Abstract
Background
The aim of this study was to assess the impact of hypertension with or without diabetes on left ventricular (LV) remodeling in rural Chinese population.
Methods
A total of 10,270 participants were classified into control group, hypertension without diabetes (HT) group, and hypertension with diabetes (HT + DM) group. We compared clinical characteristics and echocardiographic parameters, and used multivariable logistic regression analysis to assess the associations of interest.
Results
HT + DM group had higher interventricular septal thickness (IVSd), posterior wall thickness (PWTd), left ventricular mass (LVM), LVM index (LVMI), relative wall thickness (RWT), left atrial diameter (LAD), A wave and lower E wave than HT group (all P < 0.05). The prevalence rates of left ventricular hypertrophy (LVH) and abnormal geometry were statistically different among three groups (P < 0.001) and eccentric hypertrophy was the highest proportion of geometry abnormality. Logistic regression analysis suggested that subjects in HT and HT + DM groups had odds ratio (OR) values of 2.81, 4.41, 2.24 and 3.94, 7.20, 2.38 for LVH, concentric hypertrophy and eccentric hypertrophy in the total population, respectively, compared to control group. When compared with HT group, those in HT + DM group had approximately 1.40-, 1.61- and 1.38-, 1.71-fold increased risk for LVH and concentric hypertrophy in the total and female population separately, but no association of HT + DM with LVH and abnormal geometrical patterns was found in men.
Conclusions
This study demonstrated that, to varying degrees, hypertension was associated with LV remodeling in rural Chinese population, and this risk association was obviously increased for LVH and concentric hypertrophy when accompanied by diabetes, especially for women.
Similar content being viewed by others
Background
Among hypertensive patients, left ventricular hypertrophy (LVH) and abnormal geometry are both important markers of cardiac remodeling strongly associated with cardiovascular risk and prognosis [1]. There are three abnormal LV geometrical patterns including concentric remodeling, concentric hypertrophy and eccentric hypertrophy [2]. The various prevalence rates of LVH and abnormal geometrical patterns have been described in different hypertensive populations, but which type is predominated is controversial [3,4,5]. Alterations in cardiac metabolism for example are of particular interest in diabetes, where an increased reliance on fatty acid oxidation and uncoupling between glucose oxidation and glycolysis are suggested to promote LVH [6]. Hypertension coexisting with diabetes therefore can further increase the risk of target organ injury and clinical cardiovascular accident [7]. However, their combined and sex-specific effects on LV remodeling remain unclear.
Investigators have reported that patients with hypertension have high prevalence of LV remodeling or geometrical changes [8, 9], but data concerning the cardiac features of hypertensive subjects with or without diabetes remain incompletely described in large population-based samples, especially in the rural population of China, where hypertension is often popular. Therefore, we conducted this cross-sectional study to investigate the impact of hypertension with or without diabetes and its possible gender-dependent effect on LV remodeling, hypertrophy and geometry in northeast rural Chinese population.
Methods
Study population
Detail methods were described in previous studies [10,11,12,13]. From January 2012 to August 2013, a representative sample of individuals aged ≥35 years was chosen to present the prevalence, incidence and natural history of cardiovascular risk factors in rural areas of Liaoning Province, located in Northeast China. We conducted this cross-sectional study using a multi-stage, stratified randomly cluster-sampling scheme. In the first stage, three counties (Dawa, Zhangwu, and Liaoyang County) were selected from the eastern, southern, and northern region of Liaoning province. In the second stage, one town was randomly chosen from each three counties. In the third stage, 8–10 rural villages from each town were randomly selected (a total of 26 rural villages). All the eligible permanent residents aged ≥35 years from each village were invited to attend the study (a total of 14,016 participants). Of those, 11,956 participants (i.e. response rate of 85.3%) agreed and completed the study. The study was approved by the Ethics Committee of China Medical University (Shenyang, China). All procedures were performed in accordance with the ethical standards. Written consent was obtained in all participants after they had been informed of the objectives, benefits, medical items and confidentiality agreement of personal information. If the participants were illiterate, we obtained the written informed consents from their proxies. In this report, subjects were excluded if they met any of the following criteria: pregnancy, malignant tumor, mental disorder, congenital heart disease, myocardial infarction, pericardia disease, cardiomyopathy or other organic heart disease, all kinds of serious arrhythmia, heart valve disease (the valve with moderate to severe reflux or stenosis) and diabetes without hypertension. Our final sample consisted of 10,270 participants.
