Abstract
Background
Foxtail millet (Setaria italica L.) is a millet species with high tolerance to stressful environments. Plant non-specific lipid transfer proteins (nsLTPs) are a kind of small, basic proteins involved in many biological processes. So far, the genome of S. italica has been fully sequenced, and a comprehensive understanding of the evolution and expression of the nsLTP family is still lacking in foxtail millet.
Results
Forty-five nsLTP genes were identified in S. italica and clustered into 5 subfamilies except three single genes (SinsLTP38, SinsLTP7, and SinsLTP44). The proportion of SinsLTPs was different in each subfamily, and members within the same subgroup shared conserved exon–intron structures. Besides, 5 SinsLTP duplication events were investigated. Both tandem and segmental duplication contributed to nsLTP expansion in S. italica, and the duplicated SinsLTPs had mainly undergone purifying selection pressure, which suggested that the function of the duplicated SinsLTPs might not diverge much. Moreover, we identified the nsLTP members in 5 other monocots, and 41, 13, 10, 4, and 1 orthologous gene pairs were identified between S. italica and S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon, respectively. The functional divergence within the nsLTP orthologous genes might be limited. In addition, the tissue-specific expression patterns of the SinsLTPs were investigated, and the expression profiles of the SinsLTPs in response to abiotic stress were analyzed, all the 10 selected SinsLTPs were responsive to drought, salt, and cold stress. Among the selected SinsLTPs, 2 paired duplicated genes shared almost equivalent expression profiles, suggesting that these duplicated genes might retain some essential functions during subsequent evolution.
Conclusions
The present study provided the first systematic analysis for the phylogenetic classification, conserved domain and gene structure, expansion pattern, and expression profile of the nsLTP family in S. italica. These findings could pave a way for further comparative genomic and evolution analysis of nsLTP family in foxtail millet and related monocots, and lay the foundation for the functional analysis of the nsLTPs in S. italica.
Similar content being viewed by others
Introduction
Foxtail millet (Setaria italica L.) originated in China and is the second largest cultivated millet species in the world [1, 2]. It is a diploid (2n = 2x = 18) with an estimated genome size of approximately 515 Mb [1]. Foxtail millet is predominantly cultivated in arid and semiarid regions of the world as food and fodder, and displays remarkable tolerance to abiotic stress [1, 3, 4]. Along with other features such as short life-cycle, small genome, inbreeding nature, and genetic close-relatedness to several bioenergy grasses, foxtail millet has been considered as a favorable candidate for investigating the stress responsive machinery, evolutionary genomics and the system biology of millets and C4 panicoid grasses [1]. Thus, the availability of foxtail millet genome information provided excellent opportunity for researchers to initiate whole-genome annotation and perform comparative genomic study in foxtail millet [5]. Until now, foxtail millet has gained popularity among millet research community and several gene families such as AP2/ERF [1], GRAS [2], NF-Y [3], LecRLKs [4], NAC [6], WD40 [7], MYB [8], PPR [9], HSP [10], CDPK [11], BES/BZR [12], and MADS-Box [13] have been identified and characterized to investigate their role in plant abiotic stress tolerance.
Plant non-specific lipid transfer proteins (nsLTPs) are a kind of small, basic proteins, ranging in size from 6.5–10.5 kDa [14,15,16]. They are abundantly present in various plants, representing up to 4% of the total soluble protein [15]. Plant nsLTPs are able to transfer phospholipids and fatty acids between membranes in vitro, and structurally characterized by an eight cysteine motif (8CM) backbone with the general form C-Xn-C-Xn-CC-CXC-Xn-C-Xn-C [15, 17]. The cysteine residues of these peptides are linked by four disulfide bonds to stabilize a tertiary structure of a hydrophobic cavity [15, 17]. Almost all nsLTPs are synthesized as precursors with an N-terminal secretory signal peptide, thus secreted to the cell exterior for functioning [15, 16, 18]. Based on the molecular mass and connection types between the bonds, the nsLTPs were initially classified into nsLTP1 (9 kDa, Cys1-Cys6 and Cys5-Cys8) and nsLTP2 (7 kDa, Cys1-Cys5 and Cys6-Cys8). After that, a new classification according to sequence similarity and intervals of 8CM was proposed [17]. The system categorized the 267 nsLTPs from rice, wheat and Arabidopsis into nine types (Type I-IX). Then, nsLTPs in other plant species such as Brassica rapa [18], sorghum [19], cotton [20], tomato [21], tobacco [22] and Solanaceae plants [23] were also grouped according to Boutrot’s method.
In plants, there is considerable evidence showing that nsLTPs play vital roles in a range of biological processes, including cuticular wax and cutin synthesis, seed maturation, and sexual reproduction [15, 18]. On the other hand, nsLTPs also take part in the regulation of signalling and responses to abiotic/biotic stress, such as drought, high salinity, cold stress, and pathogen defense [15, 16, 18]. Thus, nsLTPs are important for plants to withstand various environmental stresses, which cause huge economic loss in agricultural production globally. In previous studies, only a small portion of nsLTPs from foxtail millet have been characterized [24], a genome-wide overview of the nsLTP family in foxtail millet has yet to be reported. Considering the importance of investigating the molecular networks, biological processes, and gene functions of nsLTP proteins, a systematic molecular evolution and expression analysis of the nsLTPs in foxtail millet is urgently required. In this study, putative nsLTPs were identified in foxtail millet. We conducted a comprehensive study on the phylogenetics, gene structure, genomic location, expansion pattern, and expression profile to evaluate the molecular evolution and biological function of the nsLTP family in foxtail millet.
