Abstract
Background
Coronavirus disease 2019 (COVID-19) has become a global pandemic. Studies showed COVID-19 affected not only the lung but also other organs. In this study, we aimed to explore the cardiac damage in patients with COVID-19.
Methods
We collected data of 100 patients diagnosed as severe type of COVID-19 from February 8 to April 10, 2020, including demographics, illness history, physical examination, laboratory test, and treatment. In-hospital mortality were observed. Cardiac damage was defined as plasma hypersensitive troponin I (hsTnI) over 34.2 pg/ml and/or N-terminal-pro brain natriuretic peptide (NTproBNP) above 450 pg/ml at the age < 50, above 900 pg/ml at the age < 75, or above 1800 pg/ml at the age ≥ 75.
Results
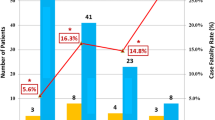
The median age of the patients was 62.0 years old. 69 (69.0%) had comorbidities, mainly presenting hypertension, diabetes, and cardiovascular disease. Fever (69 [69.0%]), cough (63 [63.0%]), chest distress (13 [13.0%]), and fatigue (12 [12.0%]) were the common initial symptoms. Cardiac damage occurred in 25 patients. In the subgroups, hsTnI was significantly higher in elder patients (≥ 60 years) than in the young (median [IQR], 5.2 [2.2–12.8] vs. 1.9 [1.9–6.2], p = 0.018) and was higher in men than in women (4.2 [1.9–12.8] vs. 2.9 [1.9–7.4], p = 0.018). The prevalence of increased NTproBNP was significantly higher in men than in women (32.1% vs. 9.1%, p = 0.006), but was similar between the elder and young patients (20.0% vs. 25.0%, p = 0.554). After multivariable analysis, male and hypertension were the risk factors of cardiac damage. The mortality was 4.0%.
Conclusions
Cardiac damage exists in patients with the severe type of COVID-19, especially in male patients with hypertension. Clinicians should pay more attention to cardiac damage.
Similar content being viewed by others
Introduction
Since the novel coronavirus–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) named by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses [1], was discovered in December 2019, it quickly spread throughout China and other countries [2,3,4,5]. As of April 30, 2020, SARS-CoV-2 has broken out in 213 countries, areas and territories with 3,090,445 confirmed cases and 217,769 deaths [6]. Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 could be classified into four clinical types: mild, moderate, severe and critical types [7]. More than 80% are mild or moderate with relatively good short-term prognosis due to the self-limiting process according to the study with 44,672 confirmed COVID-19 cases released by the Chinese Center for Disease Control and Prevention on February 17, 2020 [8]. However, 6,168 (13.8%) cases belonged to the severe type and were more likely to develop into the critical type followed by a death rate of 49%, far beyond the average mortality of 2.3%.
Researchers have reported that patients with COVID-19 had acute cardiac injury, which is associated with a higher risk of in-hospital mortality [4, 9, 10]. However, studies focusing on cardiac damage in patients with the severe COVID-19 are few.
In this study, we aim to investigate the clinical findings and cardiac damage in patients with the severe type of COVID-19, and hope to contribute to the prevention and treatment.
Methods
Study population
We retrospectively collected data of all 100 patients at the Sino-French New Town area of Tongji Hospital, Wuhan, where was aided and charged by the medical team of Beijing Hospital from February 8 to April 10, 2020. Patients were all diagnosed as COVID-19 and classified into the severe type according to the Diagnosis and Treatment of Pneumonia Infected by Novel Coronavirus (5th trial edition) pressed by the General Office of the National Health Commission and the General Office of the National Administration of Traditional Chinese Medicine [7]. Severe type met at least one of the following criteria: (1) dyspnea, respiratory frequency ≥ 30/minute, (2) blood oxygen saturation ≤ 93% at rest, (3) PaO2/FiO2 ratio ≤ 300. Critical type met at least one of the following criteria: (1) respiratory failure with mechanical ventilation, (2) septic shock, (3) transferred to the intensive care unit due to multiple organ failure. Patients with acute coronary syndrome or acute heart failure at admission or in the latest one month were excluded. This study was approved by the Ethics Commission of Beijing Hospital (2020BJYYEC-035–01).
Laboratory confirmation of SARSCoV-2 was done by real-time RT-PCR. The protocol was the same as the document published recently [4]. We also examined other respiratory viruses with real-time RT-PCR, including influenza A virus (H1N1, H3N2, H7N9), influenza B virus, respiratory syncytial virus, parainfluenza virus, adenovirus, SARS coronavirus (SARS-CoV), and MERS coronavirus (MERS-CoV). Sputum or endotracheal aspirates were obtained at admission for the identification of possible causative bacteria or fungi.
