Abstract
Background
In a Phase I study treatment with the serum amyloid P component (SAP) depleter miridesap followed by monoclonal antibody to SAP (dezamizumab) showed removal of amyloid from liver, spleen and kidney in patients with systemic amyloidosis. We report results from a Phase 2 study and concurrent immuno-positron emission tomography (PET) study assessing efficacy, pharmacodynamics, pharmacokinetics, safety and cardiac uptake (of dezamizumab) following the same intervention in patients with cardiac amyloidosis.
Methods
Both were uncontrolled open-label studies. After SAP depletion with miridesap, patients received ≤ 6 monthly doses of dezamizumab in the Phase 2 trial (n = 7), ≤ 2 doses of non-radiolabelled dezamizumab plus [89Zr]Zr-dezamizumab (total mass dose of 80 mg at session 1 and 500 mg at session 2) in the immuno-PET study (n = 2). Primary endpoints of the Phase 2 study were changed from baseline to follow-up (at 8 weeks) in left ventricular mass (LVM) by cardiac magnetic resonance imaging and safety. Primary endpoint of the immuno-PET study was [89Zr]Zr-dezamizumab cardiac uptake assessed via PET.
Results
Dezamizumab produced no appreciable or consistent reduction in LVM nor improvement in cardiac function in the Phase 2 study. In the immuno-PET study, measurable cardiac uptake of [89Zr]Zr-dezamizumab, although seen in both patients, was moderate to low. Uptake was notably lower in the patient with higher LVM. Treatment-associated rash with cutaneous small-vessel vasculitis was observed in both studies. Abdominal large-vessel vasculitis after initial dezamizumab dosing (300 mg) occurred in the first patient with immunoglobulin light chain amyloidosis enrolled in the Phase 2 study. Symptom resolution was nearly complete within 24 h of intravenous methylprednisolone and dezamizumab discontinuation; abdominal computed tomography imaging showed vasculitis resolution by 8 weeks.
Conclusions
Unlike previous observations of visceral amyloid reduction, there was no appreciable evidence of amyloid removal in patients with cardiac amyloidosis in this Phase 2 trial, potentially related to limited cardiac uptake of dezamizumab as demonstrated in the immuno-PET study. The benefit-risk assessment for dezamizumab in cardiac amyloidosis was considered unfavourable after the incidence of large-vessel vasculitis and development for this indication was terminated.
Trial registration NCT03044353 (2 February 2017) and NCT03417830 (25 January 2018).
Similar content being viewed by others
Background
Systemic amyloidosis is a progressive, usually fatal disorder in which misfolded proteins form fibrils that accumulate in vital organs leading to progressive organ dysfunction [1, 2]. The two most common types are immunoglobulin light chain (AL) and transthyretin (ATTR) amyloidosis [2]; cardiac involvement is common in both forms [1]. Prognosis is poor for both types, but significantly worse for patients with cardiac AL, with an overall median survival of approximately 9 months, or 3.5 months for patients with markedly elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cardiac troponin [3, 4].
Current treatments for cardiac amyloidosis target the production of precursor proteins by chemotherapy for AL [1, 5], and oligonucleotide gene silencing therapies [6] or targetted protein stabilisers (tafamidis) [7] for ATTR. However, no approved therapies directly remove cardiac amyloid deposits, which can range from mild to extensive on diagnosis [1, 5, 8].
In a Phase 1 clinical trial we demonstrated progressive removal of amyloid with up to three cycles of a therapy that targetted the plasma protein serum amyloid P component (SAP), which is universally present in amyloid deposits, and is therefore a target in all forms of amyloidosis [9]. For each session of treatment, normal circulating SAP was first acutely depleted by miridesap [10, 11], leaving residual SAP within amyloid deposits. This was then targetted by the anti-SAP monoclonal antibody (mAb) dezamizumab, which in mice, has been shown to lead to removal of amyloid through a macrophage giant cell response [11, 12]. Depletion of amyloid in humans was initially demonstrated in several organs (liver, spleen and kidney) in different forms of amyloidosis (AL, AA [amyloid A protein], fibrinogen and apolipoprotein A-I) [13, 14]. Based on these encouraging Phase 1 results, we designed a Phase 2 study that aimed to investigate the impact of treatment on cardiac amyloid deposits, as well as the occurrence and nature of dermatological adverse events (AEs; mainly rashes), which were seen in 58% of patients in the Phase 1 study and seemed to occur with incremental dezamizumab doses. The Phase 2 study concentrated on patients with either AL or ATTR cardiomyopathy (ATTR-CM), and subjects were intended to receive up to 6 treatments of miridesap followed by dezamizumab (anti-SAP treatment) at monthly intervals. Successful development of [89Zr]Zr-DFO-NCS-dezamizumab ([89Zr]Zr-dezamizumab) enabled a concurrent immuno-positron emission tomography (PET) study to be performed, which specifically assessed cardiac uptake and biodistribution of [89Zr]Zr-dezamizumab.
We report data from the Phase 2 trial in concert with the Phase 1 PET imaging study, to demonstrate the interplay of the data from both studies in informing a benefit-risk assessment that led to the termination of development of this anti-SAP treatment for systemic amyloidosis.
Methods
Phase 2 study
Study design and treatment
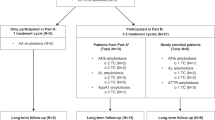
This was an open-label, non-randomised study in patients with systemic amyloidosis and cardiac dysfunction caused by cardiac amyloidosis (NCT03044353). Three groups were planned: patients with ATTR-CM (Group 1), post-chemotherapy AL amyloidosis (Group 2) and newly diagnosed Mayo stage II or IIIa AL amyloidosis (Group 3). Group 1 and Group 2 began recruitment in parallel; no patients were recruited into Group 3 due to early study termination.