Results
Genomic identification and characterization of S. italica nsLTP genes
In this study, a total of 45 nsLTP genes were identified in S. italica and designated as SinsLTPs, from SinsLTP1 to SinsLTP45 (Additional file 1). The protein structures of the identified SinsLTPs were highly diverse, and the amino acid numbers of mature peptides varied from 67 (SinsLTP13) to 120 (SinsLTP7), with the predicted molecular weight ranging from 6.9 kDa (SinsLTP13) to 12.3 kDa (SinsLTP7). The isoelectric points ranged from 4.29 (SinsLTP11 and SinsLTP12) to 10.25 (SinsLTP16). Besides, we identified 45, 32, 20, 45, and 30 nsLTP genes in S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon, respectively, and denoted them as SvnsLTPs, SbnsLTPs, ZmnsLTPs, OsnsLTPs, and BdnsLTPs, respectively (Additional file 2).
Phylogenetic analysis
The identified SinsLTP members were phylogenetically analyzed in this study (Figs. 1 and 2, Additional file 3). According to the previous classification system [17], the SinsLTPs was divided into 5 subfamilies (Type I, Type II, Type IV, Type V, and Type VI), and no Type III, Type VII, and Type IX nsLTPs were identified in S. italica. Besides, the member proportion was different in each subfamily. The Type I (31%) had the most genes, followed by Type II (20%), Type VI (18%), and Type IV (13%). Type V (11%) contained the least members (Additional file 4a). In addition, the nsLTPs in S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon were phylogenetically classified (Additional file 5), and a similar member distribution in each subfamily was found in each plant (Additional file 4b-f).
Phylogenetic analysis of the nsLTP family from foxtail millet, Arabidopsis, and rice. The neighbor-joining tree was generated using the MEGA7 program based on multiple alignments with ClustalW. The subfamilies are labeled and indicated by different colors, and the numbers in the clades are posterior probability values
Exon/intron structures and conserved protein domains of SinsLTPs
Gene structure and intron phase were investigated in the SinsLTP family (Fig. 2, Additional file 3). Results indicated low diversity in the distribution of intronic regions amid the exonic sequences. Within each subfamily, the intron patterns, formed by relative position and phase, were highly conserved. The number of introns per gene varied from 0 to 1, and no intron was identified in Type II and Type IV SinsLTPs.
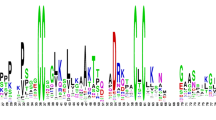
The main characteristic of plant nsLTPs is the presence of 8CM in highly conserved positions. In this study, the sequence logos of the identified SinsLTPs were generated to further confirm the conservation of amino acid residues (Fig. 3). It was found that the eight Cys residues were highly conserved in all of the 45 SinsLTPs. Besides, multiple alignments revealed a variable number of inter-cysteine amino acid residues, and 5 nsLTP subfamilies were therefore identified based on the sequence similarity and the typical spacing of the 8CM (Table 1). Between the conserved Cys1 and Cys2 residues, Type I, II, IV, and VI nsLTPs contained 7–10 residues, while Type V contained 14 residues. Between the conserved Cys4 and Cys5 residues, Type I nsLTPs contained 19 residues, while the other types contained relatively fewer residues (8–13). Between the conserved Cys7 and Cys8 residues, Type I nsLTPs contained more residues (13) than Type II nsLTPs (6). In addition, different residues were found in the central position of the Cys5XCys6 motif. Seven hydrophilic residues (Arg, Gly, Glu, Asn, Ser, Thr, and Lys) and five hydrophobic residues (Leu, Ile, Phe, Val, and Met) existed at the X position of the Cys5XCys6 motif in the 45 SinsLTPs.
Conserved domain analysis of the SinsLTPs using the WebLogo program. The height of the letter designating the amino acid residue at each position represents the degree of conservation. The numbers on the x-axis represent the sequence positions in the corresponding conserved domains. The y-axis represents the information content measured in bits
Genomic locations and gene duplications of SinsLTPs
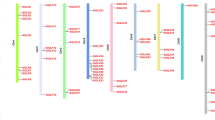
To analyze the genomic location of SinsLTPs (Additional file 6), the chromosomal distribution diagram of the SinsLTPs was generated (Fig. 4). The 45 nsLTPs were unevenly distributed on 9 chromosomes, and the number of nsLTPs on each chromosome varied widely. Chromosome 5 contained the most SinsLTPs with 10 genes, followed by Chromosome 7 and 9 with 8 members. In contrast, only 2 genes were localized on Chromosome 6, and no nsLTPs were present in Chromosome 1. Moreover, several nsLTP clusters were detected on chromosomes such as the top of Chromosome 8 and the bottom of Chromosome 7 in S. italica.
