Abstract
Background
Guidelines from the American Heart Association/American College of Cardiology recommend a higher dosage of aspirin daily following Percutaneous Coronary Intervention (PCI), whereas guidelines from the European Society of Cardiology recommend a lower dosage. This study aimed to compare the adverse clinical outcomes associated with a low dose and a high dose of aspirin following PCI.
Methods
Electronic databases were searched for studies comparing a low dose with a high dose aspirin following PCI. Adverse clinical outcomes were considered as the endpoints in this study. We calculated Odds Ratios (OR) with 95 % Confidence Intervals (CIs) for categorical variables. The pooled analyses were performed with RevMan 5.3 software.
Results
A total number of 25,083 patients were included. Results from this analysis showed that the combination of Cardiovascular (CV) death/Myocardial Infarction (MI) or stroke was not significantly different between a low and high dose of aspirin with OR: 1.08, 95 % CI: 0.98–1.18; P = 0.11. Mortality and MI were also not significantly different between these two treatment regimens following PCI with OR: 0.95, 95 % CI: 0.74–1.23; P = 0.71 and OR: 1.17, 95 % CI: 0.97–1.41; P = 0.09 respectively. However, a high dose of aspirin was associated with a significantly higher rate of Major Adverse Cardiac Events (MACEs) with OR: 1.20, 95 % CI: 1.02–1.41; P = 0.03. Thrombolysis In Myocardial Infarction (TIMI) defined minor bleeding was also significantly higher with a high dose aspirin with OR: 1.22, 95 % CI: 1.02–1.47; P = 0.03. When Stent thrombosis (ST) was compared, no significant difference was found with OR: 1.28, 95 % CI: 0.59–2.58; P = 0.53. Even if TIMI defined major bleeding favored a low dose of aspirin, with OR: 1.42, 95 % CI: 0.95–2.13; P = 0.09, or even if major bleeding was insignificantly higher with a high dose aspirin, with OR: 1.78, 95 % CI: 1.01–3.13; P = 0.05; I2 = 94 %, higher levels of heterogeneity observed in these subgroups could not be considered significant to any extent.
Conclusion
According to the results of this analysis, a high dose of aspirin following PCI was not associated with any significantly higher rate of CV death/MI/stroke, mortality or MI. However, MACEs significantly favored a low dose of aspirin. In addition, TIMI defined minor bleeding was significantly higher with a high dose of aspirin whereas the results for the major bleeding outcomes were not statistically significant. However, due to limited data availability and since the subgroups analyzing major bleeding were highly heterogeneous, further studies are recommended to completely solve this issue.
Similar content being viewed by others
Background
Percutaneous Coronary Intervention (PCI) is considered to be among the most preferred invasive procedures carried out in patients with Acute Coronary Syndrome (ACS). Dual Anti-Platelet Therapy (DAPT) with aspirin and a P2Y12 inhibitor mainly clopidogrel, showed increased benefits in reducing adverse clinical outcomes following PCI with Drug Eluting Stents (DES) or Bare Metal Stents (BMS) [1]. Therefore, the American College of Cardiology/American Heart Association [2] recommends at least one-year treatment with DAPT after PCI with DES whereas the European Society of Cardiology [3] recommends 6 to 12 months DAPT use after intracoronary stenting by DES. For BMS, the duration period for DAPT is even shorter (1 month) compared to DES. However, uncertainty regarding the optimal dosage of aspirin is still a fact which remains to be solved [4]. Guidelines from the American Heart Association/American College of Cardiology recommend higher doses of aspirin (162 to 325 mg) daily following PCI [5], whereas guidelines from the European Society of Cardiology recommend lower doses (75 to 100 mg) [6]. This current analysis aimed to compare the adverse clinical outcomes associated with a low dose and a high dose of aspirin in patients with ACS following PCI with either DES or BMS.