The study consisted of screening and baseline sessions (conducted in a 6-week period prior to the first anti-SAP treatment), up to six anti-SAP treatment sessions with patients admitted as inpatients for the first 2 weeks of each cycle (minimum of 1 month between the start of each dezamizumab treatment), and 8-week, 6-month and 12-month follow-up sessions (Fig. 1a).
Study design for the Phase 2 study (A) and immuno-PET study (B). *Blood SAP depletion could extend beyond Days –2 and –1. Note: for the Phase 2 study the start of anti-SAP monoclonal antibody treatment was denoted as Day 1, whereas for the immuno-PET study the start of miridesap treatment was denoted as Day 1. CMR cardiac magnetic resonance, ECHO echocardiogram, IV intravenous, MRI magnetic resonance imaging, PET positron emission tomography, SAP serum amyloid P component, SC subcutaneous
Each dosing session consisted of an initial depletion of circulating SAP to the target level by miridesap intravenous (IV) infusion (48 h over Days –2 and –1 but could have been administered up to 72 h; dose dependent on renal function) with maintenance of circulating SAP depletion on Day 1–11 by miridesap subcutaneous injection up to 3 times daily dependent on renal function. Dezamizumab 600 mg was selected as the initial dose to reduce the risk of haemodynamic complications from dezamizumab-associated infusion reactions in this cardiac patient population with left ventricular (LV) dysfunction. Dezamizumab was delivered in two split doses based on findings from the first-in-human study that indicated dezamizumab-associated skin rashes may be related to the maximum plasma concentration (Cmax) of the antibody [13]. Dose escalation on subsequent sessions was considered on a case-by-case basis up to a maximum of 1200 mg delivered as two infusions over 6–8 h each, on Days 1 and 3.
The first interim analysis was performed after five patients in Group 1 had completed at least three courses of anti-SAP treatment. Due to study termination, further planned interim analyses were not conducted.
Patient population
Patients were recruited from the UK and US (Additional file 1). Males and females aged 18–80 years with late-gadolinium enhancement on cardiac magnetic resonance (CMR) indicative of cardiac amyloidosis were eligible.
For Group 1, wild-type TTR patients were defined as having a cardiac or non-cardiac biopsy showing amyloid deposits that were confirmed as TTR-related by mass spectrometry or a positive technetium-dicarboxypropane diphosphonate or technetium-pyrophosphate scan in the absence of evidence of a plasma cell dyscrasia, and negative genetic screening for mutant TTR. Similar diagnostic criteria applied to those with hereditary ATTR-CM, except that they were required to have a known amyloidogenic TTR mutation recognised as primarily associated with CM demonstrated by genotyping. Patients were also required to be clinically stable (New York Heart Association [NYHA] class II or III) for 3 months pre-screening, with left ventricular mass (LVM) on CMR > 200 g.
For Group 2, patients with post-chemotherapy AL amyloidosis were required to have completed any line of chemotherapy or autologous stem cell transplantation at least 6 months previously and were required to have attained a very good partial response (reduction to < 40 mg/L in the difference in involved and uninvolved free light chain) or a complete response (normalisation of the free light chain levels and ratio, negative serum and urine immunofixation) [15]. AL amyloidosis was previously diagnosed by tissue biopsy in the setting of a plasma cell dyscrasia and appropriate amyloid typing. Patients were also required to be clinically stable (NYHA class II or III) for 3 months pre-screening with LVM on CMR > 150 g.
For Group 3, patients would have been required to have newly diagnosed AL amyloidosis and be Mayo stage II or IIIa with LVM on CMR > 150 g.
Patients were excluded from the study if any of the following criteria were met: corrected QT interval > 500 ms; history of sustained/symptomatic monomorphic or rapid polymorphic, ventricular tachycardia; unstable heart failure (emergency hospitalisation for worsening, or decompensated heart failure or syncopal episode) ≤ 1 month prior to screening.
Full inclusion and exclusion criteria are provided in the Additional file 2.
Endpoints
The primary endpoints of this study were a change in LVM over time from baseline to 8-week follow-up, and clinical safety data from AEs, clinical laboratory tests, vital signs, 12-lead electrocardiogram (ECG), cardiac monitoring and echocardiogram (ECHO) to 8-week follow-up, and incidence and grading of skin rashes classified using the Common Terminology Criteria for Adverse Events.
Secondary endpoints included investigation of the rash associated with anti-SAP treatment by histopathological and immunohistochemical examination of skin biopsies and blood biomarkers, characterisation of the pharmacokinetics (PK) of dezamizumab, evaluation of the changes to circulating biomarkers associated with pharmacodynamic (PD) effect, and evaluation of imaging markers associated with cardiac dysfunction by serial CMR and/or ECHO. Exploratory endpoints included change in cardiac extracellular volume (ECV) from baseline to 8-week follow-up. Further exploratory endpoints are listed in the Additional file 3.
Assessments
Multimodality cardiac imaging was carried out at pre-determined time points throughout the study, as shown in Fig. 1. All CMR and ECHO parameters were acquired and analysed by central imaging core laboratories [16, 17]. Parameters included, but were not limited to, strain, LV twist, stroke volume, ejection fraction, end diastolic volume, and the ratio between early mitral inflow velocity and mitral annular early diastolic velocity. All investigators and sponsor study team members, except those involved in central imaging core laboratory review, had access to the patient’s individual study treatment and dosing schedules for each constituent drug. Further ECHO imaging could be requested by the investigator at any time to monitor safety. CMR methodology is described in Additional file 4.