Gene duplication events were investigated to illustrate the expansion of the SinsLTPs. In our study, 5 gene duplication events were detected in S. italica (Table 2), and the duplication events were concentrated in Type I and Type IV. Meanwhile, based on the sequence analysis and the chromosomal distribution, 2 paired genes were identified to be involved in tandem duplication events, while the other 3 pairs were related to segmental duplication events (Fig. 4, Table 2). In addition, the Ka/Ks ratios of the duplicated SinsLTPs were calculated to estimate the molecular evolutionary rates (Table 2). The Ka/Ks ratios of 4 duplicated SinsLTPs were less than 1. Moreover, the divergence times between the duplicated gene pairs were analyzed. In S. italica, all the Ks values were less than 0.47, and their corresponding duplication age might be less than 36.01 million years ago (MYA).
In our study, orthologous relationships of nsLTPs between S. italica and 5 other monocots were analyzed, 41, 13, 10, 4, and 1 orthologous gene pairs were identified between S. italica and S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon, respectively (Fig. 5, Additional file 7). Of the orthologous gene pairs, most were distributed in Type I, Type II, Type IV, and Type V. All the Ka/Ks ratios except that of 11 orthologous gene pairs between S. italica and S. viridis were less than 1.
Regulatory element of SinsLTPs
In this study, cis-elements included stress response elements and hormone-related elements were identified in the promoter regions of SinsLTPs (Fig. 6, Additional file 8), and the promoter region of SinsLTPs from the same subfamily had the similar responsive regulatory elements. Among them, most Type I SinsLTPs showed responsive to drought stress, and SinsLTP33 had the most drought-responsive elements (4), while most Type II members showed responsive cold stress, and SinsLTP40 contained the most low-temperature-responsive elements (3). Besides, The methyl jasmonate (MeJA)-responsive elements and abscisic acid (ABA)-responsive elements were identified abundantly in the promoter regions of SinsLTPs from all subfamilies, and SinsLTP35 (Type I) and SinsLTP27 (Type V) contained the most ABA-responsive elements in the promoter region (9), while the most MeJA-responsive elements were 12 in the promoter region of SinsLTP8 (Type II) and SinsLTP19 (Type VI). In addition, among the 45 SinsLTPs, only two genes, SinsLTP25 (Type I) and SinsLTP20 (Type VI) had a wound-responsive element.
Tissue-specific expression pattern of SinsLTPs using RNA-seq data and qRT-PCR
The RNA-seq data in the SRA database at NCBI were used to examine the expression profiles of the SinsLTPs, and an expression heatmap in 7 different tissues was mapped (Fig. 7). Generally, most SinsLTPs had a broad expression spectrum, and 4 SinsLTPs (SinsLTP10, SinsLTP24, SinsLTP25, and SinsLTP28) had trace or no detected expression in the 7 tissues. Besides, SinsLTPs showed similar tissue-specific expression levels within the same subfamily. SinsLTPs in Type IV and Type V subfamily expressed at high levels in roots and stems, while SinsLTPs in Type I shared high expression levels in stems and leaves. Most Type VI SinsLTPs expressed predominately in flower organs (panicle). In addition, some genes such as SinsLTP3, SinsLTP9, SinsLTP15, and SinsLTP45 showed high transcript level in all tissues while the expression level of other genes such as SinsLTP8, SinsLTP13, SinsLTP16, and SinsLTP26 was extremely low.
Using qRT-PCR, we analyzed the expression of 10 selected SinsLTP genes in roots, stems and leaves of Yugu No.1 seedlings. As shown in Fig. 8, SinsLTP3, SinsLTP5, and SinsLTP30 were expressed at relatively high levels in the root, while SinsLTP21, SinsLTP33, SinsLTP34, SinsLTP37, and SinsLTP40 showed high transcript level in the stem. The qRT-PCR results are consistent with the former RNA-seq data available in the public database (Fig. 7).
Expression profiling of SinsLTPs during abiotic stresses treatments
In the current study, a total of 10 SinsLTPs from all the subfamilies were selected to investigate the expression patterns of SinsLTPs. The result showed that the expressions of all the 10 SinsLTPs were induced after drought and salt stress, while some SinsLTPs such as SinsLTP21, SinsLTP33, SinsLTP34, and SinsLTP40 were repressed after cold stress (Fig. 9). Moreover, the expression patterns of 2 duplicated SinsLTP gene pairs (SinsLTP3/SinsLTP5 and SinsLTP33/SinsLTP34) were compared (Additional file 9), and the 2 paired genes shared almost equivalent expression profiles after drought, salt and cold stress treatment.
Discussion
As a model monocot, the announcement of foxtail millet genome sequencing offers a good opportunity to further investigate the monocot and plant evolution in general. The present study mainly analyzed the molecular evolution and expression pattern of SinsLTPs. In this study, a total of 45 nsLTP gene members were identified in S. italica, and 45, 32, 20, 45, and 30 nsLTP genes in S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon, respectively. The encoded proteins of SinsLTPs showed significant differences in physical and chemical properties (Additional file 1), which were comparable with nsLTPs from other plant species [17,18,19,20,21,22,23]. The phylogenetic classification of nsLTPs provided comprehensive information about the gene family and facilitated further functional analysis. In the current study, the identified nsLTPs in the 6 monocots were divided into 7 subfamilies (Additional file 5), and a similar member distribution in each subfamily was found in each plant (Additional file 4). However, not all the subgroups were present in each plant. No Type III and VII nsLTPs were found in S. italica, S. viridis, and B. distachyon, and no Type VII nsLTPs existed in S. bicolor. In addition, previous studies have reported that Type VII nsLTPs were unique to monocots while Type IX nsLTPs appeared specifically in dicots [17, 20, 23]. In our study, the nsLTPs in Z. mays and O. sativa further confirmed this viewpoint, while S. italica, S. viridis, S. bicolor, and B. distachyon lost Type VII members. The result suggested that the evolution of plants not only involves gene retentions, but also is accompanied by gene losses and mutations [20]. Moreover, the proportion of nsLTPs in each subfamily indicated that Type I seemed to have contracted while Type II expanded in S. bicolor and Z. mays compared with 4 other monocots (Additional file 4). The gene retentions and losses might be associated with the related functions during plant evolution [20, 25].