Methods
Data sources and search strategy
The Cochrane Library, PubMed, Medline and EMBASE were searched for studies comparing a low dose with a high dose of aspirin following PCI by typing the words or phrases ‘low and high dose aspirin and percutaneous coronary intervention’. Another search was conducted using the words ‘aspirin and acute coronary syndrome or drug eluting stents/bare metal stents’ [aspirin + acute coronary syndrome/aspirin + percutaneous coronary intervention/aspirin + drug eluting stents/bare metal stents/low and/or high dose aspirin + percutaneous coronary intervention]. In order to enhance this search, abbreviations such as ASA, ACS, DES/BMS and PCI were also used as well as the terms ‘coronary angioplasty’, ‘coronary intervention’ and ‘single or double dose aspirin’ [ASA + PCI/ASA + percutaneous coronary intervention/ASA + acute coronary syndrome/ASA + ACS/ASA + DES/BMS/ASA + coronary angioplasty]. Medical journals which were expected to publish articles related to coronary interventions such as the Journal of Circulation, the Journal of the American College of Cardiology, Euro-intervention, the American Journal of Cardiology and BMC cardiovascular disorders were also searched using the above mentioned terms for relevant articles. Moreover, reference lists of suitable articles were also searched for relevant studies. This search was restricted to articles published in English.
Inclusion and exclusion criteria
Studies were included if:
-
(a)
They were Randomized Controlled Trials (RCTs) or observational studies comparing a low dose with a high dose of aspirin following PCI.
-
(b)
They reported adverse outcomes as their clinical endpoints.
-
(c)
They involved any dosage of aspirin, as far as a low dose was compared with a high dose.
Studies were excluded if:
-
(a)
They were meta-analyses, letter to editors and case studies.
-
(b)
They did not report adverse outcomes as their clinical endpoints.
-
(c)
They were duplicates.
Outcomes, definitions and follow ups
The clinical endpoints analyzed included:
-
(a)
Mortality
-
(b)
Myocardial Infarction (MI)
-
(c)
Cardiovascular (CV) death/MI/stroke
-
(d)
Major adverse cardiac events (MACEs) consisting of death, MI and revascularization
-
(e)
Stent Thrombosis (ST)
-
(f)
Major bleeding which was defined as bleeding that was significantly disabling for example intraocular bleeding that lead to significant vision loss, or bleeding requiring transfusion of 2 units of red blood cells or equivalent whole blood, a drop in hemoglobin concentration of 5 g/L, bleeding causing significant hypotension requiring intravenous inotropes or surgical intervention, symptomatic intracranial hemorrhage or bleeding that was fatal
-
(g)
TIMI defined major bleeding [7]
-
(h)
TIMI defined minor bleeding
The outcomes reported and the dosage of aspirin reported among the cohorts as well as their corresponding follow up periods have been summarized in Table 1.
A low dosage of aspirin was defined as any low dosage in accordance to the high dosage of aspirin reported in the same study. For example, if a dosage of aspirin greater than 200 mg was considered a high dosage, then any dosage below 200 mg in the same study should be considered as a low dosage.
Data extraction and quality assessment
Three authors (PKB, GJ and ART) independently assessed the articles selected for this analysis. Information concerning the type of study reported, the total number of patients treated with a low and high dose of aspirin respectively, data concerning the baseline characteristics of the patients, the reported outcomes and follow up periods were carefully extracted. If any disagreement about including certain data occurred, it was discussed among these three authors and if they could not reach a consensus, a final decision was made by the fourth author (WQH). Bias risk was assessed in accordance to the components recommended by the Cochrane Collaboration [8].
Methodological quality and statistical analysis
Recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement were followed since this is a meta-analysis involving mostly trials [9]. Heterogeneity across the subgroups was assessed using the Cochrane Q-statistic test (whereby a P value < 0 · 05 was considered statistically significant whereas a P value > 0.05 was considered statistically insignificant) and the I2-statistic test (whereby an I2 with low percentage represented a lower heterogeneity and an increasing percentage denoted an increasing heterogeneity). If I2 was less than 50 %, a fixed effect model was used. However, if I2 was more than 50 %, a random effect model was used. Publication bias was visually estimated by assessing funnel plots. We calculated Odds Ratios (OR) and 95 % Confidence Intervals (CIs) for categorical variables. The pooled analyses were performed with RevMan 5.3 software. Ethical approval was not required for systematic reviews and meta-analyses. All the authors had full access to the data and approved the manuscript as written.
