Abstract
Introduction
The antiinflammatory and antioxidative effects of melatonin have been established in recent years. Several studies indicate that oxidative stress and inflammation are key drivers of post-coronary artery bypass graft (CABG) surgery complications. In the present study, we aimed to investigate the effects of melatonin on cardiac injury and inflammatory biomarkers in CABG candidates.
Methods
Embase, Medline/PubMed, Web of Science, Scopus, and the Cochrane library were searched up to 5 June 2022. All randomized controlled trials examining cardiac injury and inflammatory biomarkers of CABG patients who received melatonin were included. The random-effects model was utilized to perform the analysis.
Results
A total of 947 citations were retrieved through database searches. Finally, five articles (six trials with 342 patients) were included after the screening. Melatonin supplementation led to a significant reduction in cardiac troponin I (CTnI) [weighted mean difference(WMD): −2.28 ng/ml; 95% CI −2.87, −1.69; P < 0.01; I2: 91.25%] and high sensitivity-C reactive protein (hs-CRP) levels (WMD: −0.62 mg/L; 95% CI −0.73, −0.5; P < 0.01; I2: 99.98%) in patients undergoing CABG surgery. We found a nonsignificant decrease in creatine kinase isoenzyme muscle/brain (CK-MB) levels (WMD: −2.87 ng/ml; 95% CI −5.97, 0.23; P = 0.07; I2: 99.98%) after melatonin supplementation. No publication bias was found according to Egger’s test.
Conclusion
Melatonin supplementation may be useful in reducing cardiac injury and inflammatory biomarkers in CABG candidates. Future studies should investigate the clinical significance of these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ischemic-reperfusion injury during coronary artery bypass graft (CABG) surgery is one of the most important consequences of this surgery. It can lead to serious postoperative complications such as arrhythmias and myocardial injury. |
Melatonin, a product of the pineal gland, is shown to influence the circadian rhythm and has antiinflammatory and antioxidative effects. |
Melatonin supplementation may reduce biomarkers of cardiac injury and inflammation. |
In this study, Melatonin supplementation showed cardio-protective and antiinflammatory effects in patients with CABG. |
Introduction
Ischemic-reperfusion injury during coronary artery bypass graft (CABG) is one of the most significant sequels of this surgery, which may lead to serious postoperative complications such as arrhythmia and myocardial injury [1, 2]. One of the underlying mechanisms of this phenomenon is linked to the increased reactive oxygen species secondary to impaired scavenging mechanisms, which can make the myocardium prone to inflammation and oxidative stress [1, 3]. Therefore, several studies have experimented with a wide range of antioxidative agents before or during surgery to reduce post-CABG complications [4, 5].
Melatonin, a product of the pineal gland, was first introduced as an influential factor in regulating circadian rhythm [6]. In addition, eliminating free radicals, reducing oxidative stress, and inflammation have been reported as other possible effects of melatonin [7,8,9]. In recent years, several pieces of evidence have shed light on the possible cardio-protective effects of melatonin [10,11,12]. Some researchers have indicated the presence of melatonin receptors in the vascular structure of the human body as a possible justification for its effects [13, 14].
In light of the antioxidative and antiinflammatory effects of melatonin [8, 9], several studies utilized melatonin with the purpose of reducing oxidative stress and inflammation in CABG patients. However, their results were varied [15,16,17,18,19]. The effectiveness of melatonin supplementation was assessed following treatment using markers of inflammation and cardiac muscle injury.
In the present study, for the first time, we conducted a meta-analysis on randomized controlled trials (RCTs) to illuminate the impact of melatonin therapy before CABG on reducing cardiac injury and inflammation.
