Abstract
Background
Sleep disorders and depression were recognized as independent risk factors for heart failure, whether their interaction effects also correlated with the risk of heart failure remains elusive. This study was to explore the interaction effects between sleep disorders and depression on the risk of heart failure.
Methods
This was a cross-sectional study that included data from 39,636 participants in the National Health and Nutritional Examination Survey (NHANES) database. Poisson regression model was applied to evaluate the associations of depression or sleep disorders with heart failure. The relative excess risk of interaction (RERI), attributable proportion of interaction (API) and synergy index (SI) were used to measure whether the interaction effects between depression and sleep disorders on heart failure was statistically significant.
Results
The risk of heart failure was increased in people with sleep disorders [risk ratio (RR) = 1.92, 95% confidence interval (CI): 1.68–2.19) after adjusting for confounders including age, gender, body mass index (BMI), race, marital status, education level, annual family income, drinking history, smoking history, diabetes, hypertension and stroke. The risk of heart failure was elevated in patients with depression after adjusting for confounders (RR = 1.96, 95%CI: 1.65–2.33). Patients with depression and sleep disorders were associated with increased risk of heart failure after adjusting for confounders (RR = 2.76, 95%CI: 2.23–3.42). The CIs of interactive indexes RERI was -0.42 (95%CI: -1.23–0.39), and API was -0.15 (95%CI: -0.46–0.16), which included 0. The CI of interactive indexes SI was 0.81 (95%CI: 0.54–1.21), which contained 1.
Conclusion
Depression and sleep disorders were independent risk factors for heart failure but the interaction effects between depression and sleep disorders on the occurrence of heart failure were not statistically different.
Similar content being viewed by others

Background
Heart failure is a progressive and symptomatic syndrome that has been recognized as one of the main global health problems [1]. Nearly 5.7 million adults > 20 years suffered from heart failure and the estimated prevalence is about 10% in people age > 65 years [2]. Patients with heart failure require lifelong medical treatment and great health care, and this results in a high premature mortality [3]. Heart failure decreases the quality of life in patients and brings heavy burden to the society [4]. Given the high prevalence and cost of heart failure, increasing emphasis has been put on exploring the factors associated with heart failure. A better understanding of modifiable risk factors and their interaction effects on heart failure is vital for the prevention of this disease.
Sleep disorders including sleep apnea, insomnia, restless legs, and others are frequently identified in patients with heart failure [5, 6]. Almost 75% of heart failure patients were reported to have sleep disorders [7]. Sleep duration and sleep disorders were revealed to be associated with increased risk of heart failure [8, 9]. Another modifiable risk factor for heart failure might be depression in patients. Depression is a common comorbidity in heart failure and approximately 30% of heart failure patients suffer from depression and even more have depressive symptoms [10]. Multiple evidence indicated that depressive symptoms were risk factors for heart diseases [11]. Several studies also revealed that depression not only reduces the quality of life and increases the re-hospitalization rate of heart failure patients, but also increases the morbidity and mortality of heart failure and affects the prognosis of these patients [12, 13]. Growing numbers of studies showed that depression and sleep disorders had bidirectional relationship with each other [14]. Several researchers also found that the interaction effects between depression and sleep disorders affected the occurrence of stroke and type 2 diabetes [15]. At present, sleep disorders and depression were recognized as independent risk factors for heart failure, whether their interaction effects also correlated with the risk of heart failure remains elusive.
Due to the high mortality and morbidity of heart failure, an updated and careful management of different aspects that characterize the disease such as stratifying factors associated with heart failure is essential for correctly clinical managing patients [16]. Previous studies have explored the well-known clinical, laboratory and instrumental characteristics that might influence heart failure [17, 18], the demographic characteristics such as age, gender, and body mass index (BMI) also have different influences and deserve specific insights and clarifications [19,20,21]. At this scope, we performed the subgroup analysis to investigate the interaction effects between sleep disorders and depression on the risk of heart failure in participants with different demographic characteristics.
