Abstract
Backgrounds
C2H2-type zinc finger protein (ZFPs) form a relatively large family of transcriptional regulators in plants, and play many roles in plant growth, development, and stress response. However, the comprehensive analysis of C2H2 ZFPs in cucumber (CsZFPs) and their regulation function in cucumber are still lacking.
Results
In the current study, the whole genome identification and characterization of CsZFPs, including the gene structure, genome localization, phylogenetic relationship, and gene expression were performed. Functional analysis of 4 selected genes by transient transformation were also conducted. A total of 129 full-length CsZFPs were identified, which could be classified into four groups according to the phylogenetic analysis. The 129 CsZFPs unequally distributed on 7 chromosomes. Promoter cis-element analysis showed that the CsZFPs might involve in the regulation of phytohormone and/or abiotic stress response, and 93 CsZFPs were predicted to be targeted by one to 20 miRNAs. Moreover, the subcellular localization analysis indicated that 10 tested CsZFPs located in the nucleus and the transcriptome profiling analysis of CsZFPs demonstrated that these genes are involved in root and floral development, pollination and fruit spine. Furthermore, the transient overexpression of Csa1G085390 and Csa7G071440 into Nicotiana benthamiana plants revealed that they could decrease and induce leave necrosis in response to pathogen attack, respectively, and they could enhance salt and drought stresses through the initial induction of H2O2. In addition, Csa4G642460 and Csa6G303740 could induce cell death after 5 days transformation.
Conclusions
The identification and function analysis of CsZFPs demonstrated that some key individual CsZFPs might play essential roles in response to biotic and abiotic stresses. These results could lay the foundation for understanding the role of CsZFPs in cucumber development for future genetic engineering studies.
Similar content being viewed by others
Background
Plants frequently suffer from various biotic and abiotic stresses which adversely affect plant growth and development [1, 2], while transcription factor (TFs) are important regulators that involved in various biological and environmental stress processes [3]. Among them, zinc finger proteins (ZFPs) account for a relatively large family of eukaryotic transcription factors [4]. In Arabidopsis and rice, nearly 15 and 13% transcription factors are ZFPs (176 and 189, respectively) [5, 6]. According to the number and order of the cysteine (Cys, C) and histidine (His, H) residues, the ZFPs could be classified into several subgroups, such as C2H2, C2HC, C2HC5, C3HC4, CCCH, C4, C4HC3, C6, and C8 [7, 8]. Among them, C2H2-type zinc finger proteins (C2H2-ZFPs) are classical ones which have been widely studied. The C2H2-type of zinc finger proteins, also referred to as the TFIIIA type zinc finger that contains two Cys and two His residues in the zinc finger domain, are described as CX2-4CX3FX5LX2HX3-5H, which form the conservative and best-characterized DNA-binding motif [9, 10]. In plants, C2H2 zinc finger proteins have the similar structures which differ from those in other eukaryotic organisms. The highly conserved QALGGH motif in the zinc finger helices could be detected in C2H2-ZFPs. According to the number and patterns of zinc fingers, the C2H2-ZFPs could be categorized into three groups, including triple-C2H2 (tC2H2) zinc fingers, multiple-adjacent-C2H2 (maC2H2) zinc fingers, and separated-paired-C2H2 (spC2H2) zinc fingers [11], and furthermore, each group could be divided into various subgroups [5]. In addition, C2H2-ZFPs display a wide range of structure and functions, from DNA or RNA binding to the involvement in protein-protein interactions [5], which fulfill their function as key transcriptional regulators to play important roles in adverse stresses, such as drought, low-temperature, salt, and oxidative stresses [10].