Safety assessments conducted throughout the Phase 2 study included 12-lead ECG, lead II telemetry and monitoring of vital signs. AEs were collected from the start of treatment until the 8-week follow-up. Blood samples for the analysis of drug concentration, biomarkers and PD markers were collected at various time points throughout the study. Markers analysed include complement markers and inflammatory markers (C-reactive protein [CRP]).
Phase 1 immuno-PET study
Study design and treatment
This open-label, non-randomised, single-centre, two-part [89Zr]Zr-dezamizumab PET imaging study (NCT03417830) included clinically stable patients with cardiac dysfunction caused by ATTR-CM (Fig. 1b). It was conducted concurrently with the Phase 2 study described above. A detailed description of the methods can be found in Additional file 5. In short, following SAP depletion, patients received an IV infusion of non-radiolabelled dezamizumab plus a separate, concurrent IV infusion of [89Zr]Zr-dezamizumab. This was followed by up to three serial PET scans. The protocol was originally planned to have two parts: Part A, in which three patients were to receive up to two treatment sessions, and Part B, in which up to three patients were to receive one treatment session of ~ 26 days in duration. A formal interim analysis was planned to be conducted before progression to Part B, but the study was terminated prior to completion of Part A, and as a result, neither the formal interim analysis nor Part B were conducted.
Endpoints
The primary endpoint of the PET study was standardised uptake values (SUV; measured radioactivity concentration corrected for radioactive decay and normalised for administered amount of radioactivity per body weight) in focal anatomical locations within the heart and SUV of the whole heart at different time points after [89Zr]Zr-dezamizumab administration and at different dezamizumab total mass doses.
Secondary endpoints included focal and total radioactivity uptake in different tissues at different time points and after different total mass doses; descriptive PK parameters of total and radiolabelled dezamizumab; all AEs, including the incidence and grading of skin rashes, cardiac AEs, and infusion-related reactions; and other safety information, including ECG and vital signs. For exploratory objectives of this study, see the Additional file 3.
Results
Patient disposition
Due to early study termination, recruitment was lower than planned for both studies. The Phase 2 study enrolled seven patients (six patients in Group 1 and one patient in Group 2). A further five patients were screened but not randomised. In Group 1, six patients with ATTR-CM received 3–6 dosing sessions with total dezamizumab doses of 3000–6600 mg; all patients completed the study. In Group 2, one patient with AL received a partial dose of dezamizumab 300 mg on Day 1; as described in the safety section, this patient was withdrawn due to a serious AE (SAE).
The immuno-PET study recruited two patients with ATTR-CM. Patient A received two dosing sessions (9.94 mg radiolabelled and 70 mg of non-radiolabelled dezamizumab in session 1 and 9.83 mg labelled and 490 mg non-radiolabelled dezamizumab in session 2) and completed the study; Patient B received one dosing session (10.38 mg radiolabelled and 70 mg non-radiolabelled dezamizumab) and completed dosing session 1 but was withdrawn prior to starting session 2 due to the early study termination.
Patient demographics and baseline characteristics
Baseline demographics and characteristics are presented in Table 1 for both studies. All patients were ≥ 65 years old. There was a noteworthy difference in the baseline LVM for the two patients enrolled in the immuno-PET study: 72 g for the first patient (Patient A) versus 298 g for the second patient (Patient B). Patient A’s diagnosis of ATTR-CM was based on a positive fat pad biopsy with both positive Congo Red and TTR staining, and typical changes of diastolic LV dysfunction on 2D-ECHO and a history of acute diastolic heart failure with NYHA class III clinical presentation that had improved to NYHA class II at study screening with the administration of diuretic therapy.
SAP depletion
In the Phase 2 study, depletion of circulating plasma SAP to target (< 3 mg/L) was always achieved prior to dezamizumab administration and was maintained throughout the testing period following dezamizumab administration. The same was true for the pre-dezamizumab levels in the PET study (circulating plasma SAP was not measured post-dezamizumab).
Pharmacokinetics
In the Phase 2 study, exposure to dezamizumab was similar across patients and consistent across dosing sessions at the same dose level. Derived anti-SAP mAb PK parameters including Cmax and area under the concentration–time curve until the last measurable concentration (AUC[0–t]) were stable from treatment session 2 in the Phase 2 study, and time to Cmax (Tmax) was obtained within 57 h of first dezamizumab dose in all treatment sessions (Table 2). A double ‘spike’ profile was observed, consistent with the split dosing regimen, followed by a tail-off to low levels of detection at Day 11 and beyond in each treatment (Additional file 6). Characterisation of plasma PK of radiolabelled and non-radiolabelled dezamizumab in the immuno-PET study is presented in Additional file 7.
Pharmacodynamic markers and immunogenicity (Phase 2 study)
A mean increase from pre-treatment baseline in high-sensitivity CRP and serum amyloid A protein (SAA) was evident from Day 2 (Group 2) and Day 3 (Group 1) of treatment session 1 and, for Group 1, in subsequent sessions, indicating an inflammatory response following anti-SAP mAb treatment. High-sensitivity CRP increased in the range 0.3–64.3 mg/L from Day 3 for Group 1 (e.g. Session 2, Day 3, 8H time point: Mean (SD) 30.75 (24.329) mg/L), and to 41.4 mg/L from Day 2 for the patient in Group 2. SAA values for Group 1 from Day 3 increased in the range 0.2314–430.4676 mg/L; for the patient in Group 2, SAA increased to 322.2895 mg/L from Day 2.
