Abstract
Background
We aimed to evaluate serum levels of S-100 beta (S-100β) and neuron specific enolase (NSE) in patients with coronary heart disease (CHD) after off-pump versus on-pump coronary artery bypass graft (CABG) surgery.
Methods
The PubMed (~2013) and the Chinese Biomedical Database (CBM) (1982 ~ 2013) were searched without language restrictions. After extraction of relevant data from selected studies, meta-analyses were conducted using STATA software (Version 12.0, Stata Corporation, College Station, Texas USA). Possible sources of heterogeneity were examined through univariate and multivariate meta-regression analyses and verified by Monte Carlo Simulation.
Results
Eleven studies with a total of 411 CHD patients met the inclusion criteria. Our meta-analysis showed no significant difference in serum S-100β and NSE levels between the on-pump group and the off-pump group before surgery. In the on-pump group, there was a significant difference in serum S-100β levels of CHD patients between before and after surgery, especially within the first 24 h after surgery. Furthermore, in the on-pump group, there was a significant difference in serum NSE levels of CHD patients between before and after surgery, particularly at 0 h after surgery. In the off-pump group, there was an obvious difference in serum S-100β levels between before and after surgery, especially within 24 h after surgery. Our results also demonstrated that serum S-100β and NSE levels of CHD patients in the on-pump group were significantly higher than those of patients in the off-pump group, especially within 24 h after surgery.
Conclusions
Our findings provide empirical evidence that off-pump and on-pump CABG surgeries may increase serum S-100β and NSE levels in CHD patients, which was most prominent within 24 h after on-pump CABG surgery.
Similar content being viewed by others
Background
As a major public health issue worldwide, coronary heart disease (CHD) is the primary cause of disability and death in the developed countries and is among the leading causes of disease burden in low-and middle-income countries [1, 2]. Evidence has revealed that the prevalence of CHD in persons aged 20 years or older was estimated to be 6.4 % (15.4 million) in the US in 2010, and 386,324 cases of CHD-related deaths were reported in 2009 [3]. Nowadays, three therapeutic options are generally used for patients with CHD, including medical treatment with drugs, coronary interventions such as angioplasty and coronary stent implantation, and coronary artery bypass grafting (CABG) surgery [4, 5].
CABG surgery is a surgical procedure most commonly performed to relieve angina and reduce the risk of death from CHD [6]. The CABG surgery has significantly changed over the years, from traditional surgical operations using cardiopulmonary bypass (on-pump CABG) to a newer approach in cardiovascular surgery (off-pump CABG), both of which are primarily designed to improve the outcomes in CHD patients [7, 8]. Although the operative mortality in CABG surgeries has decreased dramatically, the rate of neurologic complications remains unacceptably high; for example, neurological injury is a major perioperative risk in these patients [9, 10]. Unfortunately, the postoperative brain damage is difficult to diagnose early and mainly based on the observation of specific brain injury markers [11]. Recently, it has been reported that the cerebral biomarkers such as S-100 beta (S-100β) and neuron specific enolase (NSE) may serve as biomarkers to reflect brain damages in cardiac surgery [12]. S-100β protein, a specific protein originating from the brain, has been found in the cytosol of both glial and Schwann cells, chondrocytes and adipocytes, having both intracellular and extracellular neurotropic and also neurotoxic functions [13, 14]. Low physiological concentrations of S-100β could protect neurons against apoptosis, stimulate neurite outgrowth and astrocyte proliferation, whereas S-100β at high concentrations may result in neuronal death and exhibit properties of a damage-associated molecular pattern protein [15, 16]. In addition, elevated levels of S-100β might accurately reflect the existence of neuropathological conditions, including neurodegenerative diseases and neuronal injury [17, 18]. NSE has also been suggested to act as a specific serum marker for neuronal damage, which is mainly found in neuronal cells, especially in mature neurons of the central nervous system, and is not secreted; and thus, increased NSE in cerebrospinal fluid or blood may reflect postoperative cognitive dysfunction or structural damage to neuronal cells [19, 20]. Therefore, serum S-100β and NSE levels measured before and after on-pump and off-pump CABG could potentially be diagnostic of ongoing cerebral damage associated with these surgical procedures [21]. To date, evidence supports that both on-pump and off-pump CABG are associated with increased serum levels of NSE and S-100β, but the off-pump CABG exhibits relatively lower serum S-100β protein and NSE levels, suggesting that the off-pump CABG has less influence or impairment on neurocognitive functions in comparison to the on-pump CABG [22, 23]. However, contradictory results have also been reported in the literature. Therefore, we performed this meta-analysis aiming to evaluate serum S-100β and NSE levels in CHD patients after off-pump versus on-pump CABG surgery.