In the current study, we aimed to explore the interaction effects between sleep disorders and depression on the risk of heart failure based on the data from the National Health and Nutritional Examination Survey (NHANES) database. The independent as well as the bidirectional relationship between sleep disorders and depression were respectively investigated to deeply evaluate the associations of sleep disorders and depression with the risk of heart failure. We also stratified the analysis in terms of demographic characteristics such as age, gender, BMI and marital status.
Methods
Study design and population
This was a cross-sectional study collected the data of 39,636 participants from NHANES database. NHANES is a survey collecting the data of nationally representative samples in the United States each year and recording the detailed demographic information and comprehensive nutrition data on dietary intake, anthropometric measurements, as well as blood samples by standardized interviews and direct examination of participants [22]. In the current study, after excluding participants without the data on sleep disorders, depression questionnaires and others, 30,406 subjects were finally included.
Data collection
The data of all subjects were collected including the age (years), gender, BMI (kg/m2), race (Mexican American, Hispanic, non-Hispanic White, non-Hispanic Black, or others), marital status (married, widowed, divorced/separated, or unmarried), education [Junior high and below, High school/General Equivalent Diploma (GED), Junior college or above], annual family income (< $20,000 or ≥ $20,000), drinking history, smoking history, diabetes mellitus, stroke, hypertension, sleep disorders, depression, depression severity (no, moderate, moderate to severe and severe), and heart failure. All participants were divided into the heart failure group (n = 977) and non-heart failure group (n = 29,429).
Definitions of variables
Heart failure in patients was defined as the outcome in this study, which was determined by a response of “yes” to the household interview question asking whether they had been told to have congestive heart failure in the “Medical Conditions” module of “Questionnaire Data” in the NHANES. Sleep disorders were defined based on a response of “yes” to the question asking them whether they had been told to have sleep disorders by doctors or professional health workers. Depression was measured by the Patient Health Questionnaire 9 (PHQ-9) and PHQ-9 scores ≥ 10 was defined as depression [23]. The severity of depression was determined based on the PHQ-9 scores, including no depression (0–9), moderate depression (10–14), moderate to severe depression (15–19), and severe depression (20–27) [24]. The reliability or validity of the PHQ-9 were assessed and the Cronbach Alpha coefficient of PHQ-9 was 0.846447 after standardization.
The additive interaction effects model
Three indexes including relative excess risk of interaction (RERI), attributable proportion of interaction (API), and synergy index (SI) were used to assess the interaction effects between sleep disorders and depression on the risk of heart failure based on the addictive model. RERI = R11-R10-R01 + 1: represents the difference between the sum of the combined effects of the two factors and the sum of the separate effects. It also represents the risk degree of interaction effects in comparison with all other factors except the two factors. API = RERI/R11: represents the proportion of total effects attributed to interaction. SI = R11 (R10 × R01): the meaning is the same as RERI. No interaction effects were shown when 0 was included in the confidence intervals (CIs) of RERI and API and 1 was involved in the CI of SI.
Statistical analysis
The Shapiro–Wilk was applied for measuring the normality of the measurement data. The measurement data with normal distribution were described as Mean ± SD and comparisons between groups were subjected to t test. Non-normal distributed data were shown as [M (Q1, Q3)] and differences between groups were compared via Mann–Whitney U rank sum test. The enumeration data were described as n (%). Chi-square test (χ2) or Fisher’s exact probability method was used for comparison between the groups. Poisson regression model was applied to evaluate the associations of depression or sleep disorders with heart failure. Model 1 was the unadjusted crude model. Model 2 adjusted for demographic characteristics including age, BMI, marital status and gender. Model 3 adjusted for variables with statistical difference between heart failure group and non-heart failure group including age, gender, race, marital status, education, annual family income, drinking history, smoking history diabetes mellitus, hypertension and stroke. Stepwise regression analysis was applied in Model 3. Sensitivity analysis was performed in the data before and after deleting the missing data to explore whether the missing values influenced the results. RERI, API and SI were used to assess whether the interaction effects between depression and sleep disorders on heart failure was statistically significant. All statistical tests were performed by two-sided test and P < 0.05 were considered to be statistically significant. SAS 9.4 was used for statistical analysis, R 4.20 software was used to draw the forest plot, and GraphPad was applied to draw the graph showing the risk ratios (RR) of interaction effects term.