Methods
Search Strategy
We searched the following electronic databases systematically up to 5 June 2022: Embase, Medline/PubMed, Web of Science, Scopus, and the Cochrane library. A combination of the following terms and keywords was used to identify RCTs that examined the effects of melatonin on cardiac injury biomarkers and inflammation: patients [“Coronary Artery Bypass” OR “Coronary Artery Bypass Graft (CABG)” OR “Coronary Artery Bypass Surgery”], intervention [“melatonin” AND “use” OR “supplementation” OR “intake”], and outcomes [“Cardiac Injury biomarkers” OR “inflammatory biomarkers”]. A backward and forward reference check was performed to find any further RCTs that had not been found in our main search. The reporting of the present study was according to the standards of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines [20].
Inclusion and Exclusion Criteria
Original human RCTs concerning the effects of melatonin supplementation on cardiac injury and inflammatory biomarkers in patients undergoing CABG surgery were eligible for quantitative synthesis. Only RCTs in the English language were eligible to be included in the current study. In vitro studies, case reports, observational studies, letters, commentaries, congress abstracts without full text, studies without placebo group as control, and trials that did not report mean changes of cardiac injury biomarkers [including cardiac troponin I (CTnI), creatine kinase isoenzyme muscle/brain (CK-MB)] and inflammatory biomarkers [including high-sensitivity C-reactive protein (hs-CRP)] along with corresponding standard deviation (SD) in the intervention and control groups were excluded.
Study Selection
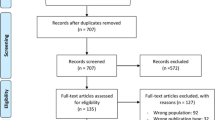
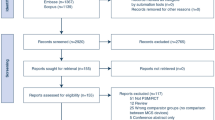
After removing duplicates, two independent authors (M.F. and E.T.) examined the citations according to the predefined criteria. Any discrepancy in study eligibility was resolved through consensus during the screening process or by consulting with a third author (M.A.).
Data Extraction and Quality Assessment
Data extraction and quality assessment of selected RCTs were conducted by two authors independently using a standard form in Excel software and the Cochrane Collaboration Risk of Bias tool, respectively. The quality of selected articles was examined through the following items: “randomization generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias.” The main baseline characteristics of each article were extracted: authors, publication year, location of study, sample size, age of participants, study method, duration of treatment, dosage and type of intervention, and the changes of the mean (SD) for outcomes including CTnI, CK-MB, and hs-CRP levels for the intervention and control groups. For studies that reported the relevant data in graphs, the authors extracted the data from graphs or figures using plot digitizer software. A discussion with a third author (M.A.) was initiated when there was any discrepancy in quality assessment or data extraction between the authors.
Data Synthesis and Analysis
Mean changes along with SDs were synthesized using the random-effects DerSimonian–Laird model in Stata version 16.0 (Stata Corp., College Station, TX, USA). The effect estimates were considered as weighted mean differences (WMDs) with a 95% confidence interval (CI). When interquartile range (IQR) or 95% confidence interval (CI) was reported among included studies, SD was calculated using “(P75-P25) × 0.7413” or “√n × (upper limit-lower limit)/4.128,” respectively, according to the Cochrane Handbook for Systematic Reviews of Interventions. Inter-study heterogeneity was evaluated using the Cochran’s Q test and I-square (I2) statistic. A P-value < 0.1 or I2 greater than 50% indicated heterogeneity across studies. In addition, sensitivity analyses were conducted using the “leave-one-out” method. The evidence of publication bias was evaluated statistically by using Egger’s test across studies.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any authors.
Results
Baseline Characteristics of Included Studies
A total of 947 citations were identified through our initial searches. Figure 1 shows the PRISMA flowchart of the screening process in detail. Overall, five articles (six trials with 342 patients) [15,16,17,18,19] were eligible for the present meta-analysis. Among these studies, the study by Dwaich et al. [18] used different doses of melatonin treatment (low versus high) for estimating the relevant outcomes, and each dose of melatonin is included as a separate trial in our meta-analysis. The daily melatonin consumption varied from 3 to 20 mg/day. Four trials have shown changes in CK-MB [17,18,19], four in hs-CRP [16,17,18], and three in CTnI [15, 18]. Detailed baseline characteristics of the selected trials are presented in Table 1. The quality assessment results of included trials are shown in Fig. 2 using the Cochrane Collaboration risk of bias tool.