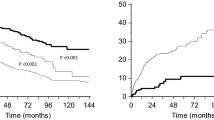
In addition, an observed reduction in plasma complement markers (notably C3 and CH50), with a return to near pre-baseline ranges before initiation of the next treatment session. In the Phase 2 study, mean reductions from pre-treatment baseline were observed for complement C3 values at most time points during each of the six treatment sessions for patients from Group 1. The largest mean reductions were observed by Day 5 or 6 within each treatment session (which were the last days of C3 measurement). Mean pre-dezamizumab values of C3 at the start of each treatment session had returned to a similar range to that observed at pre-treatment baseline (Fig. 2).
This is potentially indicative of target (SAP) engagement but it was not possible to discern the localisation of anti-SAP mAb binding in this study, and whether this was successfully achieved for target organ amyloid deposits.
An immunological response to anti-SAP mAb was evident by the presence of anti-drug antibodies at the 8-week follow-up (designated as positive or negative), with continued long-term positive assessments at 6-month follow-up visits for all patients who had received a minimum of four treatment sessions (data not shown). The only patient who did not display a positive anti-drug antibody result at these assessment times received only three treatment sessions.
LVM, LV thickness, and ECV (Phase 2 study)
In Group 1 of the Phase 2 study, there was no evidence of cardiac amyloid removal over time as measured by the primary endpoint of reduction in LVM by CMR (Fig. 3; patient-level data are shown in Additional file 8). Small mean increases in LVM were observed at all post-baseline time points for patients with ATTR-CM, although at the 8-week follow-up visit the patient from Group 2, who had a lower baseline LVM than all Group 1 patients, had a small reduction from pre-treatment baseline in LVM (change from baseline: − 32.42 g). There was no consistent mean change from baseline in LV wall thickness at any time point as monitored by ECHO. The exploratory endpoint of ECV measured on CMR showed no consistent change from baseline at the 8-week follow-up (ranging from a 7.27% decrease to a 6.61% increase).
Cardiac structural and functional endpoints (Phase 2 study)
At the 8-week follow-up time point, individual changes from baseline in cardiac functional measures for patients from Group 1 were variable. In addition, results were not always directionally consistent across CMR and ECHO at this time point where both methods were utilised; for example, LV end diastolic volume and LV stroke volume parameters showed a mean increase from baseline for the primary CMR assessment and a mean decrease from baseline when using ECHO. A small decrease from baseline in LV ejection fraction (%) was observed at 8-week follow-up using CMR and ECHO (Additional file 9).
Absolute NT-proBNP values at baseline ranged from 1631 to 5020 ng/L across patients from Group 1 with a lower value of 444 ng/L for the patient from Group 2. The corresponding baseline values for Troponin T (high sensitivity) were 32–89 ng/L (Group 1) and 27 ng/mL (Group 2). There was no consistent pattern of change from baseline in NT-proBNP values across patients or treatment sessions. Change from baseline values at the 8-week follow-up ranged from a 1067 ng/L decrease to a 2442 ng/L increase. For patients from Group 1 (with high-sensitivity assays performed), Troponin T values showed increases from baseline for most patients from Day 5 in session 4 and beyond. Increases from baseline values at the 8-week follow-up ranged from 6 to 67 ng/L.
Cardiac uptake and biodistribution
For the PET study, the study protocol specified that dezamizumab was considered to have reached the extracellular space of cardiac muscle if the cardiac-tissue-to-plasma ratio was ≥ 0.5 at any time point or any session for a given patient; this value was chosen as it is appreciably higher than the expected contribution of 0.1–0.2 from the interstitium. The uptake of [89Zr]Zr-dezamizumab in various anatomical locations within the cardiac muscle was moderately high in Patient A, who had the lower LVM, with SUV peak values mostly in the range of 2–5 (a value of 1 indicates even distribution in the body). Uptake in the cardiac muscle was also seen, but was generally lower in Patient B, who had the higher LVM (Table 3; Fig. 4). For Patient A, who participated in two dosing sessions, on the only comparable day between sessions (Day 4) a slight increase was seen in cardiac muscle SUV in the second session. The corresponding blood pool values were also slightly higher (Table 3).
Regarding overall biodistribution in the PET study, dezamizumab was principally distributed to multiple abdominal organs (Table 3; Fig. 4). At session 1, the biodistribution of [89Zr]Zr-dezamizumab was highest in the liver, adrenal gland and spleen for both patients, followed by the kidney and testes for Patient B and thyroid goiter hotspot for Patient A (Table 3). For Patient A, who participated in two dosing sessions, the uptake of [89Zr]Zr-dezamizumab in the high-uptake organs (liver, spleen and adrenals) was in general lower in session 2, and specifically on the only comparable day between sessions (Day 4) (Table 3).
Safety
No deaths were reported in either study. In the Phase 2 study, all patients reported at least one AE during the treatment phase, and all but one patient had a treatment-related AE (Table 4). One treatment-related SAE was reported and is described further below. There were no clinically significant findings from laboratory tests, vital signs, cardiac monitoring, ECHO and ECG assessments. In two patients from Group 1, ventricular tachycardia and extrasystoles in a frequency higher than seen at baseline were reported on Session 1 Day 5 and Session 3 Day 4 for one patient and on Session 1 Day 3, Session 2 Day 10 for the other. This latter patient also had increased frequency of ventricular extrasystoles on Session 2 Day 9.
In the immuno-PET study, both patients experienced AEs. There were a total of eight AEs, four of which (reported in two patients) were considered treatment-related: headache, dactylitis, injection-site bruising and urticarial vasculitis (moderate grade 2 skin rash) (Table 5). Each patient experienced at least one treatment-related AE (i.e. related to dezamizumab and/or miridesap, not related to [89Zr]Zr-dezamizumab). There were no cardiac AEs, cardiac SAEs or AEs leading to treatment discontinuation. There were no laboratory values or vital signs of potential clinical importance. Both patients had ECGs and telemetry assessments that were classed as ‘abnormal – not clinically significant’ before and after treatment.