Methods
Literature search and selection criteria
The PubMed (~2013) and the Chinese Biomedical Database (CBM) (from 1982 to 2013) were searched without language restrictions. The keywords and MeSH terms applied in combination with a highly sensitive search strategy were: (“S100 calcium binding protein beta subunit” or “nerve tissue protein S100b” or “neurotrophic protein S100beta” or “S-100β” or “S100beta protein” or “S100beta”) and (“phosphopyruvate hydratase” or “2-phospho-D-glycerate hydrolase” or “NSE” or “neuron-specific enolase” or “nervous system specific enolase” or “muscle specific enolase”) and (“coronary artery bypass” or “coronary artery bypass grafting” or “CABG” or “on-pump coronary artery bypass” or “off-pump coronary artery bypass” or “on- and off- coronary artery bypass”). Moreover, a manual search based on the references lists of the searched articles was also carried out to identify other potential articles.
The eligibility criteria for the inclusion of studies in this meta-analysis were as follows: (1) the study must report serum S-100β and NSE levels in CHD patients after off-pump versus on-pump CABG surgery; (2) all patients must have confirmed the diagnostic criteria for CHD; (3) the study must supply sufficient information on serum levels of S-100β and NSE. Studies that did not meet the inclusion criteria were excluded. In case those authors published the same subjects in several studies, the most recent study or the study with largest sample size was selected.
Data extraction and methodological assessment
Using a standardized data extraction form, two authors independently extracted the following information from the studies included: publication year of article, geographical location, language of publication, surname of the first author, sample size, the source of the subjects, design of study, follow-up time, detection method, serum levels of S-100β and NSE, etc. Methodological quality assessment was carried out respectively by two authors through the Newcastle-Ottawa Scale (NOS) criteria [24]. Three aspects were included in the NOS criteria: (1) subject selection: 0 ~ 4; (2) comparability of subject: 0 ~ 2; (3) clinical outcome: 0 ~ 3. The range of NOS scores is from 0 to 9; and a score of ≥ 7 represents a high quality.
Statistical analysis
The STATA statistical software (Version 12.0, Stata Corporation, College Station, TX, USA) was applied for our meta-analysis. Standardized mean difference (SMD) with the corresponding 95 % confidence intervals (95 % CI) was calculated. In addition, the Z test was conducted for estimation of the statistical significance of pooled SMDs. Heterogeneity among studies was estimated by the Cochran’s Q-statistic and I2 tests [25]. If the Q-test showed a P < 0.05 or the I2 test showed > 50 %, which indicate significant heterogeneity and the random-effect model was implemented, otherwise the fixed-effects model was performed [26]. Using sensitivity analysis of variables, the impact on the overall results by removing one single study was evaluated. Moreover, funnel plots and Egger’s linear regression test were applied for the investigation of publication bias [27]. Possible sources of heterogeneity were examined through univariate and multivariate meta-regression analyses and verified by Monte Carlo Simulation [28, 29].
Results
Characteristics of included studies
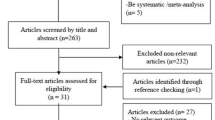
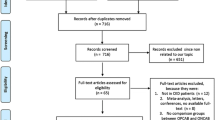
Our search strategy initially identified 138 articles. By reviewing the titles and abstracts, 67 articles were excluded. After systematically reviewing the remaining full texts, we excluded another 55 articles. In addition, 5 studies were excluded for lack of data integrity. Finally, 11 clinical cohort studies containing a total of 411 patients with CHD met the inclusion criteria used for qualitative data analysis [30, 22, 23, 31–38]. The publication years of eligible studies were between 2002 and 2013. Overall, 9 studies were based on Asians, and the other 2 studies on Caucasians. The NOS score of each included studies was ≥ 5 (moderate-high quality). The characteristics of eligible studies are summarized in Table 1.