Genome identification and functional analysis of C2H2-ZFPs have been studied in several plants and whilst some stress related C2H2-ZFPs have been characterized, including 176 members in the Arabidopsis genome [5], 189 in rice (Oryza sativa L.) [6], 122 in wheat (Triticum aestivum L.) [7], 301 in Brassica rapa L. [12], 109 in Populus trichocarpa [13], 189 in foxtail millet (Setaria italica L.) [9], 321 in soybean (Glycine max L.) [14], and 211 in maize (Zea mays L.) [15], suggesting the C2H2-ZFPs are extensively involved in plant growth, development, and defense responses [16,17,18,19]. For example, in Arabidopsis, AZF1 (At5g67450) and AZF2 (At3g19580) could function as transcriptional repressors to repress the expression of osmotic stress- and ABA-repressive genes to negatively affect plant growth [20]. ZAT18 (At3g53600) was transcriptionally induced by drought stress, and overexpression of ZAT18 improved drought tolerance while mutation of ZAT18 resulted in decreased plant tolerance to drought stress [19]. Moreover, high expression of ZAT7 in roots tissues could positively improve salt stress tolerance by activating defense genes [21]. Meanwhile, the Arabidopsis thaliana SUPERMAN (SUP) protein, one of the best studied C2H2, has been shown to regulate carpel numbers and ovule development [22], and the SUP orthologous in cucumber (CsSUP, Csa3G141870) has been identified and studied which might function importantly in the female flower buds and ovules development [23]. In Poncirus trifoliata (L.) Raf., the expression of PtrZPT2–1 was strongly induced by cold, drought, and salt stresses and overexpression of PtrZPT2–1 in tobacco enhanced its cold, drought, and salt resistance through increasing the levels of osmotic regulatory solutes and decreasing the accumulation of H2O2 [24]. In soybean, the transcription of a C2H2-type zinc finger protein, GmSCOF-1, was induced by low temperature and abscisic acid (ABA) treatments, but not by dehydration or high salinity [25]. Furthermore, overexpression of GmSCOF-1 increases expression levels of cold responsive genes to improve cold tolerance in transgenic plants. These results indicate that C2H2-ZFPs function as transcriptional activators or repressors and control the transcriptional levels of downstream genes under different stress signal transduction pathways. However, the comprehensive identification and stress related function analysis of C2H2-ZFPs in cucumber (Cucumis sativus L.) remain elusive.
Cucumber is an important vegetable crop cultivated worldwide [26]. The quality and yield of cucumber are easily affected by adverse environmental stresses, such as high drought, salinity, and nutrient deficiency, since its root largely spreads in shallow soil, [27]. In the past few years, the dramatically developed genome information and the completed genome sequencing of cucumber provide the opportunity for us to identify the interesting protein families which function importantly in various stresses at the genome-wide level [28]. Thus, to explore the function of cucumber CsZFP (Cucumissativuszinc finger protein) genes in response to biotic/abiotic stresses, we performed bioinformatics analysis to identify the main CsZFP members with further gene structure, genome localization, and phylogenetic relationship analysis. Then, the qRT-PCR analysis was conducted to abiotic stressed tissues, such as cold, salt, heat, and drought treated cucumber leaves and roots. Furthermore, the transient overexpression of selected CsZFPs into tobacco plants was performed and their subcellular localization, defense activation, and possible roles in response to drought, salt, and pathogen infection stresses was proceeded. This study provided clues for the functional characterization of CsZFPs and their potential application in cucumber molecular breeding related to stress tolerance.
Results
Genome-wide identification of ZFP genes in cucumber
Using multiple plants’ ZFP protein sequences as searching queries, a total of 144 non-redundant CsZFP sequences were identified from cucumber genome as potential aimed genes. The non-representative alternative splicing forms origin from same gene locus were treated as same gene and finally 129 CsZFP genes were retrieved from cucumber genome database (Table 1). SMART and Pfam analysis confirmed the presence of 235 C2H2 zinc finger domain in these 129 CsZFP genes. In contrast, the number of CsZFP was less than that was identified in Arabidopsis (176) [5], rice (189) [6], Brassica rapa L. (301) [12], foxtail mille (189) [9], soybean [14], and maize (211) [15], but more than that in wheat (122) [7], Populus trichocarpa [13]. Hence, the numbers of the ZFP gene family much differ among different species.
Furthermore, the detailed physiological and biochemical information of these CsZFPs, including gene ID, length of amino acid (aa), molecular masses (MW), isoelectric point (pI), and predicted subcellular localization of each proteins were shown in Table 1. Independently, the amino acid length of each CsZFP differs from 84 aa (Csa3G731900) to 1608 aa (Csa1G008490) and the average length is 338 aa. The MWs of CsZFPs ranged from 9.93 (Csa3G731900) to 181.16 (Csa1G008490) kDa with the average 37.59. The pI values, an important physicochemical property of proteins, ranged from 9.96 (Csa6G312040) to 4.69 (Csa5G625970, Csa6G150550), respectively. Then, in silico prediction suggested the most majority of CsZFPs (99) with a subcellular localization at nucleus, 28 CsZFPs located at chloroplast, one located at cell wall (Csa7G406990), and one located at endoplasmic reticulum (Csa2G292740), which was consistent with the prediction of ZFPs in maize that most were located at the nucleus except several were at the chloroplast, cytosol, or mitochondria [15].
