Abstract
Background
Studies have confirmed the prognostic value of the expression status of human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer. However, its role in early gastric cancer (EGC) remains largely unknown. This study explored the association between HER2 overexpression and clinical outcomes of patients with EGC.
Methods
A total of 211 patients who had undergone endoscopic treatment for pN0 EGC were enrolled. The HER2 expression status was assessed using immunohistochemistry (IHC).
Results
The prevalence of HER2 overexpression was 14.2%. HER2 overexpression showed a significant correlation with tumor location (P = 0.033). Multivariate analysis showed that HER2 overexpression was significantly associated with an increased risk of tumor recurrence in pN0 EGC (hazard ratio [HR] = 3.97; 95% confidence interval [CI] 1.30–12.14; P = 0.016) but not overall survival (OS) or disease-specific survival (DSS). Of the included patients, age was associated with OS (HR = 1.11; 95% CI 1.04–1.18; P = 0.002], whereas lymphovascular invasion was significantly associated with poor DSS (HR = 33.66; 95% CI 3.05–371.25; P = 0.004).
Conclusion
This study shows that HER2 overexpression is significantly associated with tumor recurrence in pN0 EGC. Hence, Her2 testing at diagnosis is important and differential treatment and/or follow up strategies for patients with Her2 overexpression may merit future study.
Similar content being viewed by others
Introduction
Gastric cancer remains an important cancer worldwide and imposes significant health-related and economic burden on the patients and society. According to the 2018 global cancer statistics, gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer-related death globally [1]. With an estimated 0.46 million cases and 0.39 million associated deaths, gastric cancer is the third most frequent cancer and the second leading cause of cancer-related death in China [2]. Early gastric cancer (EGC) can be defined as mucosal carcinoma and/or submucosa-invasive carcinoma with or without lymph node metastasis (LNM). With the recent introduction of mass endoscopy screening and advancements in diagnostic technology, the detection rate of EGC has significantly increased in China [3]. Given similar efficacy with regard to the oncologic outcomes, practicing clinicians tend to administer endoscopic treatment over open or laparoscopic surgery. Evidence suggests that endoscopic resection (ER) yields similar long-term outcomes and considerable advantages regarding surgery time, hospital stay, costs, and complications as surgical resection; however, it is also associated with several disadvantages including higher risk of local recurrence and metachronous lesions [4, 5]. Although the long-term outcomes of patients with EGC receiving ER are excellent, several conventional risk factors, including depth of tumor invasion, lymph node status, and histologic type, are associated with poor prognosis [6,7,8]. Whether other unknown risk factors of EGC exist remains unclear.
Human epidermal growth factor receptor 2 (HER2) is a member of the HER family of tyrosine receptor kinases, encoded by proto-oncogene ERBB2 on chromosome 17 [9]. Evidence suggests that HER2 can inhibit cell apoptosis and promote proliferation, thus essentially contributing to cancer cell survival and aggressiveness [10]. Recent studies have shown that HER2 expression is associated with the prognosis of advanced gastric cancer (AGC) [11]. The phase III ToGA trial established that trastuzumab, an anti-HER2 antibody, in combination with chemotherapy can be considered as a new standard treatment for patients with HER2-positive AGC [12]. However, whether HER2 is associated with the prognosis of EGC remains unclear. Therefore, this study explored whether the overexpression of HER2 is associated with poor prognosis of EGC.
Methods
Study population
Patients who were diagnosed with EGC between July 2009 and December 2013 by endoscopic screening and subsequent pathology at Chinese PLA General Hospital were included. The exclusion criteria were (1) other malignancies; (2) incomplete clinical or pathological data.
Patients were followed up by phone calls to determine survival status and cancer recurrence. The most recent follow-up was conducted in July 2020. All included patients were followed up for at least 6 years. Finally, 211 patients were included in the analysis. Additional file 1: Figure S1 shows the process of identification for participants. The study was conducted under the approval of the Ethics Board of the Chinese PLA General Hospital (ethics number: S2020-251-01), in accordance with the standards of the Declaration of Helsinki and Good Clinical Practice guidelines.