Data collection and measurements
The data collection and measurements used in this study were described in previous studies [10, 12,13,14]. Data were collected via a single visit by experienced cardiologists and trained nurses, where a standard questionnaire was used by a face-to-face interview. Before the survey was conducted, we called for all eligible investigators to undergo the organized training on the purpose of this study, how to administer the questionnaire, the standard method of measurement, the importance of standardization, and the study procedures. A strict test was performed at the end of this training, and those who scored perfectly on the test could become investigators. During data collection, our inspectors provided further instructions and support. A central steering committee with a subcommittee for quality control was organized.
Weight and height were measured to the nearest 0.1 kg and 0.1 cm respectively with the participants in light weight clothing and without shoes. Waist circumference (WC) was measured at the umbilicus using a non-elastic tape (to the nearest 0.1 cm) in standing position at the end of normal expiration. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Body surface area (BSA) was calculated as [0.0061 × height (cm) +0.0128 × weight (kg)-0.1529].
According to American Heart Association protocol, blood pressure was measured 3 times at 2-min intervals after at least 5 min of rest. Blood pressure measurements were acquired with a standardized automatic electronic sphygmomanometer (HEM-907; Omron) validated by the British Hypertension Society protocol. The participants were asked to avoid caffeinated beverages and exercise for at least 30 min before the measurement. During the measurement, participants were seated with the arm supported at the level of the heart. The mean of three BP measures was calculated for analysis. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and (or) diastolic blood pressure (DBP) ≥90 mmHg and (or) being under antihypertensive treatment according to JNC-7 report [15].
Fasting blood samples were collected in the morning after at least 12 h of fasting for all participants. Blood samples were obtained from an antecubital vein into Vacutainer tubes containing EDTA. Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose (FPG), plasma creatine (PC), hemoglobin and uric acid (UA) were analyzed enzymatically on an autoanalyzer. All laboratory equipment was calibrated, and blind, duplicate samples were used. Diabetes was defined as FPG ≥ 7 mmol/L (126 mg/dL) and/or being on treatment for diabetes according to the WHO criteria [16].
Echocardiography
Echocardiographic examination was described in our previous study [10]. The echocardiograms were obtained using a commercially available Doppler machine (Vivid, GE Healthcare, United States), with a 3.0 MHz transducer using M-mode, 2-dimensional, spectral and color Doppler transthoracic echocardiography. Echocardiogram analyses and readings were performed by three physicians majored in echocardiography.
According to the guideline of the American Society of Echocardiography [17], the parasternal long-axis view was measured to record interventricular septal thickness (IVSd), LV end-diastolic internal dimension (LVIDd), posterior wall thickness (PWTd) at end-diastole, and LV end-systolic internal dimension (LVIDs), left atrial diameter (LAD), aortic annular diameter (AOD) during systole. The mitral inflow velocities were obtained by pulsed wave Doppler at the level of mitral valve tips in the apical four-chamber view, and early diastolic peak flow (E), atrial peak flow (A) and E/A ratio were recorded. The LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were estimated by Teichholz eqs. [18]: LVEDV (ml) = LVIDd3 × 7.0/(2.4 + LVIDd), LVESV (ml) = LVIDs3 × 7.0/(2.4 + LVIDs). LV ejection fraction (EF) was calculated as [(LVEDV-LVESV)/LVEDV] × 100% and fractional shortening (FS) was determined using [(LVIDd-LVIDs)/LVIDd] × 100%.
Left ventricular mass (LVM) was estimated according to the formula: LVM = 0.8× [1.04{(IVSTd + PWTd + LVIDd)3–LVIDd3}] + 0.6 g. LVM was divided by height2.7 to obtain left ventricular mass index (LVMI). LVH was diagnosed when LVMI was >48 g/ht2.7 in men and >44 g/ht2.7 in women [19, 20]. The relative wall thickness (RWT) = (IVSd + PWTd)/LVIDd and considered increased if > 0.42 [20]. LV geometry was assessed from LVMI and RWT values [9, 20], including normal geometry with normal LVMI and normal RWT, concentric remodeling with normal LVMI and increased RWT, concentric hypertrophy with increased LVMI and increased RWT, and eccentric hypertrophy with increased LVMI and normal RWT.