Chromosomal localization, gene duplication, and phylogenetic analysis of CsZFP genes
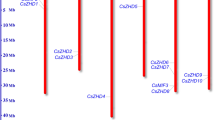
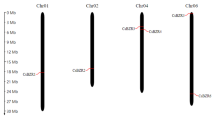
To get the global view about the distribution of C2H2-ZFP genes in cucumber, the chromosomal location of 129 CsZFPs on 7 chromosomes was shown in Fig. 1, that 17, 15, 37, 11, 21, 16, and 12 CsZFPs were presented on Chr1 (Chromosome 1), Chr2, Chr3, Chr4, Chr5, Chr6, and Chr7, respectively. Among them, Chr3 contained a maximum number of CsZFP genes (37) and Chr4 contained the minimal number of CsZFP genes (11), which demonstrated CsZFPs were widely but unevenly distributed across 7 cucumber chromosomes. In addition, gene duplication analysis revealed that no segmental and tandem duplication took place during the evolutionary process of cucumber genome. Comparing with Arabidopsis (176) and rice (189), the smaller number of CsZFPs might be attributed to the absence of segmental duplication events in cucumber genome [5, 6].
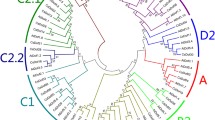
To evaluate the phylogenetic relationship of CsZFPs, the amino acid sequences were used to construct an un-rooted phylogenetic tree through multiple sequence alignment. According to the plant-specific amino acid residues and distances between conserved motif sequence as described in soybean and Populus trichocarpa [13, 14], the C2H2-ZF domains in CsZFPs can be classified into five main types, as shown in Additional file 1: Table S1: (i) Domains contained plant-specific conserved amino acid sequence “QALGGH” and a conserved spacing “X2-C-X2-C-X7-QALGGH-X3-H” were designated as Q-type, where X is any amino acid and the number indicates the consensus spacing between the conserved amino acid residues. (ii) Domains with certain modifications of the Q-type ZF, including 1 to 5 degraded amino acids in the motif “QALGGH” and certain modifications in the spacing between the two cysteines (Cys, C) and two histidines (His, H), were classified as the M-type; Based on conserved motif sequence and conserved spacing of amino acids, M-type ZF were further divided into M1 to M13 subtypes. (iii) The C2H2-ZFPs domains with more than 12 (Z1-type) and less than 12 (Z2-type) amino acid between the second Cys and the first His were classified as Z-type. (iv) The C2H2-ZFPs domains with 12 amino acid between the second Cys and the first His were classified as the C-type. (v) The D-type did not contain the second His in the C2H2-ZF region.
Furthermore, based on the types and numbers of C2H2-ZF domains, CsZFPs were further classified into nine groups as shown in Fig. 2a and Additional file 1: Table S2: (1) Cs1Q (the 1st group of Q-type CsZFP, containing 31 members) and Cs2Q (the 2nd group, containing 12 members), which contains one and two conserved motif “QALGGH” in their protein sequences, respectively. (2) Cs1M group contain two to five M-type conserved motif sequence, and 24 CsZFPs were classified into Cs1M. (3) Cs(2–5) M group contain two to five M-type conserved motif sequence, and 4 members were classified into this group. (4) Cs1C (the 1st group of C-type CsZFP) contains one C-type conserved motif sequence. And 14 CsZFPs were classified into Cs1C. (5) Cs2Mix (mixture), Cs3Mix, Cs4Mix, and Cs8Mix groups contain mixture types of conserved motif sequence. And 9, 29, 5, and 1 CsZFPs could be clustered into Cs2Mix, Cs3Mix, Cs4Mix, and Cs8Mix, respectively. In summary, Cs1Q contained the largest number of the C2H2-ZF genes (31), followed by Cs3Mix (29) and Cs1M (24), and only one gene (Csa5G623900) belonged to Cs8Mix.
The phylogenetic tree, exon and intron distribution and motif analysis of CsZFPs. a The phylogenetic analysis of CsZFP protein sequences. The MEGA7 was used to do the phylogenetic analysis with the neighbor-joining method. b Schematic diagrams of the gene structures of CsZFPs. c The motif compositions and motif logos of CsZFPs in corresponding to the phylogenetic tree. The figure was drawn by Junliang Yin
Gene structure and motifs assay
The divergence of gene structure (e.g. the exon-intron and conserved motifs diversity) provides the potential insights of gene function of evolution [29]. As shown in Fig. 2b, the number of introns varied from 0 (Csa7G432050) to 14 (Csa1G008490). In addition, the most closely related members in same groups shared similar exon/intron structure in terms of intron number, exon length and/or location (Fig. 2b). For example, most CsZFPs in Cs1Q group have none introns, whereas only the Csa2G404810 contained two introns and the same gene structure could be observed in Cs2Q group. While the largest number of introns could be found in Cs2Mix, Cs3Mix, Cs4Mix, and Cs8Mix groups with striking distinctions. These results showed that the gene structure changes of the CsZFPs might play important role in their function divergences during the evolution process.
