistic regression analyses to identify significant predictors of esophageal stricture after ESD. Univariate analysis showed that esophageal stricture after ESD was associated with age, preoperative NLR, longitudinal length of the resected mucosa, PCMD, postoperative pathological type, postoperative hormone use, positive resection margin, depth of invasion, fruit and vegetable intake, eating rate, pickled food, and high-temperature water intake (P < 0.05). In order to control for confounding factors, we performed multivariate logistic stepwise regression for the above statistically significant factors. The results showed that age of more than 60 years, long-term high-temperature water intake, preoperative high NLR, esophageal mucosal defect of more than 3/4, longitudinal diameter of resected mucosa, and depth of tissue invasion of more than M2 were independent risk factors for the esophageal stenosis after ESD. We found a cutoff value of 4 cm for the longitudinal length of the resected mucosa by SPSS software, with a Youden index of 0.603, corresponding to a sensitivity of 84.2% and a specificity of 76.1%.
Nomogram development
Collinearity analysis of age, drinking temperature, NLR, longitudinal diameter of resected mucosa, circumferential range of mucosal defects, and depth of tissue invasion resulted in variance inflation factors (VIFs) of 1.023, 1.034, 1.010, 1.466, 1.496, and 1.094, respectively, which indicated that there was no multicollinearity among the six independent risk factors. Based on the results of logistic regression analysis, we developed an individualized prediction model for esophageal stricture after ESD using R, the nomogram model (Fig. 2). The total risk score is determined by obtaining the point of each relevant factor on the nomogram, adding the scores of all variables, and directly obtaining the risk probability of developing esophageal stricture, if the risk probability is greater than 50%, the patient is predicted to develop esophageal stricture.
Nomogram validation
The fitness of the model was analyzed using the Hosmer–Lemeshow test, and the results showed that the fitness of the model was good and could be used to predict the risk of occurrence (P = 0.979). The performance of the nomogram model was verified by calculating the C-Index and plotting the ROC curve and the calibration curve. First, we first internally validate the model. We calculated the C-Index of the development group to be 0.925, plotted the ROC curve (Fig. 3), and showed that the AUC was 92.5%, and the sensitivity and specificity were 89.5% and 79.8%, respectively, indicating that the discrimination and prediction performance of this model was good. The calibration curve (Fig. 4) shown that the apparent appearance of the developed calibration curve almost overlaps with the ideal nomogram calibration curve. We then externally validated the model. We plotted ROC curves using the validation set (Fig. 5), which showed that the AUC was 86.1%, and sensitivity and specificity of 89.4% and 70.0%, respectively, indicating that the model has good predictive performance in different populations. The calibration curve of the validation group (Fig. 6) showed that it was basically consistent with the calibration curve of the development group, indicating that the prediction of the nomogram model was in good agreement with the observation results.
Discussion
In recent years, ESD has gradually become the treatment strategy of choice for superficial esophageal squamous cell carcinoma and precancerous lesions [4]. However, esophageal stricture after ESD severely affects patients' quality of life. At present, the risk factors of esophageal stricture after ESD are still unclear, the effect of patients' living habits on esophageal stricture after ESD has not been reported, and the prediction model of esophageal stricture after ESD lacks effective external validation. Therefore, this study retrospectively collected the basic data and living habits of patients, used univariate and multivariate analysis to determine the risk factors of esophageal stenosis after ESD, and then established a nomogram model to predict the risk of esophageal stenosis, and validated the model by internal and external validation.