The intron–exon pattern carries the imprint of the evolution of a gene family [26, 27]. In this study, the gene structures of SinsLTPs were highly conserved within each subgroup. Besides, the number of introns of SinsLTPs varied from 0 to 1, and no intron was found in Type II and Type IV genes (Fig. 2). Our result showed some differences from other studies, which demonstrated the generality that some nsLTPs in Type IV contained introns [17, 18, 28]. As intron loss events have been considered the main cause for the formation of new nsLTP types, and contributed to the formation of novel genes within the specific gene subgroups [28], Type IV nsLTP genes in S. italica might have evolved with no introns contained compared with the nsLTPs in other plants. Previous studies have indicated that the nsLTP family evolved in early diverged land plants, and during land plant evolution, novel types of nsLTPs generated to help plants adapt to environmental changes on land gradually [15, 28].
Like other plant nsLTPs, the nsLTPs identified in S. italica showed the presence of 8CM in highly conserved positions (Fig. 3, Table 1). In previous studies, the properties of the amino acid may determine the Cys pairing style, thus influencing the overall folding of nsLTPs [18, 28]. Generally, a hydrophilic amino acid existed separating the Cys5 and Cys6 of nsLTP1, whereas a hydrophobic residue was present in the Cys5XCys6 motif of nsLTP2 [18, 28]. In our study, the Cys5XCys6 of Type I nsLTPs harbored a hydrophilic residue in the central position, while the other subfamilies contained a hydrophobic residue in the same position. Among the five hydrophobic residues existed in the Cys5XCys6 motif, Leu was present most frequently (64.52%), and this result is consistent with previous studies [17, 18, 20].
It has been recognized that gene duplication plays a critical role in the genesis of evolutionary novelty and complexity [29, 30]. To elucidate the expanded mechanism of the nsLTP gene family in S. italica, gene duplication events were investigated in this study (Fig. 4, Table 2). We identified 5 duplicated SinsLTP gene pairs, and the duplication events were unevenly distributed across the SinsLTP subfamilies. The preferential lineage-specific expansion of Type I and Type IV subfamilies in S. italica may be associated with the expansion of the nsLTP family. Besides, the Ka/Ks ratios for 4 duplicated SinsLTPs were less than 1 (Table 2), indicating that the SinsLTP members mainly experienced purifying selection with limited functional divergence [20, 25], which was supported by their expression profiles (Additional file 9). The 2 paired genes (SinsLTP3/SinsLTP5 and SinsLTP33/SinsLTP34) shared similar expression patterns in response to drought, salt, and cold stress. These results indicated that these duplicated genes might have retained some essential functions during subsequent evolution; indeed, most duplicated plant genes are known to have similar evolutionary fates [20, 31,32,33]. It is possible that the regulatory regions, upstream of the gene, have been duplicated along with the coding region of sinsLTPs, resulting in a similar expression pattern between the duplicated genes [34,35,36]. Meanwhile, the results in our study indicated that both tandem and segmental duplication events contributed to the expansion of the nsLTP family in S. italica (Fig. 4, Table 2), and most of the nsLTP duplication events in S. italica might have occurred fewer than 36.01 MYA.
Of plants with sequenced genomes, S. italica and S. viridis are the closest relatives, together with sorghum and maize, they all belong to Panicoideae subfamily, and are suited for studies of C4 evolution and comparative grass genomics. In the present study, Sequence comparison of SinsLTP genes with other grasses like foxtail millet, sorghum, maize, as well as the two graminaceous model, rice and B. distachyon were performed and the distribution of orthologous nsLTP genes were displayed (Fig. 5). The result demonstrated that 41, 13, 10, 4, and 1 SinsLTPs had orthologs in S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon, respectively (Fig. 5, Additional file 7), taking the evolutionary tree (Additional file 5) constructed into consideration, S. italica and S. viridis nsLTPs were phylogenetically closely related compared with the nsLTPs in other grass crops, which was in accordance with expectations [5]. Besides, all Ka/Ks ratios calculated indicated the functional divergence within the nsLTP orthologous genes was limited.