Pooled Effects of Melatonin Consumption
Figure 3 shows the forest plots for the effect of melatonin consumption on cardiac injury and inflammation biomarkers. Melatonin significantly reduced CTnI (WMD: −2.28 ng/ml; 95% CI −2.87, −1.69; P < 0.01; I2: 91.25%) and hs-CRP levels (WMD: −0.62 mg/L; 95% CI −0.73, −0.5; P < 0.01; I2: 99.98%) in patients undergoing CABG surgery. We found no significant effect of melatonin on CK-MB levels (WMD: −2.87 ng/ml; 95% CI −5.97, 0.23; P = 0.07; I2: 99.98%).
Sensitivity Analysis
Regarding CTnI, after removing datasets from Dwaich et al. [18], the results became nonsignificant post analysis (a, −1.1, 95% CI −4.6, 2.4) (b, −1, 95% CI −4.3, 2.3). For hs-CRP, sensitivity analysis showed no difference between pre-and post-sensitivity analyses after removing each study from the analysis, while for CK-MB, only excluding the study by Talasaz et al. [19] showed a significant effect (−4.2, 95%CI −7.5, −1). Further information on the sensitivity analysis is available in the Supplementary Material. Additional analyses, including subgroup analysis and meta-regression, could not be conducted due to the small number of included trials for each outcome.
Publication Bias
Egger’s test was used to assess the evidence of potential publication bias across included trials. Egger’s test showed that there was no considerable publication bias for estimating the pooled effects of melatonin consumption on CTnI (B = 5.23, P = 0.21), CK-MB (B = −9.43, P = 0.90), and hs-CRP (B = −1.21, P = 0.17).
Discussion
In the present meta-analysis, we found that melatonin supplementation effectively reduces CTnI and hs-CRP in patients undergoing CABG. Also, a nonsignificant decrease was observed for CK-MB.
As research on the role of oxidative stress and inflammation in the pathophysiology of post-CABG complications has accumulated, antioxidant supplements have been utilized for both their preventative and therapeutic roles. Several studies have been performed to determine whether antioxidant supplementation (e.g., N-acetyl cysteine, ascorbic acid, polyunsaturated fatty acids, etc.) before surgery help reduce post-CABG complications; however, the results are mixed [21,22,23]. Also, a combination of antioxidant agents has been suggested to be efficient in preventing complications [15, 24]. It has been reported that CABG candidates who received N-acetyl cysteine or melatonin before surgery had a significantly lower length of hospital stay compared with the control group [15]. Our results also support the aforementioned studies on cardio-protective and antiinflammatory effects of melatonin in CABG patients as we revealed a significant reduction in CTnI and hs-CRP levels in those who received melatonin.
As a marker of inflammation, higher levels of hs-CRP were also associated with significantly higher levels of mortality and morbidity in CABG patients [25, 26]. The same pattern of higher complications was observed for CABG patients with higher CTnI [27]. Therefore, the mitigating effects of melatonin on hs-CRP, and CTnI may be helpful to prevent inflammation and subsequent complications, which should be further investigated.
Furthermore, we observed nonsignificant but decreasing CK-MB levels in CABG candidates. As a significant reduction in CTnI was identified, the nonsignificance of the CK-MB decrease may be justifiable by the lower specificity of CK-MB compared with CTnI for cardiac injuries [28, 29]. Of note, we acknowledge that this may stem from the different designs and sampling times of the studies, which resulted in over 99% heterogeneity. Hence, further studies with extended follow-up periods are required to establish a clearer picture.
Aside from supplementing with exogenous melatonin, and consistent with our findings, it has been reported that CABG surgeries that were performed in the morning were followed by lower levels of ischemic/reperfusion injury markers than surgeries that were performed at noon [30], acknowledging the fact that peak levels of melatonin occur in the morning. This underscores the significance of melatonin in preventing postoperative complications in CABG patients. However, as mentioned in the previous studies, to reach the maximum protective effects of melatonin, supraphysiological (> 3 mg) doses of melatonin may be required [19].