Results
The characteristics of all participants
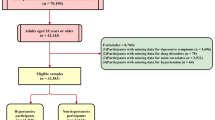
The data of 39,636 participants were extracted from NHANES database. Among them, 25 people missed the data on sleep disorders, 5579 subjects had no data on depression questionnaires and 3626 persons missed other data, and 30,406 subjects were finally included. The detailed screen process was shown in Fig. 1. The median age of all the participants was 49 years. 14,998 (49.33%) of the subjects were male. The average BMI was 29.25 kg/m2. 4679 people were Mexican–American, accounting for 15.39%, 2748 persons were Hispanic, accounting for 9.04%, 13,503 participants were non-Hispanic White, accounting for 44.41%, 6485 people were non-Hispanic Black, accounting for 21.33%, and 2991 participants were other ethnicities, accounting for 9.84%. 7831 patients had sleep disorders, accounting for 25.75%, and 2634 people had depression, accounting for 8.66%. Among patients with depression, 1642 patients were moderate depression, accounting for 5.40%, 709 people were moderate to severe depression, accounting for 2.33%, and 283 persons were severe depression, accounting for 0.93%. 977 patients had heart failure, accounting for 3.21% (Table 1).
Comparisons of the characteristics between patients with and without heart failure
The median age (69 years vs 48 years, Z = 30.471, P < 0.001), and median BMI (32.29 kg/m2 vs 29.15 kg/m2, t = 11.160, P < 0.001) of participants with heart failure were higher than those without. The proportions of males (56.70% vs 49.08%, χ2 = 21.986, P < 0.001), subjects with annual family income < $20,000 (χ2 = 152.884, P < 0.001), patients with smoking history (62.33% vs 45.10%, χ2 = 113.169, P < 0.001), patients with diabetes (χ2 = 833.809, P < 0.001), patients with stroke (20.16% vs 3.16%, χ2 = 766.006, P < 0.001), patients with sleep disorders (48.52% vs 25%, χ2 = 273.488, P < 0.001), patients with depression (18.73% vs 8.33%, χ2 = 129.320, P < 0.001) and different depression degrees (Z = 11.450, P < 0.001) in the heart failure group were higher than the non-heart failure group. The percentages of people with different education levels (Z = -8.028, P < 0.001) and drinking history (61.51% vs 69.15% χ2 = 25.748, P < 0.001) in people with heart failure were lower than those without heart failure. The differences concerning race (χ2 = 96.403, P < 0.001) and marital status (χ2 = 346.640, P < 0.001) between people with and without heart failure were statistically significant (Table 1).
Associations of sleep disorders or depression with heart failure
As observed in Fig. 2, the risk of heart failure was 2.21 times increase in patients with sleep disorders in the adjusted model for age and sex (RR = 2.21, 95%CI: 1.94–2.51). After adjusting for age, sex, BMI, race, marital status, education level, annual family income, drinking history, smoking history, diabetes, hypertension and stroke, the risk of heart failure was 1.92-fold increase in people with sleep disorders (RR = 1.92, 95%CI: 1.68–2.19). The risk of heart failure was 2.53-fold increase in patients with depression after adjusting for age and sex (RR = 2.53, 95%CI: 2.14–2.99). The risk of heart failure was 1.96-fold increase in patients with depression compared with those without (RR = 1.96, 95%CI: 1.65–2.33) after adjusting for age, sex, BMI, race, marital status, education level, annual family income, drinking history, smoking history, diabetes, hypertension and stroke (Fig. 2). For most categories of age, BMI, marital status and gender, the risk ratios for sleep disorders and depression were statistically significantly (greater than 1.0) in most cases. Exception was in underweight group. The detailed information of the subgroups was exhibited in Figs. 3 and 4.