As for multigene families, gene expression analysis often provides useful clues for function prediction. The tissue-specific expression patterns of SinsLTPs obtained from RNA-seq data (Fig. 7) and qRT-PCR (Fig. 8) indicated their important roles in performing diverse developmental and physiological functions. Among them, Type V nsLTPs expressed primarily in the vascular bundles, and they were deduced to be involved in signal transduction [19, 20]. Besides, Type VI nsLTPs showed flower-specific expression pattern in S. italica, indicating that Type VI members play an inportant role in flower development [37]. Moreover, foxtail millet has been studied as a model to understand drought, salt, and cold tolerance in plants, and nsLTP genes identified in various plant species have been proven to play crucial roles in abiotic stress response [38,39,40,41,42]. With the goal of identifying candidate abiotic stress-responsive SinsLTP genes, the analysis of expression profiles of selected SinsLTP genes was performed in the current study. As shown in Fig. 9, all the 10 selected SinsLTPs were responsive to drought, salt, and cold stress. Among them, SinsLTP40 is orthologous to OsLTPL159 [40], SinsLTP33 and SinsLTP34 are orthologous to LTP3 [41], and SinsLTP42 is orthologous to OsDIL [42], SinsLTP2, SinsLTP3, and SinsLTP5 are orthologous to DIR1 [43]. These genes mentioned above have been reported to play a role in defense signaling. Additionally, in terms of the promoter elements identified in the 10 SinsLTPs (Fig. 6), all the genes contained the regulatory elements responsive to stress or hormone, which is in line with their expression pattern to stress treatment (Fig. 9). These genes showed responsive to abiotic stress, and can be selected as candidate genes for further characterization in their functional involvement in plant resistance to abiotic stress.
Conclusions
In summary, this study identified 45 nsLTPs in foxtail millet, and comprehensively analyzed the important features of the gene family such as phylogenetic classification, expansion pattern, and expression profile. The present study deepened our understanding of the molecular evolution and expansion pattern of the nsLTP family in foxtail millet, and provided candidate genes for accelerating the genetic improvement of crops.
Materials and methods
Genomic identification of non-specific lipid transfer proteins
The genomic sequences of S. italica, S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov). The Arabidopsis nsLTP amino acid sequences were obtained from the Arabidopsis Information Resource (TAIR) (http://www.arabidopsis.org) and were used as queries by searching against the above-mentioned sequences using the BLASTP program with default parameters [44]. Then, these putative sequences were further verified to contain the conserved LTP domain using the Conserved Domain Database (CDD) program (https://www.ncbi.nlm.nih.gov/cdd). Afterwards, the candidate nsLTP sequences were manually screened step by step as described by previous studies [18, 20], and the rest of the nsLTPs were finally confirmed and used for the following analysis.
Phylogenetic classification and structural analysis
A multiple sequence alignment of the nsLTP sequences was generated using the ClustalW program [45]. Then, the neighbor joining (NJ) phylogenetic trees were constructed by MEGA7 with 1000 bootstrap iterations [46]. The alternatively spliced forms of S. italica nsLTPs were obtained from the Phytozome database, and the genomic schematic diagrams of the nsLTPs were visualized using the GSDS tool (http://gsds.cbi.pku.edu.cn/). Sequence logos of the conserved nsLTP subdomains were generated with the WebLogo program (http://weblogo.berkeley.edu/). Primary and secondary protein structures were predicted with ProtParam (http://web.expasy.org/protparam/) and SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa%20_sopma.html).
Chromosomal mapping and gene duplications
The chromosomal location information of the nsLTPs from S. italica, S. viridis, S. bicolor, Z. mays, O. sativa, and B. distachyon were extracted from the Phytozome database. Duplicated gene pairs were searched via BLASTP and phylogenetic analysis according to the previous report [47]. Briefly, the length of aligned sequence cover was > 80% of the longer gene, and the identity of the aligned regions was > 80%. Besides, only one duplication event was counted for tightly linked genes. The chromosomal distribution images of the SinsLTPs were generated using the MapInspect software (http://mapinspect.software.informer.com), and the segmental and tandem duplication events were defined based on the chromosomal locations of the genes. The orthologous nsLTP genes between S. italica and other monocots were plotted with the Circos program [48].
The evolutionary rates, Ka (non-synonymous substitution rate) and Ks (synonymous substitution rate) were estimated using the KaKs_Calculator package [49], and the Ka/Ks ratio was calculated to assess the selection pressure for each duplicated gene pair. Time (million years ago, MYA) of divergence of duplicated SinsLTPs was estimated using the formula “t = Ks/2r”, and a neutral substitution rate (r) of 6.5 × 10−9 was used in the current study [6].
Promoter analysis
The promoter sequences comprising 2000 bp of the upstream regions of SinsLTPs were extracted from the Phytozome database. Potential responsive regulatory elements of the extracted sequences were predicted with the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [50]. The distribution of the responsive regulatory elements was visualized using the TBtools software (https://github.com/CJChen/TBtools) [51].
Tissue-specific expression profile of SinsLTPs using RNA-seq data
Publicly available RNA-seq data of foxtail millet were downloaded from the NCBI-SRA database (https://www.ncbi.nlm.nih.gov/). Each RNA-seq sample had a clear annotation and its corresponding biological replicates. The RNA-seq data were derived from 7 tissues in S. italica (SRX13556037-SRX13556057), and high-quality RNA-seq data were obtained using the Trimmomatic software [52]. After that, the RNA-Seq reads were mapped to the reference S. italica genome, and the gene expression data were calculated using the pipeline of HISAT, StringTie, and Ballgown [53]. The expression level was log-transformed via the log2 (FPKM+1) function by using the values of fragments per kilobase per million read (FPKM) to reduce mean–variance dependency [54]. Lastly, the median of the expression levels of replicated samples was calculated, and the expression levels were clustered using the Cluster 3.0 software [55].