In both studies dezamizumab was associated with development of rash, which in the Phase 2 study was the most frequently reported AE during the treatment phase. Rash events deemed related to treatment were reported by four patients in the Phase 2 study and one patient in the immuno-PET study. The incident rashes were characterised as urticarial and/or maculopapular in nature. The rash AEs were grade 1 or 2. Grade 1 rashes were treated with topical steroids, antihistamine and/or analgesic as required. One patient in each trial developed a grade 2 rash; the patient in the Phase 2 trial received a reduced dose of dezamizumab on subsequent treatment sessions. Histopathological review of rash-associated skin biopsies obtained in two patients in the Phase 2 study showed leukocytoclastic vasculitis (LCV) involving small dermal vessels in the superficial dermis. The neutrophil-dependent mechanism of LCV was confirmed by strong positive neutrophil-specific elastase staining in skin rash biopsies versus negative staining in baseline biopsies (data not shown). A biopsy performed in the one patient with urticarial vasculitis in the PET study also was reported to show LCV.
In the Phase 2 study, on Day 1 of the first session in Group 2 (post-chemotherapy AL amyloidosis), an unexpected serious adverse reaction of presumptive abdominal large-vessel vasculitis that was considered related to treatment was reported following administration of dezamizumab 300 mg. The patient experienced acute abdominal pain and bloating within 24 h of drug administration. Systemic inflammatory markers were elevated, including CRP and white blood cell count with neutrophilia but no eosinophilia. There was new onset haematuria which resolved by Day 24, though there was no skin rash, and antinuclear bodies/antineutrophil cytoplasmic antibodies were negative. An abdominal CT scan revealed thickening of abdominal large arterial vessels (aorta, both iliac and renal arteries) and fat stranding in the mesentery. Symptoms were mostly resolved within 24 h of a single IV dose of methylprednisolone 1 g. An [18F] fluorodeoxyglucose (FDG)-PET scan performed 72 h after administration of methylprednisolone revealed no discernible increased [18F]FDG uptake in any large arterial vessel, which was consistent with the intended anti-inflammatory effect of high-dose steroid rescue, and prompt resolution of the patient’s abdominal symptoms. The event was reported as resolved within 16 days following methylprednisolone treatment. Ongoing clinical surveillance demonstrated no acute or residual effects on biochemical renal function or urine output; a follow-up CT scan 8 weeks later demonstrated near complete anatomical resolution of abdominal aortic wall thickening. Based on the totality of the information, the investigator and vascular radiologist concluded that the patient experienced extensive large- and small-vessel vasculitis related to the study drug, which was substantially resolved.
Discussion
In this manuscript we present data from two concurrent studies in patients with cardiac amyloid treated with dezamizumab, a mAb to SAP, following SAP depletion with miridesap. The Phase 2 study assessed the magnitude and time course of amyloid removal in the heart in patients with either ATTR or AL amyloidosis, as measured by CMR. The PET imaging study provided direct assessment of the exposure of the cardiac tissue to dezamizumab as well as its wider biodistribution, strengthening the understanding of exposure versus therapeutic evidence that was assessed in the Phase 2 trial.
The principal finding of the Phase 2 study was a lack of evidence of cardiac amyloid removal over time with anti-SAP treatment. Anti-SAP treatment was well tolerated by the six subjects with ATTR-CM, although a rash was identified as the most commonly reported AE. The one AL subject in the trial reported a SAE of large-vessel vasculitis, unexpected from the previous known profile. The occurrence of this event in conjunction with lack of cardiac signal of efficacy led to reconsideration of the benefit-risk assessment for the treatment. The principal finding of the concurrent PET imaging study was the demonstration of only low to moderate uptake of radioactive-labelled anti-SAP mAb in the cardiac tissue at the doses studied, provided supportive evidence of the basis for the Phase 2 findings.
Prior evaluation of this anti-SAP treatment demonstrated amyloid removal in patients with liver, spleen and kidney involvement [13, 14], and a link was seen between reduction in hepatic amyloid load and improvement in liver function [14]. However, our assessment of treatment impact on cardiac involvement failed to provide consistent evidence of cardiac amyloid removal or functional benefit. In particular, the exploratory endpoints of ECV, native T1 mapping, late gadolinium enhancement, and global longitudinal strain, all considered to be of prognostic utility in this disease, showed no evidence of favourable changes with treatment. If miridesap/dezamizumab, with the dosing regimens used, was effective in removing cardiac amyloid, we would expect to see a reduction in LVM within the study period based on the time frame for reductions in amyloid load that were observed in Phase 1; this reduction would be in contrast to amyloid regression with treatments that reduce the precursor source and that take several years to show an effect [18].
The decision for an early termination of the study was based on the lack of evidence of cardiac amyloid removal or functional benefit, and the reporting of a SAE of vasculitis in a subject in the Phase 2 study. The conclusion of the safety evaluation was that the risk of exposing further subjects to anti-SAP treatment outweighed the potential for benefit observed in the study.