Data collection
We obtained demographic, illness history, physical examination, laboratory test, management, and outcome data from patients’ medical records. Blood oxygen saturation was measured after oxygen therapy. In-hospital mortality were observed.
Laboratory tests were conducted within 24 h after admission, including a complete blood count, procalcitonin, interleukin-6, ferritin, coagulation profile, renal and liver function, hypersensitive troponin I (hsTnI), and N-terminal-pro brain natriuretic peptide (NTproBNP).
Definition of cardiac damage
Cardiac damage was defined as plasma hsTnI over 34.2 pg/ml and/or NTproBNP above 450 pg/ml at the age age < 50, above 900 pg/ml at the age < 75, or above 1800 pg/ml at the age ≥ 75 [11].
Statistical analysis
Continuous variables were expressed as mean ± SD when they were normally distributed or median (IQR) when they were not, and compared with the t-test or Mann–Whitney U test, respectively; categorical variables were expressed as number (%) and compared by χ2 test or Fisher’s exact test. Logistic regression analysis was performed to identify variables with a significant independent association with cardiac damage. Demographics (age and sex), potential confounders (hypertension, diabetes, cardiovascular disease, and hyperlipidemia), and variables with p ≤ 0.05 in the univariate analysis were adjusted. A two-sided α of less than 0.05 was considered statistically significant. Statistical analyses were done using the SPSS software (version 23) for all analyses.
Results
From February 8 to April 10, 2020, 100 laboratory-confirmed COVID-19 patients were classified as the severe type at the Sino-French New Town area, with an median age of 62.0 years old. 56 (56.0%) cases were men. 69.0% of the patients had comorbidities, of which hypertension, diabetes, and cardiovascular disease were the top three diseases. The initial symptoms were mainly fever, cough, chest distress, and fatigue. Ten patients had oxygen saturation below 93% after nasal oxygen supply at admission (Table 1).
Cardiac damage occurred in 25 patients. (Table 2) Increased hsTnI was in 9 (9.0%) patients; increased NTproBNP was in 22 (22.0%) patients. Subgroup analysis showed that significant age and sex differences in hsTnI. (Tables 3, 4) The elderly patients had higher plasma hsTnI levels, so did the males, who also had significantly higher NTproBNP levels than the females. Although NTproBNP level was far higher in the elder, the statistical difference disappeared when taking the effect of age on NTproBNP into consideration.
Patients with cardiac damage had a higher proportion of hypertension and diabetes, compared to those without cardiac damage. White blood count, prothrombin time, d-dimer, creatinine, interleukin-6, procalcitonin, and hsCRP levels were significantly different between the two groups. After adjusting for age, sex, hypertension, diabetes, cardiovascular disease, hyperlipidemia, and variables with significant differences, we found male and hypertension were the risk factors of cardiac damage in patients with severe COVID-19. (Tables 5, 6).
By the end of April 10, 2020, four (4.0%) patients died. In the same period, 96 patients, of which 2 cases deteriorated to critical type but ultimately recovered and discharged from the hospital according to the Criteria of Diagnosis and Treatment of Pneumonia Infected by Novel Coronavirus (5th trial edition). (Table 7).
Discussion
In this retrospective study, we analyzed data from 100 patients with severe type of laboratory-confirmed COVID-19. Fever, cough, chest distress, and fatigue were common symptoms. Patients with severe COVID-19 also presented lymphopenia, elevated interleukin-6, procalcitonin, and D-dimer. These were consistent with recent researches [3, 4, 12, 13]. More than half of the patients had comorbidities, mainly including hypertension, diabetes, and cardiovascular disease. The prevalence of cardiac damage was 25%. The mortality of severe COVID-19 was 4%.
Huang et al. reported acute cardiac and kidney injuries in COVID-19 patients. In our study, one-quarter of the patients had cardiac damage, suggesting COVID-19 was a systemic disease and SARS-CoV-2 could cause multiorgan damage. The mechanism of cardiac damage resulted from SARS-CoV-2 is unclear. We consider two possible explanations: (1) immune response elicited by the coronavirus may lead to systemic inflammatory response [14, 15]; (2) the virus exists in multiple organ systems to attack tissues [16].
The prevalence of elevated NTproBNP was 22.0% in our study, which was rarely reported previously. Published studies showed that the incidence of myocardial injury presenting elevated hsTnI was 7.2–19.7% in general COVID-19 patients and 23% in critical ill patients, higher than 9.0% in our study [9, 10, 17]. It possibly owes to different criteria of elevated hsTnI, sample sizes, and the phases of COVID-19 breakout.