Results
Search results
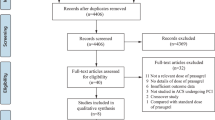
A total number of 622 articles were obtained from the Cochrane Library, PubMed, Medline and EMBASE and from reference lists of suitable articles. After a careful assessment of titles and abstracts, 584 articles were eliminated since they were not related to our topic. A further 26 articles were eliminated since they were duplicates. 12 full-text articles were assessed for eligibility. Eight more articles were eliminated: one article was a systematic review of the literature, two article did not report adverse clinical outcomes and two articles which could probably satisfy the inclusion and exclusion criteria of our study were not made available by the authors, one article did not include data which could be used in this analysis, and another two trials were the subset of other trials included in this analysis. Finally, four articles (3 trials and 1 observational study) [4, 10–13] were included in this systematic review and meta-analysis. The flow diagram for the study selection has been represented in Fig. 1.
General features of the studies included
The general features of the studies included in this meta-analysis have been listed in Table 2.
A total number of 25,083 patients (14,402 patients were assigned to a low dose of aspirin and 10,681 patients were assigned to a high dose of aspirin) were included in this analysis.
Baseline characteristics of the studies included
The baseline features of the patients have been listed in Table 3 whereas Table 4 shows the other antiplatelet/anticoagulants used by the patients during the procedure or following PCI. According to these baseline features, no significant differences were observed between patients assigned to a low dose and a high dose of aspirin respectively.
Clinical outcomes reported
Results from this analysis (Table 5) showed that the combination of CV death/MI or stroke was not significantly different between a low and a high dose of aspirin following PCI with OR: 1.08, 95 % CI: 0.98–1.18; P = 0.11, I2 = 0 %. Mortality and MI were also not significantly different between these two treatment regimens after PCI with OR: 0.95, 95 % CI: 0.74–1.23; P = 0.71, I2 = 7 % and OR: 1.17, 95 % CI: 0.97–1.41; P = 0.09, I2 = 33 % respectively. However, a high dose of aspirin was associated with a significantly higher rate of MACEs with OR: 1.20, 95 % CI: 1.02–1.41; P = 0.03, I2 = 35 %. TIMI defined minor bleeding was also significantly higher with a high dose aspirin with OR: 1.22, 95 % CI: 1.02–1.47; P = 0.03; I2 = 44 %. These results have been represented in Fig. 2.
When ST was compared between these two groups, no significant difference was found with OR: 1.28, 95 % CI: 0.59–2.58; P = 0.53, I2 = 65 %. Even if TIMI defined major bleeding favored a low dose aspirin, with OR: 1.42, 95 % CI: 0.95–2.13; P = 0.09; I2 = 59 %, the result was not statistically significant. Moreover, even if major bleeding was higher with a high dose aspirin, with OR: 1.78, 95 % CI: 1.01–3.13; P = 0.05; I2 = 94 %, the level of heterogeneity was much higher that it could not be considered significant to any extent. These results have been represented in Fig. 3.
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plot obtained, there has been little evidence of publication bias for the included studies that assessed several clinical endpoints. However, a high level of heterogeneity was observed among the subgroups analyzing stent thrombosis and the major bleeding outcomes. The funnel plot showing the sensitivity analysis has been represented in Fig. 4.
Discussion
This study aimed to compare the adverse clinical outcomes associated with a low dose and a high dose of aspirin following PCI. Results of this study showed that a high dose of aspirin was not associated with a significantly higher rate of mortality, CV death/MI/stroke and MI. ST was also not significantly different between these two dosages of aspirin. However, MACEs significantly favored a low dose aspirin. In addition, a high dose of aspirin was associated with a significantly higher rate of TIMI defined minor bleeding, without any significant increase in TIMI defined major bleeding major bleeding after PCI.
The systematic review of literature [14] which was meant to show any association between aspirin dosing and cardiac and bleeding events after treatment of ACS showed no improved clinical outcomes associated with a high dose of aspirin following PCI among the 289,330 patients analyzed. 2.1 % of patients experienced major bleeding when treated with a high dose of aspirin whereas only 1.9 % of patients treated with a low dose of aspirin following stent implantation experienced major bleeding.
Moreover, the investigators of the CURRENT OASIS 7 [11] concluded that in patients with ACS who were referred for an invasive strategy, no significant difference in primary outcome of cardiovascular death, MI or stroke was observed between a low and a high dose of aspirin. However, only a follow up period of 7 days was considered.