Statistical analysis
Descriptive statistics were calculated for all the variables. Continuous variables were reported as mean ± standard deviations, and categorical variables were reported as numbers and percentages. Differences among categories were evaluated using ANOVA, non-parametric test or the χ2-test as appropriate. Multivariable logistic regression analyses were used to identify the associations of HT, HT + DM with LVH and abnormal geometrical patterns by calculating odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS version 17.0 software, and P<0.05 indicated statistical significance.
Results
Clinical characteristics
Of the 10,270 participants, there were 4990 (48.6%) subjects without hypertension and diabetes (control group), 4462 (43.4%) subjects with hypertension without diabetes (HT group) and 818 (8.0%) subjects with hypertension and diabetes (HT + DM group). The clinical characteristics of participants in each group were described and compared in Table 1. Participants in HT + DM group tended to have higher weight, WC, BSA, BMI, SBP, TC, TG, LDL-C and FPG than control and HT groups.
Echocardiographic parameters comparison
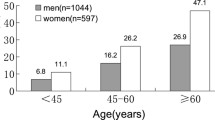
As shown in Table 2, compared to control group, IVSd, LVIDd, LVIDs, PWTd, LVM, LVMI, RWT, AOD, LAD/BSA, A wave, LVEDV, LVESV and SV were significantly higher, while E wave was lower in HT and HT + DM groups (P < 0.05). HT + DM group had higher IVSd, PWTd, LVM, LVMI, RWT, LAD, A wave and lower E wave than HT group (P < 0.05). The prevalence of LVH was statistically different in the three groups (P < 0.001) and eccentric hypertrophy was the highest proportion of geometry abnormality. The prevalence rates of eccentric hypertrophy, concentric remodeling and concentric hypertrophy were statistically different among groups. Furthermore, we compared the echocardiographic parameters of participants based on age groups. The results showed that, as age increased, IVSd, LVIDd, LVIDs, PWTd and A wave became higher, while E wave and E/A became lower from 35 to 45 year group to >65 year group (P < 0.05).
Hypertension with or without diabetes and LV geometry abnormality
Adjustment for age, gender, race, height, weight, WC, heart rate, smoking, drinking, TC, TG, LDL-C, HDL-C, PC, hemoglobin, UA,antihypertensive and antidiabetic medication, the logistic regression models showed that subjects in HT and HT + DM groups had OR values of 2.81, 4.41, 2.24 and 3.94, 7.20, 2.38 for LVH, concentric hypertrophy and eccentric hypertrophy in the total population, respectively, compared with control group. When compared with subjects in HT group, the total and female population in HT + DM group had approximately 1.40-, 1.61- and 1.38-, 1.71-fold increase in the risk of LVH and concentric hypertrophy separately, however, there existed no association of HT + DM with LVH and abnormal geometrical patterns in male population. Table 3.
Discussion
In this study, we investigated the impact of hypertension with or without diabetes on LV hypertrophy, geometry and function in northeast rural Chinese population. Our results suggested that different degree of LV remodeling was found in HT and HT + DM groups. Although there were no significant differences in LVIDd, LVIDs, LVEDV, LVESV and SV that reflected ventricular volume indexes between HT + DM and HT group, the values of IVSd, PWTd, LVM, LVMI, RWT and LAD were significantly higher in HT + DM group than HT group. It indicated that in the presence of diabetes, hypertension could produce more obvious LV hypertrophy and remodeling. Diabetes is a well-established risk factor for developing cardiovascular disease [21] and diabetic hypertensive patients may have more severe clinical and morphologic features of cardiovascular disease compared to those with hypertension only [22, 23]. Recent studies in mice models further found that diabetes by itself had no overt effect on cardiac structural change, but that combined with hypertension, diabetic heart appeared to potentiate LV hypertrophic remodeling [24,25,26]. This point could help us suppose that the diabetic condition sensitized the heart to other common pathological factors, like hypertension. Impaired LV diastolic function, followed by a decrease in early-diastolic ventricular filling and a greater atrial contribution to ventricular filling in the late-systolic period, could appear much earlier than systolic dysfunction [27]. Our study showed that diabetic hypertensive patients had worse diastolic function with lower E wave and higher A wave than non-diabetic hypertensive patients.