Plant materials, growth conditions and stress treatments
Seeds of foxtail millet cultivar Yugu No.1 obtained from Institute of Crop Sciences, Chinese Academy of Agricultural Sciences were cultivated in a growth chamber at controlled conditions (28 °C day/ 23 °C night, 14 h light/10 h dark). For tissue specific expression pattern anslysis, roots, stems, and leaves of 21-day-old seedlings were harvested. For stress treatments, 21-day-old seedlings were exposed to 250 mM NaCl (salinity), 20% PEG 6000 (dehydration) and 4 °C temperature (cold) for 6 h (early) and 24 h (late). Unstressed plants were maintained as controls. After the treatments, seedlings were immediately frozen in liquid nitrogen and stored at -80 °C until RNA isolation.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from the collected samples using the EZNA® Plant RNA Kit (Omega Bio-tek, USA), and cDNA was prepared using HiScript®ll Q RT SuperMix for qPCR(+ gDNA wiper) Kit (Vazyme). For the quantitative real-time PCR, gene-specific primers were designed (Additional file 10) and synthesized commercially (Qinke, Beijing, China). The qRT-PCR analysis was performed in the Roche LightCycler480 Real-Time PCR system by ChamQ Universal SYBR qPCR Master Mix (Vazyme), The cDNAs were amplified over 40 cycles with an annealing temperature of 60 °C. The amounts of transcript accumulated for SinsLTP genes normalized to the internal control Actin (AF288226.1) were determined using the 2−⊿⊿Ct method [56]. Each experiment was repeated in triplicate using independent RNA samples. The expression profiles of the SinsLTPs were clustered using the Cluster 3.0 software [55].
Availability of data and materials
The Arabidopsis nsLTP amino acid sequences were obtained from the Arabidopsis information source (TAIR) database (http://www.arabidopsis.org). The genomic sequences of Setaria italica, Setaria viridis, Sorghum bicolor, Zea mays, Oryza sativa, and Brachypodium distachyon were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov). The RNA-seq data of S. italica (SRX13556037-SRX13556057) were downloaded from the NCBI-SRA database (https://www.ncbi.nlm.nih.gov/). All data used during the current study are included in this published article and its additional files or are available from the corresponding author on reasonable request.
Abbreviations
- nsLTP:
-
Non-specific Lipid Transfer Protein;
- 8CM:
-
Eight Cysteine Motif;
- NCBI:
-
National Center for Biotechnology Information;
- TAIR:
-
The Arabidopsis Information Resource;
- CDD:
-
Conserved Domain Database;
- MEGA:
-
Molecular Evolutionary Genetics Analysis;
- GSDS:
-
Gene Structure Display Server;
- Ka/Ks:
-
Non-synonymous Substitution Rate/Synonymous Substitution Rate;
- SRA:
-
Sequence Read Archive;
- FPKM:
-
Fragments per Kilobase per Million Read;
- qRT-PCR:
-
Quantitative Real-time PCR
References
Lata C, Mishra AK, Muthamilarasan M, Bonthala VS, Khan Y, Prasad M. Genome-wide investigation and expression profiling of AP2/ERF transcription factor superfamily in foxtail millet (Setaria italica L.). PLoS One. 2014;9(11):e113092.
Fan Y, Wei X, Lai D, Yang H, Feng L, Li L, Niu K, Chen L, Xiang D, Ruan J, Yan J, Cheng J. Genome-wide investigation of the GRAS transcription factor family in foxtail millet (Setaria italica L.). BMC Plant Biol. 2021;21(1):1–19.
Feng ZJ, He GH, Zheng WJ, Lu PP, Chen M, Gong YM, Ma YZ, Xu ZS. Foxtail millet NF-Y families: genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front Plant Sci. 2015;6:1142.
Zhao W, Liu YW, Zhou JM, Zhao SP, Zhang XH, Min DH. Genome-wide analysis of the lectin receptor-like kinase family in foxtail millet (Setaria italica L.). Plant Cell Tiss Org. 2016;127(2):335–46.
Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, Estep M, Feng L, Vaughn JN, Grimwood J, Jenkins J, Barry K, Lindquist E, Hellsten U, Deshpande S, Wang X, Wu X, Mitros T, Triplett J, Yang X, Ye CY, Mauro-Herrera M, Wang L, Li P, Sharma M, Sharma R, Ronald PC, Panaud O, Kellogg EA, Brutnell TP, Doust AN, Tuskan GA, Rokhsar D, Devos KM. Reference genome sequence of the model plant Setaria. Nat Biotechnol. 2012;30(6):555–61.
Puranik S, Sahu PP, Mandal SN, Parida SK, Prasad M. Comprehensive genome-wide survey, genomic constitution and expression profiling of the NAC transcription factor family in foxtail millet (Setaria italica L.). PLoS One. 2013;8(5):e64594.
Mishra AK, Muthamilarasan M, Khan Y, Parida SK, Prasad M. Genome-wide investigation and expression analyses of WD40 protein family in the model plant foxtail millet (Setaria italica L.). PLoS One. 2014;9(1):e86852.
Muthamilarasan M, Khandelwal R, Yadav CB, Bonthala VS, Khan Y, Prasad M. Identification and molecular characterization of MYB transcription factor superfamily in C4 model plant foxtail millet (Setaria italica L.). PLoS One. 2014;9(10):e109920.