The MEME program was used to predict putative conversed motifs in the CsZFP sequences of cucumber, and as showed in Fig. 2c, 20 distinct motifs were identified. Generally, motif 1, 4 and motif 5, 6, 14 formed the two typical C2H2 domains, and one or both of them were found to be existed in CsZFP proteins to form the conserved reigns of finger-like fold. Motif 1 contained a plant-specific conserved “QALGGH” in the zinc finger helices, which was previously classified as the Q-type zinc finger motif and was reported playing key roles in the recognition of specific DNA bases [5]. In addition, 43 out of 129 CsZFPs had the conserved “QALGGH” motif in the zinc finger helices. Motif 2, 8 and 10 formed the SprT-like zinc ribbon domain, which was function as Zn+ binding zone (Additional file 1: Table S1, 2, Additional file 2: Fig. S1). Finally, most closely related members in the CsZFP phylogenetic tree had similar motif compositions, suggesting functional similarities among the CsZFP proteins within the same group.
Prediction of cis-acting regulatory elements
To better understand the function of CsZFPs, especilally under biotic and abiotic stresses conditions, 1.5 kb non-coding sequences upstream of the CsZFPs translation start site (TSS), which belong to the promoter region, were adopted to predict the cis-regulatory elements within PlantCARE database (Fig. 3 and Additional file 1: Table S3, 4). Results showed that the promoter sequences of CsZFPs have various cis-regulatory elements, such as CAAT-box, CCAAT-box, and TATA-box, which existed extensively in all the promoter regions, suggesting the CsZFPs involved in the regulation of plant growth and development. Moreover, the cis-regulatory elements response to biotic and abiotic stresses and phytohormone were also detected in corresponding CsZFPs, including the ABRE (abscisic acid-responsive element), CGTCA-motif, TATC-box, TGACG-motif (elements responsive to MeJA), and TCA-element (salicylic acid-responsive element), which were response to phytohormone, and AE-box, G-box, MBS and LTR etc., which were related to biotic and abiotic conditions. In addition, according to the expression level of CsZFPs during growth and development process and in response to stresses, the CsZFPs could be classified into four groups. Moreover, these group 1 CsZFPs with higher expressing levels during growth and development stages, as well as biotic and abiotic stresses, commonly contained more cis-regulatory elements in their promoter regions. The opposite results were found in group 4 (Fig. 3), that lower expressed CsZFPs contained less cis-elements, demonstrating the number of cis-regulatory elements play important role in regulating the expression of CsZFPs.
The miRNAs targeting CsZFPs transcripts
The miRNAs are highly conserved non-coding RNA which could regulate the translation of their target mRNAs [30]. Therefore, in order to get insights to the post-transcriptional adjustment of CsZFPs, the potential miRNAs targeting CsZFPs transcripts were predicted. As shown in Fig. 3 and Additional file 1: Table S5, many miRNAs could target to a total of 93 CsZFPs transcripts. The number of miRNAs target to corresponding CsZFPs transcripts ranges from 1 to 20. For example, the transcript of Csa2G382580, nine miRNAs target to it, including csa-miR-n10, csa-miRn8-3p, csa-novel-23, miRn6, PC-3p-318,270, PC-5p-100,383, PC-5p-274,347, csa-miRn6-3p, and PC-3p-45,338 with cleavage and/or translation inhibition. While Csa2G354820 was only targeted by csa-miR-n01 with translation inhibition.
Location detection of typical CsZFPs
The in silico prediction indicated that most CsZFPs located at the nucleus. In order to verify this result and reveal CsZFPs function patterns, 10 typical CsZFPs from different groups were selected to do the subcellular localization analysis. As shown in Fig. 4, all the tested CsZFPs, including Cas6G502050, Cas6G303740, Cas4G646130, Cas4G642460, Cas1G085390, Cas7G071440, Cas2G354820, Cas2G033890, Cas5G180920, and Csa6G446540, located at the nucleus, which were consistent with the prediction, suggesting the CsZFPs might contribute their biological roles in cucumber nucleus [31]. However, besides activate or suppress gene transcription in nucleus, zinc finger domain containing proteins can also involve in the regulation of protein-protein interaction, histone methylation, double stand RNA binding [32]. Thus, the precise functions of these CsZFPs need to be further experimentally explored.