Quantitative data synthesis
Our meta-analysis showed no significant difference in serum S-100β and NSE levels between the on-pump group and the off-pump group before surgery (S-100β: SMD = 0.14, 95 % CI = −0.07 ~ 0.35, P = 0.191; NSE: SMD = −0.12, 95 % CI = −0.42 ~ 0.17, P = 0.408; respectively) (Fig. 1).
In the on-pump group, there was a significant difference in serum S-100β levels of CHD patients between before and after surgery (SMD = 2.05, 95 % CI = 1.55 ~ 2.55, P < 0.001), especially within 24 h after surgery (0 h: SMD = 4.81, 95 % CI = 3.20 ~ 6.41, P < 0.001; 6 h: SMD = 2.41, 95 % CI = 1.26 ~ 3.55, P < 0.001; 24 h: SMD = 1.14, 95 % CI = 0.66 ~ 1.62, P < 0.001), while no such difference was found after 24 h post-surgery (48 h: SMD = 0.79, 95 % CI = −0.18 ~ 1.75, P = 0.109; 72 h: SMD = 0.25, 95 % CI = −0.31 ~ 0.82, P = 0.380) (Fig. 2a). In the off-pump group, there was a significant difference in serum S-100β levels between before and after surgery (SMD = 1.29, 95 % CI = 0.86 ~ 1.72, P < 0.001), especially within 24 h after surgery (0 h: SMD = 3.15, 95 % CI = 1.74 ~ 4.56, P < 0.001; 6 h: SMD = 1.48, 95 % CI = 0.53 ~ 2.44, P = 0.002; 24 h: SMD = 0.82, 95 % CI = 0.32 ~ 1.33, P = 0.001); however, there was no significant difference observed after 24 h (48 h: SMD = 0.06, 95 % CI = −0.37 ~ 0.49, P = 0.780; 72 h: SMD = 0.13, 95 % CI = −0.45 ~ 0.71, P = 0.669) (Fig. 3a). Also, our results demonstrated that the serum S-100β of CHD patients in the on-pump group were significantly higher than those of patients in the off-pump group (SMD = 1.08, 95 % CI = 0.67 ~ 1.48, P < 0.001), especially within 24 h after surgery (0 h: SMD = 2.91, 95 % CI = 1.64 ~ 4.19, P < 0.001; 6 h: SMD = 1.19, 95 % CI = 0.56 ~ 1.83, P = 0.017; 24 h: SMD = 0.51, 95 % CI = 0.09 ~ 0.92, P = 0.001); after 24 h, the results revealed no such statistical significance (48 h: SMD = 0.29, 95 % CI = −1.03 ~ 1.61, P = 0.670; 72 h: SMD = 0.02, 95 % CI = −0.55 ~ 0.59, P = 0.952) (Fig. 4a). The difference of the serum S-100β levels between on-pump and off-pump groups was the most significant 0 h after surgery, after which the difference was decreased with time (Fig. 5). According to univariate meta-regression analyses, time may be a source of heterogeneity (P = 0.016), while publication year, ethnicity and sample size did not cause heterogeneity (all P > 0.05), which was also verified by the multivariate analyses (Table 2).
Furthermore, in the on-pump group, there was a significant difference in serum NSE levels of CHD patients between before and after surgery (SMD = 1.28, 95 % CI = 0.31 ~ 2.25, P = 0.010), particularly at 0 h after surgery (SMD = 2.90, 95 % CI = 0.39 ~ 5.42, P = 0.024), while it was not significant at other time points (6 h: SMD = 0.17, 95 % CI = −2.69 ~ 3.04, P = 0.906; 24 h: SMD = 1.34, 95 % CI = −0.28 ~ 2.95, P = 0.105; 48 h: SMD = 1.75, 95 % CI = −1.10 ~ 4.59, P = 0.229; 72 h: SMD = 0.52, 95 % CI = −0.05 ~ 1.10, P = 0.076) (Fig. 2b). Nevertheless, we found no difference in serum NSE levels before and after off-pump CABG surgery (SMD = 0.42, 95 % CI = −0.24 ~ 1.08, P = 0.209) (Fig. 3b). Also, our results demonstrated that the NSE levels of CHD patients in the on-pump group were significantly higher than those of patients in the off-pump group (SMD = 1.19, 95 % CI = 0.66 ~ 1.73, P < 0.001), especially within 24 h after surgery (0 h: SMD = 1.56, 95 % CI = 0.72 ~ 2.40, P < 0.001; 6 h: SMD = 1.20, 95 % CI = 0.31 ~ 2.09, P = 0.008; 24 h: SMD = 1.24, 95 % CI = 0.01 ~ 2.48, P = 0.048), but no such difference was found after 24 h (48 h: SMD = 1.48, 95 % CI = −1.61 ~ 4.58, P = 0.348; 72 h: SMD = 0.24, 95 % CI = −1.41 ~ 1.89, P = 0.777) (Fig. 4b). Based on univariate meta-regression analyses, time, publication year, ethnicity and sample size were all not sources of heterogeneity (all P > 0.05), which was further verified by the multivariate analyses (Table 2).