Liu JM, Xu ZS, Lu PP, Li WW, Chen M, Guo CH, Ma YZ. Genome-wide investigation and expression analyses of the pentatricopeptide repeat protein gene family in foxtail millet. BMC Genomics. 2016;17(1):1–16.
Singh RK, Jaishankar J, Muthamilarasan M, Shweta S, Dangi A, Prasad M. Genome-wide analysis of heat shock proteins in C4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci Rep-UK. 2016;6(1):1–14.
Yu TF, Zhao WY, Fu JD, Liu YW, Chen M, Zhou YB, Ma YZ, Xu ZS, Xi YJ. Genome-wide analysis of CDPK family in foxtail millet and determination of SiCDPK24 functions in drought stress. Front Plant Sci. 2018;9:651.
Liu D, Cui Y, Zhao Z, Li S, Liang D, Wang C, Feng G, Wang J, Liu Z. Genome-wide identification and characterization of the BES/BZR gene family in wheat and foxtail millet. BMC Genomics. 2021;22(1):1–14.
Zhao W, Zhang LL, Xu ZS, Fu L, Pang HX, Ma YZ, Min DH. Genome-wide analysis of MADS-box genes in foxtail millet (Setaria italica L.) and functional assessment of the role of SiMADS51 in the drought stress response. Front Plant Sci. 2021;12:659474.
Kader JC. Lipid-transfer proteins in plants. Annu Rev Plant Biol. 1996;47(1):627–54.
Liu F, Zhang X, Lu C, Zeng X, Li Y, Fu D, Wu G. Non-specific lipid transfer proteins in plants: presenting new advances and an integrated functional analysis. J Exp Bot. 2015;66(19):5663–81.
Missaoui K, Gonzalez-Klein Z, Pazos-Castro D, Hernandez-Ramirez G, Garrido-Arandia M, Brini F, Diaz-Perales A, Tome-Amat J. Plant non-specific lipid transfer proteins: an overview. Plant Physiol Bioch. 2022;171:115–27.
Boutrot F, Chantret N, Gautier MF. Genome-wide analysis of the rice and Arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genomics. 2008;9(1):1–19.
Li J, Gao G, Xu K, Chen B, Yan G, Li F, Qiao J, Zhang T, Wu X. Genome-wide survey and expression analysis of the putative non-specific lipid transfer proteins in Brassica rapa L. PLoS One. 2014;9(1):e84556.
Wang HW, Hwang SG, Karuppanapandian T, Liu A, Kim W, Jang CS. Insight into the molecular evolution of non-specific lipid transfer proteins via comparative analysis between rice and sorghum. DNA Res. 2012;19(2):179–94.
Li F, Fan K, Ma F, Yue E, Bibi N, Wang M, Shen H, Hasan MM, Wang X. Genomic identification and comparative expansion analysis of the non-specific lipid transfer protein gene family in Gossypium. Sci Rep-UK. 2016;6:38948.
D’Agostino N, Buonanno M, Ayoub J, Barone A, Monti SM, Rigano MM. Identification of non-specific Lipid Transfer Protein gene family members in Solanum lycopersicum and insights into the features of Sola l 3 protein. Sci Rep-UK. 2019;9(1):1–16.
Yang Y, Li P, Liu C, Wang P, Cao P, Ye X, Li Q. Systematic analysis of the non-specific lipid transfer protein gene family in Nicotiana tabacum reveal its potential roles in stress responses. Plant Physiol Bioch. 2022;172:33–47.
Liu W, Huang D, Liu K, Hu S, Yu J, Gao G, Song S. Discovery, identification and comparative analysis of non-specific lipid transfer protein (nsLtp) family in Solanaceae. Genom Proteom Bioinf. 2010;8(4):229–37.
Pan Y, Li J, Jiao L, Li C, Zhu D, Yu J. A non-specific Setaria italica lipid transfer protein gene plays a critical role under abiotic stress. Front Plant Sci. 2016;2016(7):1752.
Li F, Guo X, Liu J, Zhou F, Liu W, Wu J, Zhang H, Cao H, Su H, Wen R. Genome-wide identification, characterization, and expression analysis of the NAC transcription factor in Chenopodium quinoa. Genes. 2019;10(7):500.
Lynch M. Intron evolution as a population-genetic process. P Nat Acad Sci. 2002;99(9):6118–23.
Del Campo EM, Casano LM, Barreno E. Evolutionary implications of intron-exon distribution and the properties and sequences of the RPL10A gene in eukaryotes. Mol Phylogenet Evol. 2013;66(3):857–67.
Edstam MM, Viitanen L, Salminen TA, Edqvist J. Evolutionary history of the non-specific lipid transfer proteins. Mol Plant. 2011;4(6):947–64.
Moore RC, Purugganan MD. The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol. 2005;8(2):122–8.
Flagel LE, Wendel JF. Gene duplication and evolutionary novelty in plants. New Phytol. 2009;183(3):557–64.
Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–5.
Adams KL. Evolution of duplicate gene expression in polyploid and hybrid plants. J Hered. 2007;98(2):136–41.
Fan K, Shen H, Bibi N, Li F, Yuan S, Wang M, Wang X. Molecular evolution and species-specific expansion of the NAP members in plants. J Integr Plant Biol. 2015;57(8):673–87.