The immuno-PET study data are severely limited given enrolment of just two patients. In the study protocol, cardiac uptake was defined as a cardiac-tissue-to-plasma ratio > 0.5 at any time point or any session, which for a haematocrit of approximately 0.5, equates to a tissue-to-blood ratio > 1.0. If there was only a vascular contribution to the signal in the heart, the tissue-to-blood ratio of the myocardium would be expected to be < 0.1 [19]. By this definition, dezamizumab was shown to be able to access the interstitial space, although at best the uptake was moderate to low. A high uptake could conceivably be possible if there was good tissue access and a significant binding to SAP–amyloid complexes. Furthermore, such high uptake could diminish in a second study due to competition by a high amount of non-radiolabelled dezamizumab. Cardiac uptake was seen in both dosing sessions in Patient A but uptake in various anatomical locations within the myocardium was limited in comparison to liver and spleen, with most SUV peak values in the range of 2–5. In Patient B, who was the more typical ATTR-CM patient, only one dezamizumab dose was received and only minor uptake was seen in the LV septum. These limited data suggest that, although dezamizumab does have the ability to be taken up into the myocardial interstitium, it is at a low level with the dosing regimen tested and with a significant ‘sink’ effect, i.e. preferential antibody binding in non-cardiac organs (primarily, but not limited to, liver and spleen). The mechanisms behind the high accumulation of radiolabelled antibody in these organs is not clear, but a noteworthy aspect is that the uptake in these organs is proportionally decreased at the higher mass dose in Patient A. The effect likely had a significant impact on the degree of cardiac penetration, and it is unknown whether longer administration would lead to increased cardiac penetration, once the peripheral sink is saturated. Of note, the different myocardial uptake profiles in the two wild-type ATTR-CM patients enrolled may be a result of the different features of their disease as evidenced by their LVM values: 72 g for Patient A, and 298 g for Patient B. It is possible that Patient A was in earlier stages of cardiac amyloidosis, or that the CM may be (at least partially) attributed to other causes, such as pre-existing diabetes, whereas Patient B had more pronounced cardiac amyloid deposition.
Coronary microvascular perfusion is known to be impaired in TTR amyloidosis. Thus, one additional reason for the discriminatory imaging findings between the two patients in the immuno-PET study is that since LVM is indicative of the extent of amyloid in the interstitial space of the myocardium, extensive amyloid infiltration in Patient B could have led to severe impairment in microvascular perfusion of the myocardium, leading to limited dezamizumab distribution to the heart. This is further supported by the finding in the Phase 2 study that all the ATTR-CM patients who had a similar cardiac amyloid mass to Patient B in the PET study had low coronary perfusion parameters at rest on baseline magnetic resonance imaging (data not shown). However, further correlative pathological imaging research would be needed to confirm the pathophysiological basis for the poor cardiac distribution of dezamizumab and the difference in distribution between the two patients.
In the previous first-in-human study, evidence of amyloid clearance was generally preceded by a fall in C3 (and to a lesser extent C4) [13, 14]. This is consistent with the mechanism of action of dezamizumab [12]. In the Phase 2 study presented here, there was an observed reduction in plasma total C3 levels with a return to the near pre-baseline range before initiation of the next treatment session. Although these findings are indicative and potentially sentinel for dezamizumab–SAP engagement, we are unable to comment on the quality of immunological engagement since the depth of circulating C3 activation was not evaluated by measurement for plasma C3 activation products. As such, since we could not evaluate the precise tissue localisation of dezamizumab–SAP binding (I.e. amyloid deposits vs normal tissue), we cannot provide a complete PD characterisation of dezamizumab in patients with cardiac amyloidosis.
It is possible that the observed reduction in plasma total C3 levels represents PD activity in extra-cardiac organs. The preliminary results of the PET study suggest that while dezamizumab can enter cardiac tissue the amounts are small, which provides a rationale for the lack of impact of dezamizumab on cardiac amyloid load (LVM) or the functional parameters explored in the Phase 2 study. Although amyloid reduction was demonstrated in the first-in-human study in other visceral organs, the necessary triggering of the PD response for removal of amyloid fibrils in the heart is not possible without adequate delivery of the mAb to cardiac tissue to achieve a sufficient density of binding of mAb to cardiac amyloid deposits. In addition to impaired cardiac microvascular perfusion, this may also be a result of the continuous structure of the endothelium in cardiac tissue in contrast to the fenestrated endothelium found in the liver and spleen, the organs in which the most striking treatment effects had previously been seen triggering of a complement-dependent macrophage giant cell response by dezamizumab is dependent on achieving a sufficient density of binding of mAb to target, and the amount of mAb binding to SAP in cardiac tissue may have been insufficient to trigger the response. Alternative explanations could be inadequate entry of complement or macrophage/monocytes for similar reasons. Finally, the depletion of circulating SAP by miridesap necessary for the safe administration of dezamizumab also has been shown to remove SAP from amyloid deposits. It is therefore possible that the extent to which SAP was lowered in these studies affected the extent to which cardiac entry of dezamizumab could achieve target engagement and initiate amyloid removal.
SAP is also present in some vascular basement membranes and is part of the microfibrillar mantle of elastic fibres in normal human tissue [20,21,22]. The potential binding of the mAb to this SAP is the basis of dermal LCV manifesting as rash. Rash was common across these studies and phenotypically similar to that observed in the Phase 1 study. Crucially, this was mainly grade 1 or 2. The single patient with AL enrolled in the Phase 2 study (who had almost exclusively clinically-determined cardiac amyloid), reported an index event of suspected large abdominal vessel vasculitis. In considering the event to be large-vessel vasculitis/aortitis, we acknowledge that CT angiographic imaging has not previously been reported in patients with systemic amyloidosis in patients treated with miridesap and dezamizumab in the current studies or the Phase 1 study. We therefore cannot be sure whether the abnormalities, which were consistent with vasculitis, actually reflect that pathology or indeed whether vasculitis is a more frequent, if asymptomatic, occurrence. There is the potential for overlap between dermatological manifestations of LCV and an event of large-vessel vasculitis, as it is possible for vasculitis to affect small arteries [23]. However, the risk of large-vessel vasculitis is a significant concern due to potential catastrophic clinical implications (such as cerebrovascular, myocardial, or mesenteric ischaemia), and the potential for varied and sometimes vague clinical presentation (as in the index case). These implications (e.g. severity of sequelae, difficulty in monitoring, and lack of clear markers predicting its occurrence) led to the conclusion that the risk of exposing further patients to anti-SAP treatment was not justifiable given the lack of evidence for potential benefit from the Phase 2 interim data (i.e. no reductions in amyloid in the heart nor improvement in cardiac function) and the low cardiac update demonstrated in the PET study.