We conducted the present meta-analysis according to the standard PRISMA guidelines. We found no publication bias, which strengthens our results. These strengths notwithstanding, the heterogeneity between the studies was significantly high in the analysis. The follow-up intervals of some studies were too short, so alterations in the laboratory values might have gone unnoticed. The included studies encompassed a narrow range of ethnicities, so the results should be interpreted cautiously. Also, the number of the included studies is relatively low, which can act as a call for action for further studies given the cardio-protective effects of melatonin in CABG candidates. Finally, we acknowledge that the observed effects were relatively small and their clinical significance should be further investigated.
Conclusions
Melatonin supplementation could be helpful in reducing cardiac injury and inflammatory biomarkers in patients undergoing CABG. Further studies in light of our findings should be done to elaborate the knowledge regarding antiinflammatory and antioxidative effects of melatonin to reduce CABG complications.
References
Weman SM, Karhunen PJ, Penttilä A, Järvinen AA, Salminen US. Reperfusion injury associated with one-fourth of deaths after coronary artery bypass grafting. Ann Thorac Surg. 2000;70(3):807–12.
Pooria A, Pourya A, Gheini A. Postoperative complications associated with coronary artery bypass graft surgery and their therapeutic interventions. Future Cardiol. 2020;16(5):481–96.
Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–8.
Zakkar M, Guida G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2015: 189863.
Geng J, Qian J, Si W, Cheng H, Ji F, Shen Z. The clinical benefits of perioperative antioxidant vitamin therapy in patients undergoing cardiac surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. 2017;25(6):966–74.
Pévet P. Melatonin. Dialogues Clin Neurosci. 2002;4(1):57–72.
Pal R, Gulati K, Banerjee BD, Ray A. Pharmacological and biochemical studies on the protective effects of melatonin during stress-induced behavioral and immunological changes in relation to oxidative stress in rats. Can J Physiol Pharmacol. 2016;94(3):296–301.
Emamgholipour S, Hossein-Nezhad A, Ansari M. Can melatonin act as an antioxidant in hydrogen peroxide-induced oxidative stress model in human peripheral blood mononuclear cells? Biochem Res Int. 2016;2016:5857940.
Cho JH, Bhutani S, Kim CH, Irwin MR. Anti-inflammatory effects of melatonin: a systematic review and meta-analysis of clinical trials. Brain Behav Immun. 2021;93:245–53.
Song Y-J, Zhong C-B, Wu W. Cardioprotective effects of melatonin: focusing on its roles against diabetic cardiomyopathy. Biomed Pharmacother. 2020;128: 110260.
Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44(1):16–25.
Gögenur I, Kücükakin B, Panduro Jensen L, Reiter RJ, Rosenberg J. Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: a randomized, placebo-controlled, clinical trial. J Pineal Res. 2014;57(1):10–5.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010;49(1):14–22.
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. 2018;64(3): e12471.
Shafiei E, Bahtoei M, Raj P, Ostovar A, Iranpour D, Akbarzadeh S, et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: a randomized, open-labeled, placebo-controlled trial. Medicine (Baltimore). 2018;97(30): e11383.
Javanmard SH, Heshmat-Ghahdarijani K, Mirmohammad-Sadeghi M, Sonbolestan SA, Ziayi A. The effect of melatonin on endothelial dysfunction in patient undergoing coronary artery bypass grafting surgery. Adv Biomed Res. 2016;5:174.
Barati S, Jahangirifard A, Ahmadi ZH, Tavakoli-Ardakani M, Dastan F. The effects of melatonin on the oxidative stress and duration of atrial fibrillation after coronary artery bypass graft surgery: a randomized controlled trial. Endocr Metab Immune Disord Drug Targets. 2021;21(6):1142–9.