Immunohistochemistry
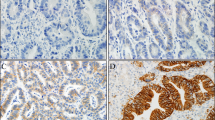
HER2 expression status was determined using immunohistochemistry (IHC). Rabbit anti-human HER2 antibody was used (Roche Diagnostics Japan. Tokyo, Japan). Two pathologists individually scored the HER2 expression. Conflicts were resolved by a third pathologist’s interpretation. A semiquantitative approach, recommended by the National Comprehensive Cancer Network guidelines, was used to classify the expression status of HER2. We classified HER2 expression status into 2 groups: negative (0 and 1 +) and overexpression (2 + and 3 +) [13]. The definition for each was as follows: 0 = no membrane staining, or membrane responds to staining with < 10% of tumor cells; 1 + = faint staining or barely recognizable staining in more than 10% of tumor cells; 2 + = weak to moderate staining of the entire membrane in > 10% of tumor cells; 3 + = > 10% of tumor cells show a strong complete staining reaction in the entire membrane.
Pathologic characteristics and definitions
Pathologic characteristics included depth of infiltration, lesion location, lesion morphology, lesion differentiation, tumor size, and lymphovascular infiltration. Depth of infiltration was categorized as mucosal or submucosal; lesion location as upper third, middle third, or lower third based on the lesion location on the longitudinal axis of the stomach; and lesion morphology as elevated (types 0-I, 0-IIa, 0-I + IIa, 0-IIa + IIb, 0-IIa + IIc), flat (type 0-IIb), or depressed (types 0-IIc, 0-III, 0-IIc + IIa, and 0-III + IIa) [14]. Histologically, tumors were characterized into 2 types: differentiated (papillary adenocarcinoma or moderately- and well-differentiated tubular adenocarcinoma) or undifferentiated (poorly differentiated tubular adenocarcinoma, signet-ring cell carcinoma, or mucinous adenocarcinoma). Tumor size was measured by the longest diameter of the lesion in the specimen and categorized into 2 groups: > 2 cm or ≤ 2 cm. Lymphovascular infiltration was classified into 2 groups: present or absent. In this study, three other clinical variables were also included: resection margin (positive or negative), current gastric ulcer (yes or no) and current H. pylori (Hp) infection when admitted to our hospital for EGC. Patients had previous Hp infection and have received successful Hp eradication therapy were classified as not having current Hp infection. Methodology of Hp diagnostic included serum antibody, rapid urease test, immunohistochemistry, 13C or 14C urea breath test, or stool antigen test.
Prognostic outcomes
Prognostic outcomes of this study included overall survival (OS), disease-specific survival (DSS), and tumor recurrence. OS and DSS was ascertained by asking the family members the detailed information recorded on the death certificate of the patients.The OS period was determined from the date of endoscopic treatment for EGC until the date of death from any cause. The DSS period was determined from the date of endoscopic treatment for EGC to the date of death due to tumor recurrence (patients experienced recurrent GC, and died from dyscrasia or massive hemorrhage due to GC, or died from malignant event caused by GC distant metastasis, including severe lung infection, cerebral edema or hernia, or hepatic failure). The recurrence pattern in this study included both metachronous recurrence and metastatic recurrence. The tumor recurrence period was defined as the time from endoscopic treatment for EGC to the date of detection of new gastric cancer. Tumor recurrence was identified using routine abdomen-pelvis computed tomography and endoscopy after endoscopic treatment.