While the Phase I study showed finding suggesting that anti-SAP therapy can remove amyloid for other organs [14], the current data show that the dosing used in these studies we report is inadequate to target the myocardium. The PET study did not identify localisation of anti-SAP mAb binding in target organ and therefore localisation to other organs cannot be ruled out as an explanation for the lack of change in cardiac measures. Despite these disappointing results in terms of cardiac therapy, we believe that this experience has furthered insight into the potential pathophysiological challenges of delivering anti-amyloid therapy to the heart, an organ in which the effect of amyloid infiltration influences both myocardial structure and perfusion [24, 25].
The main limitation of these studies was the low sample number, with six patients completing the Phase 2 study, and one patient completing the immuno-PET study. Although the initial sample sizes were consistent with the objectives of the studies, sample sizes were further reduced by the early termination of the studies. Furthermore, there was no control data available from the Phase 2 study to quantify anticipated increases in cardiac amyloid attributable to the natural time course of the disease during the study period and hence interpretation of this finding is limited.
Conclusions
In summary, the encouraging Phase 1 results with miridesap/dezamizumab showing removal of amyloid in several organs provided proof of mechanism to conduct the Phase 2 study in cardiac amyloidosis, the major determinant of prognosis in the two most common forms of systemic amyloidosis (ATTR and AL). Recognising the differences in antibody access to the myocardial interstitium compared with liver or spleen, we also conducted an exploratory PET study in parallel to confirm cardiac localisation. Data from the Phase 2 study from seven patients after up to six treatment sessions showed no convincing evidence of amyloid removal with the systemic doses of antibody used. The concurrent immuno-PET study showed only moderate to low uptake in the heart, while inflammatory responses observed in skin and vascular tissue, including the event of large abdominal vessel vasculitis in the Phase 2 study, suggested non-cardiac binding. With the unexpected observation of large-vessel vasculitis, it was considered unwise to test higher doses of dezamizumab in an attempt to deliver more antibody to the heart due to the potential increase in the risk of on-target off-amyloid binding. Given the findings of these studies, we considered the benefit-risk in cardiac amyloid was no longer favourable; therefore, the studies and the development of this treatment for cardiac amyloidosis were terminated.
Availability of data and materials
Anonymized individual participant data from this study plus the annotated case report form, protocol, reporting and analysis plan, data set specifications, raw dataset, analysis-ready dataset, and clinical study report are available for research proposals approved by an independent review committee. Proposals should be submitted to www.clinicalstudydatarequest.com. A data access agreement will be required.
Abbreviations
- AA:
-
Amyloid A protein
- AE:
-
Adverse event
- AL:
-
Light chain
- AUC:
-
Area under the concentration–time curve
- CI:
-
Confidence interval
- CM:
-
Cardiomyopathy
- Cmax :
-
Maximum plasma concentration
- CMR:
-
Cardiac magnetic resonance
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- ECHO:
-
Echocardiogram
- ECG:
-
Electrocardiogram
- ECV:
-
Extracellular volume
- FDG:
-
Fluorodeoxyglucose
- IV:
-
Intravenous
- LCV:
-
Leukocytoclastic vasculitis
- LVM:
-
Left ventricular mass
- mAb:
-
Monoclonal antibody
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- NYHA:
-
New York Heart Association
- PD:
-
Pharmacodynamic
- PET:
-
Positron emission tomography
- PK:
-
Pharmacokinetic
- SAA:
-
Serum amyloid A protein
- SAE:
-
Serious AE
- SAP:
-
Serum amyloid P
- SUV:
-
Standardised uptake values
- Tmax :
-
Time to Cmax
- TTR:
-
Transthyretin
References
Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.
Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24(6):343–50.
Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36(4):244–51.
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–7.
Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–41.
Hayashi Y, Jono H. Recent advances in oligonucleotide-based therapy for transthyretin amyloidosis: clinical impact and future prospects. Biol Pharm Bull. 2018;41(12):1737–44.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16.
Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79–89.
MacRaild CA, Stewart CR, Mok YF, Gunzburg MJ, Perugini MA, Lawrence LJ, et al. Non-fibrillar components of amyloid deposits mediate the self-association and tangling of amyloid fibrils. J Biol Chem. 2004;279(20):21038–45.
Pepys MB, Herbert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417(6886):254–9.
Sahota T, Berges A, Barton S, Cookson L, Zamuner S, Richards D. Target mediated drug disposition model of CPHPC in patients with systemic amyloidosis. CPT Pharmacom Syst Pharmacol. 2015;4(2):15.
Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–7.
Richards DB, Cookson LM, Barton SV, Liefaard L, Lane T, Hutt DF, et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018;10(422):65.
Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373(12):1106–14.
Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9.
Martinez-Naharro A, Abdel-Gadir A, Treibel TA, Zumbo G, Knight DS, Rosmini S, et al. CMR-verified regression of cardiac AL amyloid after chemotherapy. JACC Cardiovasc Imaging. 2018;11(1):152–4.
Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70(4):466–77.
Salehpour M, Håkansson K, Westermark P, Antoni G, Wikström G, Possnert G. Life science applications utilizing radiocarbon tracing. Radiocarbon. 2016;55(2):865–73.
Shah DK, Betts AM. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs. 2013;5(2):297–305.