Dwaich KH, Al-Amran FG, Al-Sheibani BI, Yousif NG, Hadi NR. The cardioprotective role of melatonin against myocardial injury in patients undergoing coronary artery bypass grafting surgery. Vasc Invest Ther. 2018;1(2):41.
Hajhossein-Talasaz A, Dianatkhah M, Salehiomran A, Ghaeli P, Dianatkhah M. Possible effects of melatonin on reperfusion injury following coronary artery bypass graft surgery. ARYA Atheroscler J. 2022;18(March):1–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Kara H, Yasim A. Effects of high-dose vitamin D supplementation on the occurrence of post-operative atrial fibrillation after coronary artery bypass grafting: randomized controlled trial. Gen Thorac Cardiovasc Surg. 2020;68(5):477–84.
Antonic M, Lipovec R, Gregorcic F, Juric P, Kosir G. Perioperative ascorbic acid supplementation does not reduce the incidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J Cardiol. 2017;69(1):98–102.
Mozaffarian D, Marchioli R, Gardner T, Ferrazzi P, O’Gara P, Latini R, et al. The ω-3 fatty acids for prevention of post-operative atrial fibrillation trial—rationale and design. Am Heart J. 2011;162(1):56-63.e3.
Rodrigo R, Korantzopoulos P, Cereceda M, Asenjo R, Zamorano J, Villalabeitia E, et al. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013;62(16):1457–65.
Min JJ, Nam K, Kim TK, Kim HJ, Seo JH, Hwang HY, et al. Relationship between early postoperative C-reactive protein elevation and long-term postoperative major adverse cardiovascular and cerebral events in patients undergoing off-pump coronary artery bypass graft surgery: a retrospective study. Br J Anaesth. 2014;113(3):391–401.
Olesen OJ, Vinding NE, Østergaard L, Butt JH, Gislason GH, Torp-Pedersen C, et al. C-reactive protein after coronary artery bypass graft surgery and its relationship with postoperative atrial fibrillation. Europace. 2020;22(8):1182–8.
Li Y, Li Y, Hu Q, Zheng S, Tian B, Meng F, et al. Association of early elevated cardiac troponin I concentration and longitudinal change after off-pump coronary artery bypass grafting and adverse events: a prospective cohort study. J Thorac Dis. 2020;12(11):6542–51.
Chang CC, Ip MP, Hsu RM, Vrobel T. Evaluation of a proposed panel of cardiac markers for the diagnosis of acute myocardial infarction in patients with atraumatic chest pain. Arch Pathol Lab Med. 1998;122(4):320–4.
Pervaiz S, Anderson FP, Lohmann TP, Lawson CJ, Feng YJ, Waskiewicz D, et al. Comparative analysis of cardiac troponin I and creatine kinase-MB as markers of acute myocardial infarction. Clin Cardiol. 1997;20(3):269–71.
Sokullu O, Sanioğlu S, Kurç E, Sargin M, Deniz H, Tartan Z, et al. Does the circadian rhythm of melatonin affect ischemia-reperfusion injury after coronary artery bypass grafting? Heart Surg Forum. 2009;12(2):E95–9.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Melika Farshidianfar, Erfan Taherifard, Reza Tabrizi and Maryam Akbari. The first draft of the manuscript was written by Melika Farshidianfar, and Ali Ardekani, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Melika Farshidianfar, Ali Ardekani, Reza Tabrizi, Kamran B Lankarani, Erfan Taherifard, Ashkan Abdollahi, Arezou Azizi, and Maryam Akbari have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Farshidianfar, M., Ardekani, A., Tabrizi, R. et al. Effects of Melatonin on Cardiac Injury and Inflammatory Biomarkers in Patients Undergoing Coronary Artery Bypass Graft Surgery: a Meta-analysis. Cardiol Ther 12, 11–20 (2023). https://doi.org/10.1007/s40119-022-00287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00287-1