In subgroups, there was no significant age or sex difference in cardiac damage, but a higher proportion of comorbidities, especially hypertension and diabetes, existed in patients with cardiac damage. After multivariable analysis, we found males and hypertension were the risk factors of cardiac damage. It is consistent with Shi’s report [10]. As all the patients in our study didn’t have an acute coronary syndrome or acute heart failure at admission or in the latest one month, it may suggest that male patients with hypertension are more susceptible to cardiac damage. The possible mechanism is that hypertension-induced cardiac damage is associated with mitochondrial injury, which can be caused by SARS-COV-2 [18, 19]. Estrogen may also play an important protective role in the process [20]. Researchers found cardiac troponins elevation was associated with the male [21, 22].
As of April 10, 96% of the patients were discharged from the hospital. The mortality was 4%, less than the average mortality of severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS), which were 11% and 35% [23, 24]. It indicates the prognosis of COVID-19 is generally good. There was no difference in mortality between patients with cardiac damage and those without (0 vs. 5.3%, p = 0.556).
Limitations
Our study has several limitations. First, it’s a study with a small sample size, confounding factors and selection bias are inevitable. Second, we had no data on medication history, electrocardiography, and echocardiography and we can’t describe and discuss the results adequately. Third, we didn’t include the outcomes after patients were discharged from the hospital.
Conclusion
COVID-19 is a systemic disease. Cardiac damage exists in patients with the severe type of COVID-19, especially in male patients with hypertension. Clinicians should pay more attention to cardiac damage. Further studies with large sample size are needed to verify our findings.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019;
- SARS-CoV:
-
SARS coronavirus
- MERS-CoV:
-
MERS coronavirus
- hsTnI:
-
Hypersensitive troponin I
- NTproBNP:
-
N-terminal-pro brain natriuretic peptide
References
1ICTV. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44.
Chan JF, Yuan S, Kok K, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23.
Xu X, Wu X, Jiang X, Xu K, Ying L, Ma C, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497–506.
Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Dig Health. 2020;2:e201–8.
6WHO COVID-19 Dashboard.2020. https://covid19.who.int/ Accessed 1 May, 2020
7China NHCO. The Diagnosis and Treatment of Pneumonia Infected by Novel Coronavirus (5th trial edition). 5 February, 2020. https://39.137.20.198/cache/www.nhc.gov.cn/xcs/zhengcwj/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf?ich_args2=46-24145100054382_b8ed1a1a481d778c43503cdcde88e45c_10001002_9c896c2ed2c1f6d1913c518939a83798_10cabae9adc42948a617b3b1c71d6fff. Accessed 24 February, 2020
Team TNCP. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19) in China. Chin J Epidemiol. 2020;41:145–51.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China. JAMA. 2020;323:1061–9.
10Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients WithCOVID-19 in Wuhan, China. JAMA Cardiol. 2020;e200950.
Chinese Medical Association. Chinese Medical Journals Publishing House, Chinese Society of General Practice, Editorial Board of Chinese Journal of General Practitioners of Chinese Medical Association, Expert Group of Guidelines for Primary Care of Cardiovascular Disease. Guideline for primary care of acute heart failure. Chin J Gen Pract. 2019;18:925–30.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–71.
Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol. 2020;30:e2107.
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–4.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med. 2020;8:475–81.
Eirin A, Lerman A, Lerman LO. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. EUR HEART J. 2014;35:3258–66.
Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. MITOCHONDRION. 2020;54:1–07.
Patrizio M, Marano G. Gender differences in cardiac hypertrophic remodeling. Ann Ist Super Sanita. 2016;52:223–9.
Suthahar N, Meems LMG, Ho JE, de Boer RA. Sex-related differences in contemporary biomarkers for heart failure: a review. Eur J Heart Fail. 2020;22:775–88.
Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–65.
Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, Ip MS. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(Suppl):S36-40.
Badawi A, Ryoo SG. Prevalence of comorbidities in the middle east respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33.
Acknowledgments
None.
Funding
The study was funded by the Beijing Municipal Natural Science Foundation (No. 7192078).
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. LJ, GJ and LJ conceived and designed the study; LJ, ZYH, GJ and LJ analyzed the data and drafted the manuscript. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Commission of Beijing Hospital (2020BJYYEC-035–01). Individual informed consent was waived by the ethics committee listed above because this study used currently existing sample collected during the course of routine medical care and did not pose any additional risks to the patients.
Consent for publication
Not applicable.
Competing interests
All authors have nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Zhang, Y., Wang, F. et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID-19). BMC Cardiovasc Disord 20, 479 (2020). https://doi.org/10.1186/s12872-020-01758-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01758-w