Sensitivity analysis and publication bias
Results of sensitivity analyses indicated that all the included publications had no significant influence on SMD (Fig. 6). Funnel plots revealed no obvious asymmetry (Fig. 7). Also, Egger’s test didn’t illustrate strong statistical evidence of publication bias (all P > 0.05).
Sensitivity analyses to evaluate the impact of removing one single study on the overall results. a: Serum S100ß level before surgery (On-pump versus Off-pump); b: Serum NSE level before surgery (On-pump versus Off-pump); c: Serum S100ß level in the on-pump group (Post-surgery versus Pre-surgery); d: Serum NSE level in the on-pump group (Post-surgery versus Pre-surgery); e: Serum S100ß level in the off-pump group (Post-surgery versus Pre-surgery); f: Serum NSE level in the off-pump group (Post-surgery versus Pre-surgery); g: Serum S100ß level after surgery (On-pump versus Off-pump); h: Serum NSE level after surgery (On-pump versus Off-pump)
Funnel plots for the differences in serum S-100 beta (S-100ß) and neuron specific enolase (NSE) levels before and after on-pump versus off-pump coronary artery bypass graft surgeries. a: Serum S100ß level before surgery (On-pump versus Off-pump); b: Serum NSE level before surgery (On-pump versus Off-pump); c: Serum S100ß level in the on-pump group (Post-surgery versus Pre-surgery); d: Serum NSE level in the on-pump group (Post-surgery versus Pre-surgery); e: Serum S100ß level in the off-pump group (Post-surgery versus Pre-surgery); f: Serum NSE level in the off-pump group (Post-surgery versus Pre-surgery); g: Serum S100ß level after surgery (On-pump versus Off-pump); h: Serum NSE level after surgery (On-pump versus Off-pump)
Discussion
The present meta-analysis was identified the influence of off-pump and on-pump CABG surgeries on serum levels of S-100β and NSE in patients with CHD. The findings revealed no significant difference in preoperative serum S-100β and NSE levels between off-pump and on-pump CABG groups. The S-100β and NSE proteins cannot be detected in the serum under normal circumstances; however, they can be detected in serum following traumatic cerebral injury, stroke and cardiopulmonary bypass surgery due to impairment of blood–brain barrier (BBB) [39]. We presume that the lack of significant difference in serum S-100β and NSE protein levels before surgery indicates an intact BBB in the patients. Previous evidence showed a positive correlation between serum levels of S-100β and NSE and the neurocognitive dysfunction, because increased S-100β and NSE proteins could leak out from structurally damaged nerve cells into cerebrospinal fluid and secondarily across the BBB [40]. Interestingly, in previous studies the time of cardiopulmonary bypass has been proved strongly correlated with the peak release of S-100β and NSE, and the restrictive fluid management may reduce perioperative cerebral injury [23, 30].
The results also showed that the postoperative serum S-100β and NSE levels were markedly elevated in the on-pump group, especially within 24 h after surgery. Cerebral damage remains one of the major problems associated with open-heart surgery and the contribution of on-pump CABG to cerebral damage is still only partially understood. We hypothesized that during extracorporeal circulation in on-pump CABG, blood and its constituents are likely in contact with foreign surfaces, which may activate inflammation, potentially leading to respiratory insufficiency and damage to lung and brain [22]. Furthermore, brain damage may cause disruption of BBB, which may induce dilatation of small capillaries and arterioles in the brain and then both S-100β and NSE protein may be allowed to release from cerebrospinal fluid to blood fluid in the patients [41]. Thereby, serum levels of S-100β and NSE may increase markedly after on-pump CABG surgery, confirmed by our results, further suggesting that perioperative care should be modified accordingly to control such adverse effects. Additionally, another mechanism of neurocognitive dysfunction in patients undergoing on-pump CABG is cerebral microembolization, which mostly generates from pump circuits and is partially related to the manipulation and instrumentation of the heart using surgical instrumentation, especially the aorta [42]. These embolic events can also result in increased serum S-100β and NSE levels postoperatively in patients [43].