This study found that age of more than 60 years, long-term high-temperature water intake, preoperative high NLR, esophageal mucosal defect of more than 3/4, longitudinal diameter of resected mucosa, and depth of tissue invasion of more than M2 were independent risk factors for esophageal stenosis after ESD (P < 0.05). Previous studies have shown that the extent of esophageal mucosal defects, the depth of tissue invasion, and the length of longitudinal mucosal defects are reliable independent risk factors for esophageal stricture after ESD [7, 8, 18,19,20,21], but the extent of specific mucosal defects and the length of longitudinal mucosal defects still vary individually in different research reports. Katada et al. found that the frequency of esophageal strictures with mucosal defect circumference above 3/4 and longitudinal diameter > 30 mm was significantly higher in patients [18], thus confirming a correlation between the extent and size of mucosal resection and esophageal strictures. Tang et al. in investigating the risk factors for refractory stricture after extensive ESD, found that the length of longitudinal resection > 50 mm and the depth of tissue invasion above M2 were independent risk factors for refractory esophageal stricture [22]. Ono et al. used stepwise logistic regression analysis to investigate predictors of stricture after ESD and found that circumferential resection > 3/4, longitudinal diameter > 30 mm, and histological depth of M2 were reliable predictors of postoperative esophageal stricture [8]. The results of this study are basically consistent with the conclusions of previous literature.
In previous studies, inflammatory markers such as NLR, PLR, and LMR are often used to predict the stage, differentiation, invasion, and prognosis of tumors, and have good predictive value [23, 24]. The above indicators were included in this study. Statistical analysis showed that preoperative high NLR was an independent risk factor for esophageal stenosis after ESD, and preoperative high NLR was more likely to have postoperative esophageal stenosis (P = 0.003, OR = 5.65, 95% CI: 1.782—17.917). In fact, the formation of esophageal stricture itself requires the participation of inflammatory mediators, which play an important role in scar repair [25]. Therefore, inflammatory markers may be one of the risk factors for esophageal stricture.
The incidence of cancer increases with age, which is a risk factor for esophageal cancer and is commonly used to predict the occurrence and prognosis of cancer [26, 27]. The effect of age on esophageal stricture was rarely reported in previous studies, but the results of this study indicated that an age older than 60 years was an independent risk factor for esophageal stricture (P = 0.003, OR = 11.229, 95% CI: 2.315—54.466). Funakawa et al. found that there was no significant difference in age, gender, alcohol consumption and smoking, and tumor location between the esophageal stenosis group and the non-stenosis group after ESD [28]. However, this study obtained that age was an independent risk factor for esophageal stricture based on excluding confounding factors, which had some accuracy and reliability. The increased risk of esophageal stricture in elderly patients probably is caused by factors such as trace elements and vitamin deficiency related to the mucosal repair and poor local blood circulation [29,30,31].
Liu et al. established an age-stratified risk prediction model for esophageal squamous cell carcinoma and its precancerous lesions on the basis of a population-based large endoscopic screening trial, which included predictors of lifestyle habits and dietary status [16]. In recent years, no report has been found that the patient's living habits are included in the risk factors and prediction model of esophageal stenosis. This study, the living habits of patients were included in this study for the first time, and the results of multivariate logistic stepwise regression revealed that long-term high-temperature water intake was an independent risk factor for esophageal stenosis after ESD (P < 0.001, OR = 13.378, 95% CI: 3.987—44.882), but not with high-protein diet, fruit and vegetable intake, pickled food, fried food, eating speed, long-term smoking and alcohol consumption (P > 0.05). High temperature have been reported to lead to esophageal mucosal edema and inflammation formation, long-term will lead to surrounding fibrous hyperplasia, collagen accumulation, scar contracture, and ultimately esophageal scar stenosis [32]. Therefore, high-temperature water intake probably is one of the causes of esophageal stricture after ESD. However, its accuracy and effectiveness need to be verified by more clinical studies in the future.
In this study, nomogram models were established to predict the risk of esophageal stricture after ESD, and the models were internally and externally validated by calculating C-Index, plotting ROC curves, and calibration curves. The c-index of the development group and the validation group were 0.925 and 0.861, respectively, and the ROC curves of the two groups suggested that the discrimination and prediction performance of the model were good. The two groups of calibration curves were basically consistent and almost overlapped with the ideal calibration curve, indicating that the predictions of the nomogram model were in good agreement with the observations. Since the data sources between the development group and validation group are different, there are some differences in the patient's living habits, physician technical level, etc., but this does not significantly affect the discrimination and calibration of this prediction model. External validation showed that the model had good performance in predicting esophageal stricture after ESD, indicating that the model can be well-suitable for different populations and medical centers, which is important to reduce or avoid the occurrence of esophageal stricture.