Interaction effects between sleep disorders and depression on heart failure
The additive interaction effects terms of sleep disorders and depression were established, including no depression and no sleep disorders, no depression and sleep disorders, depression and no sleep disorders, depression and sleep disorders. The detailed sample size of each interaction effects term was displayed in Table 2. Patients with depression and sleep disorders were associated with increased risk of heart failure after adjusting for age and gender (RR = 3.68, 95% CI: 2.99–4.54), or adjusting for age, gender, BMI, race, marital status, education level, annual family income, drinking history, smoking history, diabetes, hypertension and stroke (RR = 2.76, 95%CI: 2.23–3.42) (Fig. 2). For most categories of age, BMI, marital status and gender, the risk ratios for people with sleep disorders and depression were statistically significantly (> 1.0) in most cases (Fig. 5). Sensitivity analysis depicted that there was no statistical difference of the results between the data before and after deleting the missing values (Supplementary Table 1). The CIs of interactive indexes RERI was -0.42 (95%CI: -1.23–0.39), and API was -0.15 (95%CI: -0.46–0.16), which included 0. The CIs of interactive indexes SI was 0.81 (95%CI: 0.54–1.21), which contained 1 (Table 3). These indicated that the interaction effects between sleep disorders and depression on heart failure was not statistically significant (Fig. 6). Subgroup analysis concerning the demographic characteristics exhibited no statistical differences in terms of the interaction effects between depression and sleep disorders on the risk of heart failure (Table 3).
Discussion
In the present study, 30,406 eligible participants were enrolled from NHANES, including 977 people with heart failure and 29,429 without heart failure. The results depicted that depression and sleep disorders were independently associated with increased risk of heart failure. No synergic and additive interaction effects between depression and sleep disorders on the occurrence of heart failure was obtained. The findings of this study might make it more clear about the effects between depression and sleep disorders on the occurrence of heart failure, and help the clinicians to make appropriate interventions on patients with depression or/and sleep disorders.
Depression is a chronic medical illness affecting thoughts, mood, and physical health, which decreases the ability of individuals to function in their daily life [25]. A review by Celano et al. uncovered that depression was associated with the development and progression of heart failure via mediating the physiologic and behavioral mechanisms [26]. Another prospective observational study including about 2 million healthy adults demonstrated that depression was prospectively associated with an 18% increased risk of heart failure [27]. These findings gave support to the results of our study, which showed that depression was a risk factor for the occurrence of heart failure. This may be because patients with depression was linked to the hypothalamic–pituitary–adrenal gland dysfunction, increased pro-inflammatory and pro-thrombotic factor activity, reduced heart rate variability and physical inactivity [28]. Depressive symptoms and major depression were associated with elevated levels of inflammatory biomarkers such as C-reactive protein (CRP), interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) [29]. The immune system and inflammation were reported to be involved in the pathogenesis of heart failure [30]. To early screen out patients with depression can timely provide proper treatments such as anti-depressive medications. In the current study, sleep disorders were recognized to be associated with a higher risk of heart failure. Javaheri et al. discovered that insomnia, especially when accompanied by short sleep duration was linked with increased risk of heart failure [31]. A prospective population-based study reported that obstructive sleep apnea had a twofold increase in the risk of heart failure [32]. The potential mechanisms might be that sleep disorders including sleep-disordered breathing is associated with increased sympathetic activation, vagal withdrawal, altered haemodynamic loading conditions, and hypoxaemia, which is one of the most common risk factor for cardiac failure [33]. To improve the quality of sleep in general population might be a strategy for the prevention of heart failure. Subgroup analysis showed that depression and sleep disorders were associated with higher risk of heart failure in both patients aged ≥ 65 years and 30–65 years, married or others, males or females. These suggested that patients with sleep disorders or depression should be cautious of the risk of heart failure despite the age, marital status and gender. As for underweight patients, no significant association was found between depression or sleep disorders and heart failure. This maybe because the sample size in underweight group was small [underweight group (n = 475) vs normal BMI (n = 8521) vs overweight (n = 9945) vs obesity (n = 11,465)].