Statistical analysis
We reported and compared the baseline clinicopathologic characteristics of included patients according to HER2 expression status, using the chi-square test or Fisher exact test for categorical data and the Student t test for continuous data. Multivariate Cox regression with stepwise backward selection was applied to identify factors associated with prognostic outcomes. For OS and DSS, 8 factors (age, sex, tumor size, depth of invasion, resection margin status, lymphovascular invasion, tumor differentiation, and HER2 expression status) were included in the model. For tumor recurrence, 2 additional factors (recent history of gastric ulcer and HP infection) were added in the model. These factors were chosen because they were considered potentially clinically relevant for each outcome. Survival curves for each prognostic outcome were generated using the Kaplan–Meier method and were compared using the log-rank test. Statistical analyses were performed using SPSS 26.0 for Windows (SPSS, Inc., Chicago, IL). Survival curves were graphed using GraphPad Prism 8 (GraphPad Prism version 8.1, GraphPad Software, La Jolla, CA). Two-tailed P values < 0.05 were considered statistically significant.
Results
Baseline characteristics of patients with EGC
A total of 211 patients who received endoscopic treatment for EGC were finally included. Baseline characteristics of included patients are summarized in Table 1. Of the 211 patients, 162 (76.8%) were men, and the mean age was 61.6 years (range 40–83 years). Of total, 30 patients (14.2%) had gastric ulcer, and 26 (12.3%) had a HP infection before endoscopic treatment. The most common lesion location was the lower third (116, 55.0%) on the longitudinal axis. The most common gross appearance of the lesion was of the elevated type (93, 44.1%). With regard to invasion depth, 193 patients (91.4%) had mucosal cancer. The differentiated-type EGC was significantly more prevalent than the undifferentiated-type EGC (n = 200, 94.8%), and 182 patients (86.3%) had a tumor size ≤ 2 cm. Furthermore, only 4 patients (1.9%) had lymphovascular infiltration. Finally, according to our definition, 30 patients (14.2%) had HER2 overexpression.
Clinicopathologic characteristics according to HER2 protein expression status
The clinicopathologic characteristics per HER2 protein expression status as assessed using IHC are summarized in Table 2. A significant correlation was observed between HER2 status and tumor location (P = 0.033) (Table 2). No significant associations were seen between HER2 expression and age, sex, history of gastric ulcer, HP infection, morphologic type, invasion depth, differentiated type EGC, proportions of large tumors (> 2 cm), and lymphovascular infiltration.
Association between HER2 expression and prognostic outcomes
To examine the association between HER2 expression and prognostic outcomes, multivariate analyses were performed using the Cox proportional hazard model with backward elimination. The analyses revealed significant associations between age and poor OS (hazard ratio [HR] = 1.11; 95% confidence interval [CI] 1.04–1.18; P = 0.002), lymphovascular invasion and poor DSS (HR = 33.66; 95% CI 3.05–371.25; P = 0.004), and HER2 overexpression and tumor recurrence (HR = 3.97; 95% CI 1.30–12.14; P = 0.016)(Table 3). The log-rank test revealed a significant difference in tumor recurrence but not OS and DSS between HER2-negative patients and patients with HER2 overexpression (Figs. 1, 2, 3).
Sensitivity analysis
A sensitivity analysis was conducted by excluding the patients lost to follow-up. Sensitivity analysis showed the similar results (Additional file 2: Table S1).
Discussion
This study aimed to examine the prognostic utility of HER2 expression status in patients with EGC who received endoscopic treatment. We followed up 211 patients with EGC for a period of 72 months and found that HER2 overexpression was only associated with increased risk of tumor recurrence, not OS or DSS. These results are novel and would inform future studies.
Prevalence of HER2 positivity in patients with gastric cancer varies across studies. A review by Boku et al. showed that the prevalence ranged from 3.8 to 36.6% [15]. However, owing to the lack of studies, determining the true prevalence of HER2 positivity in patients with EGC is challenging. In this study, we found that the prevalence of HER2 overexpression is 14.2%, which is in line with that reported by Yan et al. [16]. In our study, the HER2 expression status was only associated with tumor location. However, Yan et al. and Han et al. both found that the HER2 positivity rate was associated with differentiated types, unlike in our study [16, 17]. This difference could be attributed to the inclusion of only those patients who received endoscopic treatment and a small proportion of patients with the undifferentiated type (5.7%) in our study. The studies by Yan et al. and Han et al. included patients who received surgical treatment, and the proportions of patients with the undifferentiated type were relatively high (35.8% and 53.5%, respectively) in their studies [16, 17]. Future studies are warranted to further explore the association between clinicopathologic characteristics and HER2 status.