The LV adaptation to hemodynamic changes takes four different geometric patterns using LVMI together with RWT, which is determined to a great degree by whether pressure or volume overload is predominating. In our study, about 35.7% of hypertensive subjects without diabetes and 45.6% of hypertensive ones with diabetes had abnormal geometrical changes, in which eccentric hypertrophy was the highest proportion. Eccentric hypertrophy may be the early cardiac presentation of a pathological process without an intervention of concentric hypertrophy [1] and its high prevalence may be associated with the status of volume overload which plays a critical role in LV adaptation in hypertension with or without diabetes [28, 29]. A common belief was that concentric remodeling or hypertrophy was the predominant type of abnormal LV geometry among hypertensive population [30,31,32]. On the contrary, data from some epidemiologic studies have indicated that eccentric hypertrophy was even more common than concentric hypertrophy in hypertensive population [33], which is similar to our finding. The differences in geometric patterns might be due to population heterogeneity, genetic variability, diagnostic criteria of LVH and risk factors [9, 34].
Furthermore, after multivariable adjustment, results from logistic regression analysis indicated a positive and significant association of LVH and three abnormal geometrical patterns with non-diabetic hypertension. Hypertension coexisting with diabetes has been shown to be correlated with concentric hypertrophy rather than eccentric hypertrophy [7, 35, 36]. However, in our study, when compared with control group, we demonstrated that hypertension with diabetes was not only significantly associated with concentric hypertrophy, but also with LVH and eccentric hypertrophy in the total, male and female population, respectively. Moreover, we found that subjects in HT + DM group had about 1.5-fold higher risk for LVH and concentric hypertrophy than those from HT group in the total and female population, but no such association for men. Thus, we further provided evidence that hypertension and diabetes had additive effects of pressure load and sugar metabolic disorder on LV hypertrophy and abnormal geometrical changes. Of note, hypertension had strong effects on LV remodeling, and an additional contribution of diabetes was obtained only in women but not in men [37]. Such a sex-specific difference might be contributed to the presence of sensitive estrogen receptors in cardiomyocytes and insulin resistance in diabetes counterbalancing the beneficial cardiovascular influences of estrogen in women [38]. Therefore, there is an urgent need to strengthen the control of hypertension with diabetes, especially for female patients, in order to avoid subsequent serious cardiovascular events and burden on the rural population.
Our study has some limitations. First, based on a cross-sectional design, we were unable to draw causal inferences exactly from the results. Second, although we have a strict operation and diagnostic criteria, small and inevitable differences in performance technique among doctors might influence results. Third, detailed and complete records of glycated hemoglobin (HBA1c) level, types of hypertensive drugs, dose and compliance were not available. Fourth, diagnosis of diabetes relied on FPG level and/or being on treatment for diabetes without integrating HbA1c into the diagnostic criteria, which suggests that the prevalence of hypertension with diabetes may have been underestimated [39].
Conclusions
In summary, there existed LV remodeling in hypertensive subjects with or without diabetes in rural Chinese population, and eccentric hypertrophy was the commonest abnormal geometrical pattern. Hypertension was an independent risk factor for LVH and geometrical changes, and this risk was increased by different degrees when accompanied with diabetes. Compared to non-diabetic hypertension, hypertension with diabetes was more important for an increase in the risk of LVH and concentric hypertrophy in women, but not in men. Moreover, further prospective studies are required to understand the predictive usefulness of LVH and geometrical changes in risk assessment in these patient groups.
Abbreviations
- A:
-
Atrial peak flow
- AOD:
-
Aortic annular diameter
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- DBP:
-
Diastolic blood pressure
- E:
-
Early transmitral diastolic peak flow
- EF:
-
Ejection fraction
- FPG:
-
Fasting plasma glucose
- FS:
-
Fractional shortening
- HDL-C:
-
High-density lipoprotein cholesterol
- IVSd:
-
Interventricular septal thickness
- LAD:
-
Left atrial diameter
- LDL-C:
-
Low-density lipoprotein cholesterol
- LV:
-
Left ventricular
- LVEDV:
-
LV end-diastolic volume
- LVESV:
-
LV end-systolic volume
- LVH:
-
Left ventricular hypertrophy
- LVIDd:
-
LV end-diastolic internal dimension
- LVIDs:
-
LV end-systolic internal dimension
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- PC:
-
Plasma creatine
- PWTd:
-
Posterior wall thickness
- RWT:
-
Relative wall thickness
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- WC:
-
Waist circumference
References
Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. New insights into the relationship of left ventricular geometry and left ventricular mass with cardiac function: a population study of hypertensive subjects. Eur Heart J. 2010;31(5):588–94.