Breathnach SM, Bhogal B, Dyck RF, De Beer FC, Black MM, Pepys MB. Immunohistochemical demonstration of amyloid P component in skin of normal subjects and patients with cutaneous amyloidosis. Br J Dermatol. 1981;105(2):115–24.
Breathnach SM, Melrose SM, Bhogal B, de Beer FC, Dyck RF, Tennentt G, et al. Amyloid P component is located on elastic fibre microfibrils in normal human tissue. Nature. 1981;293(5834):652–4.
Dyck RF, Lockwood CM, Kershaw M, McHugh N, Duance VC, Baltz ML, et al. Amyloid P-component is a constituent of normal human glomerular basement membrane. J Exp Med. 1980;152(5):1162–74.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
Edwards CV, Rao N, Bhutani D, Mapara MY, Radhakrishnan J, Shames S, et al. Phase 1a/b study of monoclonal antibody CAEL-101 (11–1F4) in patients with AL amyloidosis. Blood. 2021;6:66.
Gertz MA, Cohen AD, Comenzo RL, Du Mond C, Kastritis E, Landau HJ, et al. Results of the Phase 3 VITAL Study of NEOD001 (Birtamimab) plus standard of care in patients with light chain (AL) amyloidosis suggest survival benefit for Mayo stage IV patients. Blood. 2019;134(1):3166.
Acknowledgements
Not applicable.
Funding
This study was funded by GlaxoSmithKline (GSK). GSK was involved in the design of the study and collection, analysis and interpretation of data and in writing the manuscript. CTC coordinated the study in Uppsala and was funded by GSK. JC acknowledges funding support from the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Medical writing support was provided by Chloe Stevenson, MSci, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation and review of the manuscript and approved the final version. Contributions by studies are summarised below. Phase 2 study: RHF and AW1 contributed to the conception and design of the study, data analysis and interpretation and acquisition of data. JC, RYK and SDS contributed to the data analysis and interpretation and acquisition of data. SB, LC, PG, RLJ, DR, JS2 and DT contributed to the conception and design of the study and the data analysis and interpretation. CC, MAL and HM contributed to the data analysis and interpretation. JC and RHF were principal investigators, and AW1 was the chief investigator. PET study: JS1 and AW2 contributed to the conception and design of the study, data analysis and interpretation and acquisition of data. GA, MB, SB, CC, LC, PG, RLJ, DR, IS and DT contributed to the conception and design of the study and data analysis and interpretation. WAA, MAL and HM contributed to the data analysis and interpretation. MC contributed to the conception and design of the study. GvD and DJV contributed to the conception and design of the study, and also contributed to the synthesis of [89Zr]Zr-dezamizumab (PET tracer). GW contributed to the data analysis and interpretation and acquisition of data and was the chief investigator. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Both studies were conducted in accordance with the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice ethical principles, as outlined in the Declaration of Helsinki. The Phase 2 study protocol and procedures were reviewed and approved by UK Medicines and Healthcare Products Regulatory Agency, Food and Drug Administration's Center for Drug Evaluation and Research and National Research Ethics committee of Services Committee South West—Central Bristol, Partners Human Research Committee—Somerville. The PET study protocol and procedures were reviewed and approved by the Swedish Medical Products Agency and the Regional Ethical Review Board, Uppsala University Dnr 2017/498. Written informed consent was obtained from each patient for both studies.
Consent for publication
Not relevant.
Competing interests
AW1 has received consultancy fees from GSK. WA-A, MB, SB, CC, MC, LC, MAL, HM, DR, IS, JS2, RLJ, PG, and DT are employees of or hold stocks/shares in GSK. JC is a fulltime Cambridge University Hospitals NHS Foundation Trust employee who was seconded for 50% of his NHS time to the GSK unit in Cambridge until September 2020. He received no employee benefits from GSK. RHF, RYK received research funding from GSK in support of the work provided by the CMR core laboratory. GW has received lecture fees from Novartis, Orion-Pharma, and Actelion. Outside of office hours Professor Wikström received remuneration from CTC. JS1 has no competing interests. SDS has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, and Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, and American Regent. GA and AW2: report no conflicts of interest. GvD: received funding from GSK for [89Zr]Zr-dezamizumab development and production. He has an unpaid position of Chief Scientific Officer (CSO) at LinXis biopharmaceuticals. DJV: received funding from GSK for [89Zr]Zr-dezamizumab development and production.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Patient population details.
Additional file 2.
Full inclusion and exclusion criteria.
Additional file 3.
Study objectives.
Additional file 4.
Phase 2 CMR methodology.
Additional file 5.
Phase 1 immuno-PET study methods.
Additional file 6.
Median plasma dezamizumab concentration–time plots by treatment cycle (Phase 2 study; safety population).
Additional file 7.
Plasma PK of [89Zr]Zr-dezamizumab and total dezamizumab (non-radiolabelled dezamizumab and [89Zr]Zr-dezamizumab (immuno-PET study; PK population).
Additional file 8
. Changes in LVM across treatment sessions for each patient (Phase 2 study).
Additional file 9.
Summary of imaging markers of cardiac dysfunction as measured by CMR and/or ECHO imaging over time (safety population).Summary of imaging markers of cardiac dysfunction as measured by CMR and/or ECHO imaging over time (safety population).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wechalekar, A., Antoni, G., Al Azzam, W. et al. Pharmacodynamic evaluation and safety assessment of treatment with antibodies to serum amyloid P component in patients with cardiac amyloidosis: an open-label Phase 2 study and an adjunctive immuno-PET imaging study. BMC Cardiovasc Disord 22, 49 (2022). https://doi.org/10.1186/s12872-021-02407-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02407-6