Abdullah, Faraji S, Mehmood F, Malik HMT, Ahmed I, Heidari P, Poczai P. The GASA gene family in cacao (Theobroma cacao, Malvaceae): genome wide identification and expression analysis. Agronomy. 2021;11(7):1425.
Faraji S, Heidari P, Amouei H, Filiz E, Poczai P. Investigation and computational analysis of the sulfotransferase (SOT) gene family in potato (Solanum tuberosum): insights into sulfur adjustment for proper development and stimuli responses. Plants. 2021;10(12):2597.
Heidari P, Faraji S, Ahmadizadeh M, Ahmar S, Mora-Poblete F. New insights into structure and function of TIFY genes in Zea mays and Solanum lycopersicum: a genome-wide comprehensive analysis. Front Genet. 2021;12:534.
Liu F, Xiong X, Wu L, Fu D, Hayward A, Zeng X, Cao Y, Wu Y, Li Y, Wu G. BraLTP1, a lipid transfer protein gene involved in epicuticular wax deposition, cell proliferation and flower development in Brassica napus. PLoS One. 2014;9(10):e110272.
Gangadhar BH, Sajeesh K, Venkatesh J, Baskar V, Abhinandan K, Yu JW, Prasad R, Mishra RK. Enhanced tolerance of transgenic potato plants over-expressing non-specific lipid transfer protein-1 (StnsLTP1) against multiple abiotic stresses. Front Plant Sci. 2016;7:1228.
Hairat S, Baranwal VK, Khurana P. Identification of Triticum aestivum nsLTPs and functional validation of two members in development and stress mitigation roles. Plant Physiol Bioch. 2018;130:418–30.
Zhao J, Wang S, Qin J, Sun C, Liu F. The lipid transfer protein Os LTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol J. 2020;18(3):756–69.
Guo L, Yang H, Zhang X, Yang S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J Exp Bot. 2013;64(6):1755–67.
Guo C, Ge X, Ma H. The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol Biol. 2013;82(3):239–53.
Champigny MJ, Isaacs M, Carella P, Faubert J, Fobert PR, Cameron RK. Long distance movement of DIR1 and investigation of the role of DIR1-like during systemic acquired resistance in Arabidopsis. Front Plant Sci. 2013;4:230.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–80.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Liu W, Li W, He Q, Daud MK, Chen J, Zhu S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS One. 2014;9(6):e98189.
Krzywinski MI, Schein JE, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45.
Zhang Z, Li J, Zhao XQ, Wang J, Wong GKS, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genom Proteom Bioinf. 2006;4(4):259–63.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Chen C, Xia R, Chen H, He Y. Toolkit for biologists integrating various HTS-data handling tools with a user-friendly interface. BioRxiv Preprint. 2018;289660(10.1101):289660.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT. StringTie and Ballgown Nat Protoc. 2016;11(9):1650–67.
Ma S, Ding Z, Li P. Maize network analysis revealed gene modules involved in development, nutrients utilization, metabolism, and stress response. BMC Plant Biol. 2017;17(1):1–17.
De Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20(9):1453–4.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−⊿⊿Ct method. Methods. 2001;25(4):402–8.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Applied Basic Research Project of Shanxi Province (201801D221239), the Doctoral Scientific Research Foundation of Shanxi Datong University (2017-B-18), the Program for Scientific and Technological Innovation of Higher Education Institutions in Shanxi (2021L379), and the Research Project of Industry-Education Integration of Shanxi Datong University (2019CXK16). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
FL conceived and designed research. FL, KF, and PL contributed new reagents or analytical tools. FL, KF, XG, and KZ analyzed data. FL, XG, and JL conducted experiments. FL, KF, and XG contributed to the writing of the manuscript. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental studies on plants were complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The structural analysis of nsLTPs identified in S. italica.
Additional file 2.
The nsLTPs identified in S. viridis, S. bicolor, Z. mays, O. sativa and B. distachyon in this study.
Additional file 3.
The classification and gene structures of nsLTPs in S. italica.
Additional file 4.
The percentage of members in each nsLTP subfamily in S. italica (a), S. viridis (b), S. bicolor (c), Z. mays (d), O. sativa (e) and B. distachyon (f).
Additional file 5.
Phylogenetic relationships of the nsLTPs in S. italica, S. viridis, S. bicolor, Z. mays, O. sativa, B. distachyon and Arabidopsis. Amino acid sequences were aligned using ClustalW and the neighbor-joining tree was generated through the MEGA7 program. The subfamilies are labeled and denoted by different colors and the numbers at nodes represent bootstrap support values from 1000 replicates.
Additional file 6.
Genomic locations of nsLTPs in S. italica.
Additional file 7.
Ka/Ks analysis for orthologous nsLTP gene pairs between between S. italica and S. viridis, S. bicolor, Z. mays, O. sativa and B. distachyon.
Additional file 8.
Number of the responsive-regulatory elements in the promoter regions of SinsLTPs.
Additional file 9.
Expression patterns of some duplicated SinsLTP genes after drought (a, b), salt (c, d) and cold stress treatment (e, f).
Additional file 10.
PCR primers used for qRT-PCR in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, F., Fan, K., Guo, X. et al. Genome-wide identification, molecular evolution and expression analysis of the non-specific lipid transfer protein (nsLTP) family in Setaria italica. BMC Plant Biol 22, 547 (2022). https://doi.org/10.1186/s12870-022-03921-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03921-1