Conrady AO, Rudomanov OG, Zaharov DV, Krutikov AN, Vahrameeva NV, Yakovleva O, et al. Prevalence and determinants of left ventricular hypertrophy and remodelling patterns in hypertensive patients: the St. Petersburg study. Blood Press. 2004;13(2):101–9.
Li H, Pei F, Shao L, Chen J, Sun K, Zhang X, et al. Prevalence and risk factors of abnormal left ventricular geometrical patterns in untreated hypertensive patients. BMC Cardiovasc Disord. 2014;14:136.
Zabalgoitia M, Berning J, Koren MJ, Støylen A, Nieminen MS, Dahlöf B, et al. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol. 2001;88(6):646–50.
Richey PA, Disessa TG, Somes GW, Alpert BS, Jones DP. Left ventricular geometry in children and adolescents with primary hypertension. Am J Hypertens. 2010;23(1):24–9.
De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33(7):860–71.
Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: hyper-tension genetic epidemiology network (HyperGEN) study. Circulation. 2001;103(1):102–7.
Park HE, Youn TJ, Kim HK, Kim YJ, Sohn DW, Oh BH, et al. Different pattern of carotid and myocardial changes according to left ventricular geometry in hypertensive patients. J Hum Hypertens. 2013;27(1):7–12.
Silangei LK, Maro VP, Diefenthal H, Kapanda G, Dewhurst M, Mwandolela H, et al. Assessment of left ventricular geometrical patterns and function among hypertensive patients at a tertiary hospital, Northern Tanzania. BMC Cardiovasc Disord. 2012;12:109.
Li T, Yang J, Guo X, Chen S, Sun Y. Geometrical and functional changes of left heart in adults with prehypertension and hypertension: a cross-sectional study from China. BMC Cardiovasc Disord. 2016;16:114.
Guo X, Li Z, Sun G, Guo L, Zheng L, Yu S, et al. Comparison of four nontraditional lipid profiles in relation to ischemic stroke among hypertensive Chinese population. Int J Cardiol. 2015;201:123–5.
Chen S, Guo X, Yu S, Sun G, Li Z, Sun Y. Association between the Hypertriglyceridemic waist phenotype, prediabetes, and diabetes mellitus in rural Chinese population: a cross-sectional study. Int J Environ Res Public Health. 2016;13(4):368.
Yu S, Guo X, Yang H, Zheng L, Sun Y. Soybeans or soybean products consumption and depressive symptoms in older residents in rural Northeast China: a cross-sectional study. J Nutr Health Aging. 2015;19(9):884–93.
Li Z, Guo X, Sun G, Zheng L, Sun Y, Liu Y, et al. Plasma homocysteine levels associated with a corrected QT interval. BMC Cardiovasc Disord. 2017;17(1):182.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52.
World Health Organization and International Diabetes Fedaration. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva, Switzerland: WHO, 2006; p. 1–3.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
de Simone G, Devereux RB, Ganau A, Hahn RT, Saba PS, Mureddu GF, et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78(7):801–7.
de Ramirez SS, Enquobahrie DA, Nyadzi G, Mjungu D, Magombo F, Ramirez M, et al. Prevalence and correlates of hypertension: a cross-sectional study among rural populations in sub-Saharan Africa. J Hum Hypertens. 2010;24(12):786–95.
Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727–54.
Alpaydın MS, Aksakal E, Erol MK, Simşek Z, Açıkel M, Arslan S, et al. Assessment of regional left ventricular functions by strain and strain rate echocardiography in type II diabetes mellitus patients without microvascular complications. Turk Kardiyol Dern Ars. 2011;39(5):378–84.
Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67.