In this study, the interaction effects between depression and sleep disorders on heart failure were also explored, and no synergic interaction effects between depression and sleep disorders was identified on the occurrence of heart failure. This maybe because the disease course of heart failure was progressive and long [34], and the interaction effects of depression and sleep disorders on heart failure was not significant during the long disease course. The interaction effects between depression and sleep disorders on heart failure might be more obvious if more relevant clinical biomarkers were including in the subgroup analysis. Inflammation plays an important part in depression, sleep disorders and heart failure [35, 36], and depression and sleep disorders might be linked to heart failure through the pathway of inflammation. Depression and heart failure also share some mechanisms and risk factors, including dysregulation of platelet reactivity, neuroendocrine function, arrhythmias, high-risk behaviors, and social factors [10]. Severe depression was reported to be associated with diastolic dysfunction and left ventricular hypertrophy, which increase the risk of heart failure [37]. For patients with depression, early interventions should be provided to decrease the risk of heart failure in those patients. Interestingly, we found in people with normal BMI, there might be interaction effects of depression and sleep disorders on heart failure. This maybe because abnormal BMI might involve in more mechanisms associated with the occurrence of heart failure [38], and the interaction effects of depression and sleep disorders on heart failure might be not significant.
This study explored the interaction effects between depression and sleep disorders on heart failure, which obtained convincing results based on the nationally representative NHANES database with a large sample size. Several limitations existed in our study. Firstly, this was a cross-sectional study, which could only identify the associations but not the causal relationship between depression or sleep disorders and heart failure. Secondly, the history of sleep disorders and heart failure were based on the self-reported data of participants in the NHANES, which might cause bias. Thirdly, subgroup analysis was only conducted in terms of gender, race and age, more heart failure related subgroups should be performed to clearly identify the interaction effects between depression and sleep disorders on heart failure in different populations. In the future, more case–control studies on deeply exploring the interaction effects of depression and sleep disorders on heart failure were required to verify the findings in the current study.
Conclusions
Our study analyzed the effect of depression and sleep disorders on the risk of heart failure based on the data of 30,406 participants from NHANES. The findings revealed that depression and sleep disorders were independent risk factors of heart failure but the interaction effects between depression and sleep disorders were not statistically different on the occurrence of heart failure. The results of our study revealed the co-existing of sleep disorder and depression does not seem to have a synergistic effect on the occurrence of heart failure.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/.
Abbreviations
- NHANES:
-
National Health and Nutritional Examination Survey
- GED:
-
General Equivalent Diploma
- PHQ-9:
-
Patient Health Questionnaire 9
- RERI:
-
Relative excess risk of interaction
- API:
-
Attributable proportion of interaction
- SI:
-
Synergy index
- CIs:
-
Confidence intervals
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–492.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292.
Wiley JF, Chan YK, Ahamed Y, Ball J, Carrington MJ, Riegel B, et al. Multimorbidity and the Risk of All-Cause 30-Day Readmission in the Setting of Multidisciplinary Management of Chronic Heart Failure: A Retrospective Analysis of 830 Hospitalized Patients in Australia. J Cardiovasc Nurs. 2018;33(5):437–45.
Goonesekera S, Rudnicka-Noulin D, Isherwood A. The burden of heart failure in North America and Western Europe. Future Cardiol. 2021;17(4):637–46.
Teo YH, Tam WW, Koo CY, Aung AT, Sia CH, Wong RCC, Kong W, Poh KK, Kofidis T, Kojodjojo P, et al. Sleep apnea and recurrent heart failure hospitalizations after coronary artery bypass grafting. J Clin Sleep Med. 2021;17(12):2399–407.