Previous studies have shown that HER2 expression status is strongly associated with the prognosis of patients with AGC [11, 18]; however, only few studies have focused on the association between HER2 expression status and the clinical outcomes of patients with EGC. Yan et al. assessed the expression of HER2 in surgical specimens of 67 patients with EGC and without LNM who received surgical resection and investigated the association between HER2 expression status and OS [16]. They found that the prevalence of HER2 positivity was 16.4% and that HER2 positivity was associated with poor OS for EGC (HR = 1.384; 95.0% CI 1.142–1.897; P = 0.005) [16]. However, our results showed no association between HER2 overexpression and OS or DSS. These differences could likely be explained by the following reasons. First, Yan et al. included patients treated surgically, whereas we only included patients receiving endoscopic treatment. Second, the study by Yan et al. had a small sample size, which could have affected the reliability of the statistical estimation of the study outcome.
A significant result of our study is the finding that HER2 overexpression is correlated with a > fourfold higher risk of tumor recurrence in patients with EGC. The potential underlying mechanisms of this result are unclear. However, evidence suggests that HER2 immunoexpression is significantly associated with the development of micrometastases, which could lead to tumor recurrence [19]. In addition, Han et al. retrospectively reviewed 727 patients who underwent surgical treatment for EGC and evaluated the clinical significance of HER2 overexpression in these patients. Their results indicate that HER2 overexpression as a good predictive marker of LNM in patients with undifferentiated EGC (odds ratio, 6.49; 95% CI 1.12–37.37) [17]. LNM has long been confirmed as a significant risk factor for tumor recurrence. Moreover, previous studies have shown that HER2 overexpression is associated with recurrence or recurrence-free survival in breast cancer and gastric cancer [20,21,22,23]. These reports along with our results indicate that further studies are needed to clarify the clinical significance of HER2 overexpression in tumor recurrence in patients with EGC.
According to the ToGA trial, HER2 is a target in patients with AGC with HER2 overexpression [12]. An HER2 test is recommended when metastatic gastric adenocarcinoma is present or suspected. Overall, studies investigating the association between HER2 overexpression and prognosis of EGC are yet in the preliminary stages. However, these studies indicate that the importance of HER2 overexpression in EGC should not be overlooked. Implementing strict endoscopic follow-up after endoscopic treatment or surgical intervention rather than endoscopic treatment may be necessary given HER2 overexpression may play a significant role in EGC, as it does in AGC. We recommend performing an HER2 expression test in all patients with EGC.
Our study has several limitations. First, this was a single-center study. Second, statistical power might be insufficient because of the small sample size and outcome events. Third, many patients were lost to follow-up, which could have affected the results. Finally, fluorescence in situ hybridization (FISH) was not performed for IHC 2 + patients, which could have resulted in the misclassification of IHC 2 + and FISH ( −) patients into the HER2 overexpression group. However, in our study, 6 patients in the HER2 overexpression group were IHC 2 + . Hence, the effect of this misclassification was probably minimal.
Conclusion
In conclusion, HER2 overexpression is an independent risk factor for tumor recurrence in patients with EGC who receive endoscopic treatment. Therefore, HER2 may have clinical significance in EGC as it does in AGC. Clinicians should hence be cautious of the possibility of tumor recurrence in patients with EGC who have HER2 overexpression. Further studies are warranted to confirm the prognostic role of HER2 expression in patients with EGC.
Availability of data and materials
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Feng RM, Zong YN, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22.
Wang SM, Zheng RS, Zhang SW, et al. Epidemiological characteristics of gastric cancer in China, 2015. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(12):1517–21 (Article in Chinese).