Kalaycıoğlu E, Gökdeniz T, Aykan AÇ, Gül I, Dursun I, Kiriş G, et al. The relationship between dipper/nondipper pattern and cardioankle vascular index in hypertensive diabetic patients. Blood Press Monit. 2013;18(4):188–94.
Daniels A, Linz D, van Bilsen M, Rütten H, Sadowski T, Ruf S, et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail. 2012;14(2):193–201.
Daniels A, van Bilsen M, Janssen BJ, Brouns AE, Cleutjens JP, Roemen TH, et al. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol (Oxf). 2010;200(1):11–22.
van Bilsen M, Daniels A, Brouwers O, Janssen BJ, Derks WJ, Brouns AE, et al. Hypertension is a conditional factor for the development of cardiac hypertrophy in type 2 diabetic mice. PLoS One. 2014;9(1):e85078.
Füth R, Dinh W, Bansemir L, Ziegler G, Bufe A, Wolfertz J, et al. Newly detected glucose disturbance is associated with a high prevalence of diastolic dysfunction: double risk for the development of heart failure? Acta Diabetol. 2009;46(4):335–8.
Mancia G, Carugo S, Grassi G, Lanzarotti A, Schiavina R, Cesana G, et al. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: data from the PAMELA population. Hypertension. 2002;39(3):744–9.
Litwin M, Sladowska J, Antoniewicz J, Niemirska A, Wierzbicka A, Daszkowska J, et al. Metabolic abnormalities, insulin resistance and metabolic syndrome in children with primary hypertension. Am J Hypertens. 2007;20(8):875–82.
Adamu UG, Kolo PM, Katibi IA, Opadijo GO, Omotosho AB, Araoye MA. Relationship between left ventricular diastolic function and geometric patterns in Nigerians with newly diagnosed systemic hypertension. Cardiovasc J Afr. 2009;20(3):173–7.
Wang SX, Xue H, Zou YB, Sun K, Fu CY, Wang H, et al. Prevalence and risk factors for left ventricular hypertrophy and left ventricular geometric abnormality in the patients with hypertension among Han Chinese. Chin Med J. 2012;125(1):21–6.
Akintunde AA, Akinwusi PO, Opadijo GO. Relationship between Tei index of myocardial performance and left ventricular geometry in Nigerians with systemic hypertension. Cardiovasc J Afr. 2011;22(3):124–7.
Oktay AA, Lavie CJ, Milani RV, Ventura HO, Gilliland YE, Shah S, et al. Current perspectives on left ventricular geometry in systemic hypertension. Prog Cardiovasc Dis. 2016;59(3):235–46.
Aje A, Adebiyi AA, Oladapo OO, Dada A, Ogah OS, Ojji DB, et al. Left ventricular geometric patterns in newly presenting Nigerian hypertensives: an echocardiographic study. BMC Cardiovasc Discords. 2006;6:4.
Eguchi K, Kario K, Hoshide S, Ishikawa J, Morinari M, Shimada K. Type 2 diabetes is associated with left ventricular concentric remodeling in hypertensive patients. Am J Hypertens. 2005;18(1):23–9.
Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, et al. Left ventricular geometric patterns in the Jackson cohort of the atherosclerotic risk in communities (ARIC) study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153(2):238–44.
Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, et al. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension. 2006;47(5):881–6.
Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham heart study. Circulation. 2003;107(3):448–54.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–9.
Acknowledgments
The authors would like to thank Professor Yingxian Sun responsible for enabling the project completion. The authors also thank Yonghong Zhang, Liying Xing and Guowei Pan for their assistance.
Funding
This study was supported by grants from the “Twelfth Five-Year” project funds (National Science and Technology Support Program of China, Grant #2012BAJ18B02) and Pro Yingxian Sun was responsible for enabling the project completion.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
TL participated in the echocardiographic measurements and drafted the manuscript. SC contributed to the statistical analysis. XG and JY took responsible for revising the manuscript critically for important intellectual content. YS was involved in supervisory role in study design. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of China Medical University. And informed consent was signed by all the participants.
Consent for publication
Consent for publication was obtained in all participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, T., Chen, S., Guo, X. et al. Impact of hypertension with or without diabetes on left ventricular remodeling in rural Chinese population: a cross-sectional study. BMC Cardiovasc Disord 17, 206 (2017). https://doi.org/10.1186/s12872-017-0642-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-017-0642-y