We found a significant difference in the serum S-100β levels before and after off-pump CABG surgery, while no significant difference in serum NSE levels were observed in the off-pump group before and after surgery. In a previous study, the peak release of S-100β occurs at 6 h postoperatively and signifies perioperative brain damage, while the NSE peak serum levels occurred beyond 24 h after the surgery in patients undergoing off-pump CABG [44, 45]. Therefore, the different peak times of the peak serum S-100β and NSE levels may be a the reason that significant changes are observed in S-100β levels and not in NSE levels before and after off-pump CABG. Bonacchi et al. also found that in the off-pump group, serum levels of S-100β and NSE were almost within the normal range preoperatively; but only the S-100β serum levels increased significantly postoperatively [23].
Another principal finding in our meta-analysis is that postoperative serum S-100β and NSE protein levels were significantly higher in the on-pump group than those in the off-pump group, especially within 24 h after surgery, implying that off-pump CABG may be associated with lower risk of neurocognitive dysfunction than on-pump CABG. Although the precise mechanism through which off-pump CABG reduces systemic inflammation in brain damage and postoperative mortality is still not fully understood, it may be reasonable to postulate that off-pump CABG and decrease the frequency of cerebral embolism [46]. Huseyin Bayram et al. has showed that the postoperative serum S-100β levels in the off-pump group were significantly lower than that in the on-pump CABG group [22]. Similarly, Lee et al. have observed that off-pump CABG surgery may decrease neurological and clinical morbidity in comparison to on-pump CABG in a randomized group of 60 patients undergoing on-pump and off-pump procedures and complemented by neurocognitive testing before surgery and 2 week/1 year after surgery [47]. By contrast, Edwards compared on-pump and off-pump CABG with a year of follow-up study, reporting that on-pump CABG is superior to off-pump CABG, although off-pump CABG had advantages of time on mechanical ventilation, bleeding and need for reoperation etc. [48]. Despite these contradictory results on whether off-pump CABG is superior to the on-pump CABG [49], our results are in accordance with several studies that demonstrated that the preoperative brain injury evaluated by the release of NSE and S-100β protein is significantly higher in patients undergoing off-pump CABG than patients receiving on-pump CABG. Our study has limitations which should be interpreted. First, through searching the databases, only 5 randomized controlled trials relevant to the topic were identified (the other 6 studies were non-randomized controlled trials), which may cause bias due to the small sample size. Second, because all the included randomized controlled trials could not demonstrated any significant impairment of cognitive function after both on-pump and off-pump surgeries, these studies are more likely to provide “academic” rather than clinical evidence, therefore future clinical evidence are needed. Third, the meta-analysis could not acquire the original data and information on the techniques used in the surgeries was limitedly provided in the studies included, which may restrict further evaluation of the plausible effect of off-pump and on-pump CABG on serum S-100β and NSE levels. Moreover, Due to the lack of data on neurological complications in the enrolled studies, we failed to identify a relationship between higher marker levels and neurological events. Even though there are several limitations, our study is the first meta-analysis on the comparison of serum levels of S-100β and NSE between patients treated with on-pump and off-pump CABG. More importantly, a literature search strategy with high sensitivity was implemented for electronic databases. In order to identify other potential articles, we also manually searched the reference lists of relevant articles, and the eligible articles were selected on the basis of strict inclusion and exclusion criteria. Besides, pooling of information from each study is founded on rigorous statistical analysis.
Conclusions
Our findings revealed that off-pump and on-pump CABG surgeries may increase serum S-100β and NSE levels in CHD patients, especially within 24 h of on-pump CABG surgery. However, more researches with more detailed data and large sample size are necessary to confirm our findings and validate the clinical use of S-100β and NSE as reliable biomarkers to predict outcomes.