However, this study still has some limitations. This study was a multi-center, retrospective, small-sample design, so the clinical effectiveness needed to be verified in a large-sample, prospective study; secondly, the evaluation of the patient's living habits and other indicators had certain subjectivity, and the relevant factors should be quantified by the standard for accurate analysis; lastly, due to the limited factors included in this study, such as the technical level of endoscopists, patient's work, living environment, and other factors may also affect the study outcome, it needs to be considered in the future relevant studies.
Conclusion
In this study, a nomogram model was established to predict the risk of esophageal stenosis after ESD. Both internal and external validations showed that the model has good predictive performance and can be well adapted to different populations and medical centers. It is a free tool beneficial to clinical risk assessment, which has a positive significance for preventing esophageal stenosis and guiding clinical practice. However, more prospective validation is needed in the future.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available for the sake of considering the principle of protecting patient privacy but are available from the respective authors upon reasonable request.
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- ESD:
-
Endoscopic submucosal dissection
- LGIN:
-
Low-grade intraepithelial neoplasia
- HGIN:
-
High-grade intraepithelial neoplasia
- NLR:
-
Neutrophil–lymphocyte ratio
- PLR:
-
Platelet-lymphocyte ratio
- LMR:
-
Lympho-monocyte ratio
- VIFs:
-
Variance Inflation Factors
- OR:
-
Odds ratio
- 95%CI:
-
95% Credibility Interval
- ROC:
-
Receiver Operating Characteristic
- AUC:
-
Area Under ROC Curve
- BMI:
-
Body mass index
- L-D:
-
Longitudinal diameter of the resected specimen
- PCMD:
-
Percentage of the circumferential mucosal defect
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin. 2020;70(4):313.
Lao-Sirieix P, Fitzgerald RC. Screening for oesophageal cancer[J]. Nat Rev Clin Oncol. 2012;9(5):278–87.
Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer[J]. J Gastrointest Oncol. 2015;6(1):20–30.
Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer[J]. Ann Cardiothorac Surg. 2017;6(2):88–98.
Mahawongkajit P, Tomtitchong P, Boochangkool N, Limpavitayaporn P, Kanlerd A, Mingmalairak C, et al. Risk Factors for Esophageal Stricture in Grade 2b and 3a Corrosive Esophageal Injuries[J]. J Gastrointest Surg. 2018;22(10):1659–64.
Aadam AA, Abe S. Endoscopic submucosal dissection for superficial esophageal cancer[J]. Dis Esophagus. 2018;31(7). https://doi.org/10.1093/dote/doy021.
Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, et al. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma[J]. Endoscopy. 2014;46(8):640–4.
Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms[J]. Endoscopy. 2009;41(08):661–5.
Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, et al. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma[J]. Surg Endosc. 2016;30(9):4049–56.
Cheng H, Sun X, Ji X, Zhang J, Lv J, Li T, et al. Risk factors and the potential of nomogram for predicting hospital-acquired pressure injuries[J]. Int Wound J. 2020;17(4):974–86.
Peng K, Wang S, Gao L, You H. A nomogram incorporated lifestyle indicators for predicting nonalcoholic fatty liver disease[J]. Medicine (Baltimore). 2021;100(26): e26415.
Chao YK, Chang HK, Tseng CK, Liu YH, Wen YW. Development of a nomogram for the prediction of pathological complete response after neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma[J]. Dis Esophagus. 2017;30(2):1–8.
Gabbert HE, Shimoda T, Hainaut P, Nakamura Y, Field JK, Inoue H. Squamous cell carcinoma of the oesophagus. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics. Tumours of the digestive system. Lyon: IARC Press; 2000. p. 8–19.
Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer[J]. J Thorac Oncol. 2017;12(1):36–42.
Lambert R, Lightdale C. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002[J]. Gastrointest Endosc. 2003;58(6 Suppl):S3–43.
Liu M, Liu Z, Cai H, Guo C, Li X, Zhang C, et al. A Model To Identify Individuals at High Risk for Esophageal Squamous Cell Carcinoma and Precancerous Lesions in Regions of High Prevalence in China[J]. Clin Gastroenterol Hepatol. 2017;15(10):1538–46.