Mahmood A, Ray M, Dobalian A, Ward KD, Ahn S. Insomnia symptoms and incident heart failure: a population-based cohort study. Eur Heart J. 2021;42(40):4169–76.
Jeon S, Redeker NS. Sleep Disturbance, Daytime Symptoms, and Functional Performance in Patients With Stable Heart Failure: A Mediation Analysis. Nurs Res. 2016;65(4):259–67.
Yan B, Li R, Li J, Jin X, Gao F, Gao Y, et al. Sleep Timing May Predict Congestive Heart Failure: A Community-Based Cohort Study. J Am Heart Assoc. 2021;10(6):e018385.
Wang ID, Chien WC, Chung CH, Tsai PY, Chang SY, Meng FC, et al. Non-Apnea Sleep Disorder associates with increased risk of incident heart failure-A nationwide population-based cohort study. PLoS ONE. 2019;14(1):e0209673.
Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and heart failure: the lonely comorbidity. European journal of heart failure. 2020;22(11):2007–17. Epub 2020/05/30. https://doi.org/10.1002/ejhf.1865. PubMed PMID: 32468714.
Deschênes SS, Burns RJ, Graham E, Schmitz N. Depressive symptoms and sleep problems as risk factors for heart disease: a prospective community study. Epidemiology and psychiatric sciences. 2019;29:e50.
Freedland KE, Hesseler MJ, Carney RM, Steinmeyer BC, Skala JA, Dávila-Román VG, et al. Major Depression and Long-Term Survival of Patients With Heart Failure. Psychosom Med. 2016;78(8):896–903.
Pushkarev GS, Kuznetsov VA, Fisher YA, Soldatova AM, Enina TN. Depression and all-cause mortality in patients with congestive heart failure and an implanted cardiac device. Turk Kardiyol Dern Ars. 2018;46(6):479–87.
Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–32.
Zhao J, Li XL, Han K, Tao ZQ, Wu ZM. Biological interaction between sleep quality and depression in type 2 diabetes. Eur Rev Med Pharmacol Sci. 2016;20(14):3087–91.
Sciomer S, Moscucci F, Salvioni E, Marchese G, Corrà U, Piepoli MF. Role of gender, age and BMI in prognosis of heart failure. Eur J Prev Cardiol. 2020;27(2_suppl):46–51.
Matsue Y, Sama IE, Postmus D, Metra M, Greenberg BH, Cotter G, Davison BA, Felker GM, Filippatos G, Pang P, et al. Association of Early Blood Pressure Decrease and Renal Function With Prognosis in Acute Heart Failure. JACC Heart failure. 2021;9(12):890–903.
Limpitikul WB, Dewland TA, Vittinghoff E, Soliman E, Nah G, Fang C, Siscovick DS, Psaty BM, Sotoodehnia N, Heckbert S, et al. Premature ventricular complexes and development of heart failure in a community-based population. Heart (British Cardiac Society). 2022;108(2):105–10.
Lainščak M, Milinković I, Polovina M, Crespo-Leiro MG, Lund LH, Anker SD, Laroche C, Ferrari R, Coats AJS, McDonagh T, et al. Sex- and age-related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long-Term Registry. Eur J Heart Fail. 2020;22(1):92–102.
Maeda D, Matsue Y, Kagiyama N, Jujo K, Saito K, Kamiya K, Saito H, Ogasahara Y, Maekawa E, Konishi M, et al. Sex differences in the prevalence and prognostic impact of physical frailty and sarcopenia among older patients with heart failure. Nutr Metab Cardiovasc Dis. 2022;32(2):365–72.
Bygdell M, Ohlsson C, Lilja L, Celind J, Martikainen J, Rosengren A, Kindblom JM. Birth weight and young adult body mass index for predicting the risk of developing adult heart failure in men. Eur J Prev Cardiol. 2022;29(6):971–8.