Also, the study published by Joyal et al. [15] demonstrating the influence of a low dose (81 mg) versus a high dose (325 mg) of aspirin on the incidence of sirolimus eluting stents showed a similar rate of ST to be associated with either a low or a high dose of aspirin. The Ottawa Heart Institute PCI Registry [16] which involved 930 patients discharged on 325 mg aspirin and 910 patients discharged on 81 mg aspirin showed no difference in death or MI at 1 year between these two different dosages of aspirin. In addition, another study investigating the influence of low dose aspirin (81 mg) on the incidence of definite stent thrombosis in patients receiving BMS and DES concluded that a low dose of aspirin following PCI was not associated with any increase in definite stent thrombosis compared to a high dose [17].
However, results from the Dual Antiplatelet Therapy Study [18] showed that a high dose of aspirin might be associated with adverse events and the authors suggested that a low dose of aspirin might be the target to improve clinical outcomes after PCI reflecting the results of this current analysis.
Nevertheless, when prasugrel was compared with clopidogrel, with a high and low dose aspirin respectively, prasugrel was associated with better clinical outcomes irrespective of the dosage of aspirin as demonstrated in the TRITON TIMI 38 trial whereby 12,674 patients were classified into a low and high dose aspirin groups [19]. No meaningful interaction of aspirin with clopidogrel was observed. However, this current analysis was different and was focused mainly on comparing a low with a high dose of aspirin following PCI.
Novelty
This study is new in the way that it is among the first systematic review and meta-analyses comparing a low dose with a high dose of aspirin following PCI. Moreover, several adverse outcomes have been analyzed. This study also included a large number of patients from randomized trials compared to patients from observational studies and reported a low or moderate level of heterogeneity among several subgroups assessing these clinical endpoints. Since dosage of aspirin following PCI could be an important issue in several types of patients, this analysis could inspire other scientists to conduct further research in this particular field.
Limitations
This study also has limitations. First of all, due to the limited number of patients and studies, this analysis might not provide robust results. Secondly, because this analysis also included data obtained from observational studies along with data obtained from randomized trials, selection bias could possibly have been introduced. Moreover, one study had a follow-up period of 3 years and another one had a follow up period of 1 month. They have been included among other studies with a follow-up period of 1 year and analyzed altogether. This could be another limitation. In addition, several subgroups analyzed only compared data from two or three studies which could strictly affect the results and should be considered another limiting factor in this meta-analysis. A high level of heterogeneity observed among the several subgroups analyzing stent thrombosis, and major bleedings could be another limitation in this study. In addition, different studies reported different dosage of aspirin. This could also be a limiting factor in this study. Also, only the percentage of patients representing the female population were included from the trial CURRENT OASIS 7, because its large number of patients could influence the results of this analysis. Therefore, this could also be a limitation in this study. It should also be noted that patients involved in this analysis varied from stable coronary diseases, ST segment elevated MI or non-ST segment elevated MI representing another probable limitation to this study. However, the main focus was on patients who underwent PCI. Also, almost all of the studies were sub-group analysis of trials whereby patients were not randomized specifically to a low and high dosage of aspirin respectively, therefore, the groups might not necessarily be comparable thus rendering the introduction of potentially confounding variables possible, which could have a great impact on the results of this current analysis. Another limitation could be the influence of glycoprotein IIb/IIIa inhibitors used peri-operatively, and other anticoagulants which were used differently following PCI in different studies, possibly affecting the bleeding outcomes.
Conclusion
According to the results of this analysis, a high dose of aspirin following PCI was not associated with any significantly higher rate of CV death/MI/stroke, mortality or MI. However, MACEs significantly favored a low dose of aspirin. In addition, TIMI defined minor bleeding was significantly higher with a high dose of aspirin whereas the results for the major bleeding outcomes were not statistically significant. However, due to limited data availability and since the subgroups analyzing major bleeding were highly heterogeneous, further studies are recommended to completely solve this issue.
Abbreviations
- ACS:
-
Acute coronary syndrome
- MACEs:
-
Major adverse cardiac events
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- TIMI defined bleeding:
-
Thrombolysis in myocardial infarction defined bleeding
References
Bhatt DL. Intensifying platelet inhibition--navigating between Scylla and charybdis. N Engl J Med. 2007;357(20):2078–81.