Liu Q, Ding L, Qiu X, et al. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: a systematic review and meta-analysis. Int J Surg. 2020;73:28–41.
Sun W, Han X, Siyuan Wu, et al. Endoscopic resection versus surgical resection for early gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(43): e1649.
Zhou Y, Li XB. Endoscopic prediction of tumor margin and invasive depth in early gastric cancer. J Dig Dis. 2015;16(6):303–10.
Yin XY, Pang T, Liu Y, et al. Development and validation of a nomogram for preoperative prediction of lymph node metastasis in early gastric cancer. World J Surg Oncol. 2020;18(1):2.
Zhao B, Huang R, Lu H, et al. Risk of lymph node metastasis and prognostic outcome in early gastric cancer patients with mixed histologic type. Curr Probl Cancer. 2020;44(6): 100579.
Wagner AD, Özdemir BC, Rüschoff J. Human epidermal growth factor receptor 2-positive digestive tumors. Curr Opin Oncol. 2019;31(4):354–61.
Tafe LJ, Tsongalis GJ. The human epidermal growth factor receptor 2 (HER2). Clin Chem Lab Med. 2011;50(1):23–30.
Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer. 2012;3:137–44.
Bang YJ, Cutsem EV, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Zhang XL, Yang YS, Xu DP, et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg. 2009;33(10):2112–8.
Expert Group of Study on Treatment Standard of Early Gastric Cancer. Expert consensus on standardized endoscopic resection of early gastric cancer (2018, Beijing). Chin J Dig Endosc. 2019;36(6):381–92 (Article in Chinese).
Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17(1):1–12.
Yan YJ, Lu L, Liu CN, et al. HER2/neu over-expression predicts poor outcome in early gastric cancer without lymph node metastasis. Clin Res Hepatol Gastroenterol. 2015;39(1):121–6.
Han S, Park S, An J, et al. HER2 as a potential biomarker of lymph node metastasis in undifferentiated early gastric cancer. Sci Rep. 2020;10(1):5270.
Lei YY, Huang JY, Zhao QR, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol. 2017;15(1):68.
Albagoush SA, Limaiem F. HER2. Treasure Island, FL: StatPearls Publishing; 2020.
Otsu H, Oki E, Ikawa-Yoshida A, et al. Correlation of HER2 expression with clinicopathological characteristics and prognosis in resectable gastric cancer. Anticancer Res. 2015;35(4):2441–6.
Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—a systematic review. Int J Cancer. 2012;130(12):2845–56.
Petrelli F, Barni S. Role of HER2-neu as a prognostic factor for survival and relapse in pT1a-bN0M0 breast cancer: a systematic review of the literature with a pooled-analysis. Med Oncol. 2012;29(4):2586–93.
Guo H, Wei B, Zhang HY, et al. HER2 expression and its prognostic implication in lymph node negative breast carcinoma: a meta-analysis. Zhonghua Bing Li Xue Za Zhi. 2005;34(3):140–6 (Article in Chinese).
Acknowledgements
We are grateful to those who offered any help in this article.
Funding
The study was supported by Beijing Municipal Science & Technology Commission (Grant No. Z181100001718177).
Author information
Authors and Affiliations
Contributions
HL, LSL, NZ, ZXW and NX collected the data. HL and LSL analyzed the data and wrote the manuscript draft. EQLH and NLC designed and supervised this study. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Chinese PLA General Hospital (S2020-251-01) approved the study. Written informed consent was obtained from all participants. All methods were carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Flowchart of participants identification.
Additional file 2.
Results of sensitivity analysis by excluding the patients lost to follow-up.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Li, L., Zhang, N. et al. Relationship between HER2 overexpression and long-term outcomes of early gastric cancer: a prospective observational study with a 6-year follow-up. BMC Gastroenterol 22, 238 (2022). https://doi.org/10.1186/s12876-022-02309-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02309-7