Fain JA. NHANES. Diabetes Educ. 2017;43(2):151.
Jorgensen D, White GE, Sekikawa A, Gianaros P. Higher dietary inflammation is associated with increased odds of depression independent of Framingham Risk Score in the National Health and Nutrition Examination Survey. Nutr Res (New York, NY). 2018;54:23–32.
Kung S, Alarcon RD, Williams MD, Poppe KA, Jo Moore M, Frye MA. Comparing the Beck Depression Inventory-II (BDI-II) and Patient Health Questionnaire (PHQ-9) depression measures in an integrated mood disorders practice. J Affect Disord. 2013;145(3):341–3.
Cui R. Editorial: A Systematic Review of Depression. Curr Neuropharmacol. 2015;13(4):480.
Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and Anxiety in Heart Failure: A Review. Harv Rev Psychiatry. 2018;26(4):175–84.
Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D, et al. Depression as a Risk Factor for the Initial Presentation of Twelve Cardiac, Cerebrovascular, and Peripheral Arterial Diseases: Data Linkage Study of 1.9 Million Women and Men. PloS one. 2016;11(4):e0153838.
Raič M. Depression and Heart Diseases: Leading Health Problems. Psychiatr Danub. 2017;29(Suppl 4):770–7.
Lorkiewicz P, Waszkiewicz N. Biomarkers of Post-COVID Depression. J Clin Med. 2021;10(18):4142. https://doi.org/10.3390/jcm10184142.
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–85.
Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435–44.
Ljunggren M, Byberg L, Theorell-Haglöw J, Lindahl B, Michaëlsson K, Lindberg E. Increased risk of heart failure in women with symptoms of sleep-disordered breathing. Sleep Med. 2016;17:32–7.
Parati G, Lombardi C, Castagna F, Mattaliano P, Filardi PP, Agostoni P. Heart failure and sleep disorders. Nat Rev Cardiol. 2016;13(7):389–403.
Wagner M, Tiffe T, Morbach C, Gelbrich G, Störk S, Heuschmann PU. Characteristics and Course of Heart Failure Stages A-B and Determinants of Progression - design and rationale of the STAAB cohort study. Eur J Prev Cardiol. 2017;24(5):468–79.
Redeker NS, Conley S, Anderson G, Cline J, Andrews L, Mohsenin V, et al. Effects of Cognitive Behavioral Therapy for Insomnia on Sleep, Symptoms, Stress, and Autonomic Function Among Patients With Heart Failure. Behav Sleep Med. 2020;18(2):190–202.
Heo S, Moser DK, Pressler SJ, Dunbar SB, Dekker RL, Lennie TA. Depressive symptoms and the relationship of inflammation to physical signs and symptoms in heart failure patients. Am J Crit Care. 2014;23(5):404–13.
Kim YH, Kim SH, Lim SY, Cho GY, Baik IK, Lim HE, et al. Relationship between depression and subclinical left ventricular changes in the general population. Heart (British Cardiac Society). 2012;98(18):1378–83.
Benn M, Marott SCW, Tybjærg-Hansen A, Nordestgaard BG. Obesity increases heart failure incidence and mortality: observational and Mendelian randomization studies totalling over 1 million individuals. Cardiovasc Res. 2023;118(18):3576–85. https://doi.org/10.1093/cvr/cvab368. IF:13.081 Q1.
Acknowledgements
We thank the participants included in our study for their contributions.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TF and DS designed the study. TF wrote the manuscript. TF and DS collected, analyzed and interpreted the data. DS critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research analyzed de-identified information downloaded from the National Health and Nutrition Examination Survey public database, which is exempt from future Institutional Review Board approval. All methods were performed in accordance with Declarations of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table1.
Comparisons of the results between the data before and after deleting themissing values.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, T., Su, D. Interaction effects between sleep disorders and depression on heart failure. BMC Cardiovasc Disord 23, 132 (2023). https://doi.org/10.1186/s12872-023-03147-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03147-5