Kishida Y, Kakushima N, Kawata N, Tanaka M, Takizawa K, Imai K, et al. Complications of endoscopic dilation for esophageal stenosis after endoscopic submucosal dissection of superficial esophageal cancer[J]. Surg Endosc. 2015;29(10):2953–9.
Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions[J]. Gastrointest Endosc. 2003;57(2):165–9.
Lewis JJ, Rubenstein JH, Singal AG, Elmunzer BJ, Kwon RS, Piraka CR. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus[J]. Gastrointest Endosc. 2011;74(4):753–60.
Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer[J]. Dis Esophagus. 2009;22(7):626–31.
Chen M, Dang Y, Ding C, Yang J, Si X, Zhang G. Lesion size and circumferential range identified as independent risk factors for esophageal stricture after endoscopic submucosal dissection[J]. Surg Endosc. 2020;34(9):4065–71.
Tang J, Kong F, Li J, Liu F, Kong X, Li Z. Independent risk factors for esophageal refractory stricture after extensive endoscopic submucosal dissection[J]. Surg Endosc. 2021;35(7):3618–27.
Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis[J]. Cancer Manag Res. 2018;10:6167–79.
Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis[J]. Ann Surg Oncol. 2016;23(2):646–54.
Broughton GN, Janis JE, Attinger CE. The basic science of wound healing[J]. Plast Reconstr Surg. 2006;117(7 Suppl):12S-34S.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin. 2016;66(2):115–32.
Pasquer A, Gronnier C, Renaud F, Duhamel A, Théreaux J, Carrere N, et al. Impact of Adjuvant Chemotherapy on Patients with Lymph Node-Positive Esophageal Cancer who are primarily Treated with Surgery[J]. Ann Surg Oncol. 2015;22(Suppl 3):S1340–9.
Funakawa K, Uto H, Sasaki F, Nasu Y, Mawatari S, Arima S, et al. Effect of endoscopic submucosal dissection for superficial esophageal neoplasms and risk factors for postoperative stricture[J]. Medicine (Baltimore). 2015;94(1):e373.
Gunin AG, Petrov VV, Golubtzova NN, Vasilieva OV, Kornilova NK. Age-related changes in angiogenesis in human dermis[J]. Exp Gerontol. 2014;55:143–51.
Shimoda Y, Matsuo K, Ono K, Soma Y, Ueyama T, Matoba S, et al. Aging differentially alters the expression of angiogenic genes in a tissue-dependent manner[J]. Biochem Biophys Res Commun. 2014;446(4):1243–9.
Gana W, De Luca A, Debacq C, Poitau F, Poupin P, Aidoud A, et al. Analysis of the Impact of Selected Vitamins Deficiencies on the Risk of Disability in Older People[J]. Nutrients. 2021;13(9):3163.
Zhao SL, Gu CD. Advances in the research of scar stricture after esophageal burn. Zhonghua Shao Shang Za Zhi. 2013;29(5):459–62.
Acknowledgements
The authors thank Prof Yang Guodong for the excellent assistance.
Authors’ information
All authors participated in this study.
Funding
This study was supported by the Construction of Key Experimental Platform for Pancreatic Diseases in Sichuan Province (18SXHZ0371) and the Hospital-level project of the Affiliated Hospital of North Sichuan Medical College (2022ZD005). The funders rationally apply scientific research funds to the project.
Author information
Authors and Affiliations
Contributions
MZ, TX conducted this study design and manuscript drafting. WJT, CYL, CK, and RXY contributed to the acquisition of clinical data. YGD provided the statistical analysis and review this paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was performed at The Affiliated Hospital of North Sichuan Medical College under the Declaration of Helsinki. Verbal consent was obtained from the participants and approved by the ethics committee of The Affiliated Hospital of North Sichuan Medical College for this study(ethical batch number: 2022ER018-1).
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mu, Z., Tang, X., Wang, J. et al. Construction and external validation of a nomogram model for predicting the risk of esophageal stricture after endoscopic submucosal dissection: a multicenter case–control study. BMC Gastroenterol 23, 226 (2023). https://doi.org/10.1186/s12876-023-02855-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02855-8