Levine GN, Bates ER, Blankenship JC, et al. American college of cardiology foundation; American heart association task force on PracticeGuidelines; society for cardiovascular angiography and interventions. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American college ofCardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011;58(24):e44–122. doi:10.1016/j.jacc.2011.08.007.
Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularizationof the European society of cardiology (ESC) and the European association for cardio-thoracic surgery(EACTS)developed with the special contribution of the European association of percutaneous CardiovascularInterventions (EAPCI). Eur Heart J. 2014;35(37):2541–619. doi:10.1093/eurheartj/ehu278.
Peters RJ, Mehta SR, Fox KA, et al. Clopidogrel in unstable angina to preventRecurrent events (CURE) trial investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the clopidogrel in unstable angina to prevent recurrent events (CURE) study. Circulation. 2003;108:1682–7.
King 3rd SB, Smith Jr SC, Hirshfeld Jr JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: are port of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2008;51(2):172–209.
Silber S, Albertsson P, Avilés FF, et al. Task force for percutaneous coronary interventions of the European society of cardiology. Guidelines for percutaneous coronary interventions. The task force for percutaneous coronary interventions of the European society of cardiology. Eur Heart J. 2005;26(8):804–47.
The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312(14):932–6.
Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Wiley;2008. p. 187–241.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ. 2009;339:b2700.
Harjai KJ, Shenoy C, Orshaw P, et al. Low-dose versus high-dose aspirin after percutaneous coronary intervention: analysis from the guthrie health off-label StenT (GHOST) registry. J Interv Cardiol. 2011;24(4):307–14.
CURRENT-OASIS 7 Investigators, Mehta SR, Bassand JP, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363(10):930–42.
Jolly SS, Pogue J, Haladyn K, et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: insightsfrom the PCI-CURE study. Eur Heart J. 2009;30(8):900–7. doi:10.1093/eurheartj/ehn417.
Yu J, Mehran R, Dangas GD, et al. Safety and efficacy of high- versus low-dose aspirin after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2012;5(12):1231–8.
Berger JS, Sallum RH, Katona B, et al. Is there an association between aspirin dosing and cardiac and bleeding events after treatment of acute coronarysyndrome? A systematic review of the literature. Am Heart J. 2012;164(2):153-162.e5.
Joyal D, Freihage JH, Cohoon K, et al. The influence of low (81 mg) versus high (325 mg) doses of ASA on the incidence of sirolimus-eluting stent thrombosis. J Invasive Cardiol. 2007;19(7):291–4.
So D, Cook EF, Le May M, et al. Association of aspirin dosage to clinical outcomes after percutaneous coronary intervention: observations from the Ottawa Heart Institute PCI Registry. J Invasive Cardiol. 2009;21(3):121–7.
Lotfi A, Cui J, Wartak S, Columbo J, Mulvey S, Davis M, Schweiger M, Giugliano GR. Influence of low-dose aspirin (81 mg) on the incidence of definite stent thrombosis in patients receiving bare-metal and drug-eluting stents. Clin Cardiol. 2011;34(9):567–71.
Matteau A, Yeh RW, Kereiakes D, et al. Frequency of the use of low- versus high-dose aspirin in dual antiplatelet therapy after percutaneous coronaryintervention (from the Dual Antiplatelet Therapy study). Am J Cardiol. 2014;113(7):1146–52.
Kohli P, Udell JA, Murphy SA, Cannon CP, Antman EM, Braunwald E, Wiviott SD. Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated withprasugrel versus clopidogrel: an analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). J Am Coll Cardiol. 2014;63(3):225–32.
Acknowledgements
There was no external source of funding for this research and no writing assistance was required.
Funding
There was no external source of funding for this research.
Availability of data and materials
All data and materials used in this research are freely available. References have been provided.
Authors’ contributions
PKB, GJ, ART and WQH were responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. PKB wrote this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was not applicable for this systematic review and meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bundhun, P.K., Janoo, G., Teeluck, A.R. et al. Adverse clinical outcomes associated with a low dose and a high dose of aspirin following percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord 16, 169 (2016). https://doi.org/10.1186/s12872-016-0347-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-016-0347-7