Abbreviations
- CABG:
-
Coronary artery bypass graft
- CHD:
-
Coronary heart disease
- NSE:
-
Neuron specific enolase
- CBM:
-
Chinese Biomedical Database
- NOS:
-
The Newcastle-Ottawa Scale
- SMD:
-
Standardized mean difference
- CI:
-
Confidence intervals
- BBB:
-
Blood–brain barrier
References
Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115.
Odegaard AO. Coronary heart disease: what hope for the developing world? Heart. 2013;99(17):1227–9.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–72.
Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–35.
ElBardissi AW, Aranki SF, Sheng S, O'Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143(2):273–81.
Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366(16):1489–97.
Palmerini T, Biondi-Zoccai G, Riva DD, Mariani A, Savini C, Di Eusanio M, et al. Risk of stroke with percutaneous coronary intervention compared with on-pump and off-pump coronary artery bypass graft surgery: Evidence from a comprehensive network meta-analysis. Am Heart J. 2013;165(6):910–7. e914.
Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366(3):250–7.
Kennedy ED, Choy KC, Alston RP, Chen S, Farhan-Alanie MM, Anderson J, et al. Cognitive outcome after on- and off-pump coronary artery bypass grafting surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2013;27(2):253–65.
Dabrowski W, Rzecki Z, Czajkowski M, Pilat J, Wacinski P, Kotlinska E, et al. Volatile anesthetics reduce biochemical markers of brain injury and brain magnesium disorders in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26(3):395–402.
Yuan SM. Biomarkers of cerebral injury in cardiac surgery. Anadolu Kardiyol Derg. 2014;14(7):638–45.
Astrand R, Unden J, Romner B. Clinical use of the calcium-binding S100B protein. Methods Mol Biol. 2013;963:373–84.
Seco M, Edelman JJ, Wilson MK, Bannon PG, Vallely MP. Serum biomarkers of neurologic injury in cardiac operations. Ann Thorac Surg. 2012;94(3):1026–33.
Sorci G, Bianchi R, Riuzzi F, Tubaro C, Arcuri C, Giambanco I, Donato R: S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc Psychiatry Neurol 2010, 2010; PMID: 20827421.
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57.
Beharier O, Kahn J, Shusterman E, Sheiner E. S100B - a potential biomarker for early detection of neonatal brain damage following asphyxia. J Matern Fetal Neonatal Med. 2012;25(9):1523–8.
Michetti F, Corvino V, Geloso MC, Lattanzi W, Bernardini C, Serpero L, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120(5):644–59.
Yee KM, Ross-Cisneros FN, Lee JG, Da Rosa AB, Salomao SR, Berezovsky A, et al. Neuron-specific enolase is elevated in asymptomatic carriers of Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012;53(10):6389–92.
Streitburger DP, Arelin K, Kratzsch J, Thiery J, Steiner J, Villringer A, et al. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain - a combined serum, gene expression and MRI study. PLoS One. 2012;7(8), e43284.
Kobayashi J, Tashiro T, Ochi M, Yaku H, Watanabe G, Satoh T, et al. Early outcome of a randomized comparison of off-pump and on-pump multiple arterial coronary revascularization. Circulation. 2005;112(9 Suppl):I338–43.
Bayram H, Hidiroglu M, Cetin L, Kucuker A, Iriz E, Uguz E, et al. Comparing S-100 beta protein levels and neurocognitive functions between patients undergoing on-pump and off-pump coronary artery bypass grafting. J Surg Res. 2013;182(2):198–202.
Bonacchi M, Prifti E, Maiani M, Bartolozzi F, Di Eusanio M, Leacche M. Does off-pump coronary revascularization reduce the release of the cerebral markers, S-100beta and NSE? Heart Lung Circ. 2006;15(5):314–9.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3.
Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–37.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80.
Huizenga HM, Visser I, Dolan CV. Testing overall and moderator effects in random effects meta-regression. Br J Math Stat Psychol. 2011;64(Pt 1):1–19.
Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–20.
van Boven WJ, Morariu A, Salzberg SP, Gerritsen WB, Waanders FG, Korse TC, et al. Impact of different surgical strategies on perioperative protein S100beta release in elderly patients undergoing coronary artery bypass grafting. Innovations (Phila). 2013;8(3):230–6.
Zhai YJ, Wang XL, Ji SY, Liu YR. Brain injuries in patients during cornonary artery bypass graft with or without cardiopulmonary bypass. Academic J Guangzhou Med Col. 2008;36(5):13–5.
Yan XZ, Yang SY, Sun ZL, Huang FJ, Zhang DG, Xiang DK, et al. Off-pump coronary artery bypass grafting and neuronal injury. Guizhou Med J. 2002;26(6):483–5.
Tian LQ, Cheng ZY, Li XH, Liu C. Effects of on-pump and off-pump coronary artery bypass grafting on plasma neuron specific enolase and S100 protein levels. Chin J Practical Nerv Dis. 2012;15(1):17–9.
Liu JT, Li HY, He GX, Wang SB, Pu RF, Zhou F. The change and meaning of serum s100β protein during CABG with or without extracorporeal circulation. Chin J Extracorporeal Circulation. 2007;5(2):81–3. 120.
Hong T, Wen DX, Hang YN. Effects of off-pump and on-pump on cerebral neurologic injuries in elder patients undergoing coronary artery bypass grafting. Jiangsu Med J. 2008;34(2):117–9.
Hong F, Peng JM, Zhang RX, Chen JP. Serum concentration of S100 -B protein in patients during CABG with or without cardiopulmonary bypass. Chin J Pathophysiol. 2007;23(7):1293–5.
Guo XY, Luo AL, Yin YQ, Ren HZ, Ye TH, Huang YG, et al. Perioperative plasma concentrations of neuron-specific enolase and postoperative cognitive function in patients undergoing coronary artery bypass grafting surgery. Basic & Clin Med. 2005;25(10):934–7.
Gao CQ, He T, Li BJ, Wang G, Li JC, Mu YL. Comparison of cerebral Injury in patients during coronary artery bypass graft without versus with cardiopulmonary bypass. Chin Circulation J. 2003;18(6):449–52.
Iriz E, Kolbakir F, Akar H, Adam B, Keceligil HT. Comparison of hydroxyethyl starch and ringer lactate as a prime solution regarding S-100beta protein levels and informative cognitive tests in cerebral injury. Ann Thorac Surg. 2005;79(2):666–71.
Berger RP, Pierce MC, Wisniewski SR, Adelson PD, Clark RS, Ruppel RA, et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109(2), E31.
Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, et al. Serum S-100beta as a possible marker of blood–brain barrier disruption. Brain Res. 2002;940(1–2):102–4.
Mackensen GB, Ti LK, Phillips-Bute BG, Mathew JP, Newman MF, Grocott HP. Cerebral embolization during cardiac surgery: impact of aortic atheroma burden. Br J Anaesth. 2003;91(5):656–61.
Schoenburg M, Kraus B, Muehling A, Taborski U, Hofmann H, Erhardt G, et al. The dynamic air bubble trap reduces cerebral microembolism during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;126(5):1455–60.
Grocott HP, Arrowsmith JE. Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth. 2001;86(2):289–90.
Woertgen C, Rothoerl RD, Brawanski A. Neuron-specific enolase serum levels after controlled cortical impact injury in the rat. J Neurotrauma. 2001;18(5):569–73.
Bowles BJ, Lee JD, Dang CR, Taoka SN, Johnson EW, Lau EM, et al. Coronary artery bypass performed without the use of cardiopulmonary bypass is associated with reduced cerebral microemboli and improved clinical results. Chest. 2001;119(1):25–30.
Lee JD, Lee SJ, Tsushima WT, Yamauchi H, Lau WT, Popper J, et al. Benefits of off-pump bypass on neurologic and clinical morbidity: a prospective randomized trial. Ann Thorac Surg. 2003;76(1):18–25. discussion 25–16.
Edwards JH, Huang DT. Using pump for bypass surgery–on-off-on again? Crit Care. 2010;14(5):319.
Velissaris T, Jonas MM, Ohri SK. Hemodynamic advantages of right heart decompression during off-pump surgery. Asian Cardiovasc Thorac Ann. 2010;18(1):17–21.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LZ, Q-M F designed, conceived and supervised the study. LZ and Z-Y W selected the studies. Z-Y W performed the statistical analysis and interpreted the results. All authors drafted and revised the paper. All authors read and approved the final paper.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zheng, L., Fan, QM. & Wei, ZY. Serum S-100β and NSE levels after off-pump versus on-pump coronary artery bypass graft surgery. BMC Cardiovasc Disord 15, 70 (2015). https://doi.org/10.1186/s12872-015-0050-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-015-0050-0