Expression profiling of CsZFP genes in cucumber different growth and developmental stages
In attempt to detect the role of CsZFPs during the cucumber different growth and developmental stages, the expression levels of each CsZFPs were analyzed by the available RNA-seq transcriptomic data. According to the log2-transformed FPKM (fragments per kilobase of transcript per million fragments mapped) values of the dataset, the different expression pattern of CsZFPs were shown in heatmap figure (Fig. 5 and Additional file 1: Table S6). These CsZFPs expressed in different tissues, mainly including root, cotyledon, hypocotyl, stem, leaves, flower, seed. According to their expression levels, these CsZFPs could be clustered into four groups that the genes in group 4 show none or low expression level in all the tissues, but the expression level of genes in group 1 were much higher and significantly responsive to different treatments (Fig. 3). For instance, after 4 days of planting, the expression level of CsZFPs show different patterns in three root developmental regions, such as differentiation zone, elongation zone and meristematic zone. Among them, the expression levels of Csa1G657510 and Csa6G150550 were highly induced in the three parts, in contrast, 26 of CsZFPs showed none expression. While, two genes (Csa1G132120 and Csa5G548160) were abundantly expressed in root differentiation zone, but were not in meristematic zone. In cotyledon and hypocotyl of 4 weeks seedlings, it is notable that Csa1G657510 was significantly highly expressed. In addition, the expression levels of these genes were different in stem, stalk, and pedicle. Gene expression values for Csa1G657510 were relatively higher in stem (maximum FPKM 626.52), in stalk (maximum FPKM 2958.85), and in pedicle (maximum FPKM 1661.78), respectively. Among the old and young leaves, the FPKM expression level of each CsZFP ranges from 0 (Csa1G524710) to 603.39 (Csa1G657510) and 0 (Csa2G190780) to 362.59 (Csa1G657510), respectively. In the fruit and fruit peels, Csa6G303740 and Csa7G406990 showed high expression levels in fruit, but not in fruit peels. All these results shown that the different expression of CsZFPs might function importantly and diversely in various tissues.
The RNA-seq analysis of different expression pattern of CsZFPs within heatmap pattern in response cucumber growth, development and biotic and abiotic stresses. The log2-transformed FPKM (Fragments Per Kilobase of transcript per Million fragments mapped) values of the dataset were used to do the expression analysis. The figure was drew by Junliang Yin
Except the detection of expression level of each CsZFP in different tissues, their functions were also analyzed during tissue developmental stages, such as different time points after pollination, fruit spine on different fruits. After pollination of 28, 37, 45, and 60 days, most of the CsZFPs showed increasing expression patterns with time development. Of them, the expression level of Csa6G150550 increased significantly (15.41 to 335.51) from 28 to 60 days after pollination. While the Csa7G450800 shown opposite expressing pattern that its expressing level decreased from day 28 (3.31) to day 60 (1.12). Moreover, the expression level of CsZFPs in fruit spine on fruits of 0.5 and 1.6 cm long showed distinctive expressing pattern. The expression levels of Csa1G560650 and Csa2G139840 showed none significant difference, but again most of them showed increasing patterns with the development of fruit.
Expression analyses of the CsZFPs under biotic and abiotic stresses
To gain insights into the expression of CsZFPs in response to biotic and abiotic stresses, firstly the log2-transformed FPKM values of the RNA-seq transcriptomic dataset were mined as shown in Fig. 5. Within the gibberellin (GA) treatment, most of the CsZFPs shown none significant expressing changes in 12 h treatment, but the expression level of Csa4G642460 increased strongly from 253.87 to 647, as well as other CsZFPs (Csa4G166950 and Csa7G450800) which showed similar expression pattern in response to GA treatment. While, Csa6G446540, Csa5G623900, Csa3G748810, and Csa3G098560 showed decrease patterns in their respective transcription levels. In response to salt stress, Csa1G600970, Csa2G404810, and Csa7G419600 were highly induced. Moreover, in response to biotic stresses, most of the CsZFPs showed increase or decrease expressing patterns in 4 weeks old leaf infected with downy mildew, indicating these CsZFPs function differently in response to downy mildew. To sum up, the CsZFPs from group 1, 2, and 3 likely play more important roles during cucumber growth and development, and responses to biotic and abiotic stresses.
Furthermore, nine CsZFPs, which showed relatively higher expression levels and/or had orthologous been functional studied in other plants (e.g. Arabidopsis, rice) were selected to do the qRT-PCR analysis of their expression patterns in responding to salt, drought, heat, and cold stresses (Fig. 6). Seven CsZFPs were significantly induced in 3 d salt treatment and two CsZFPs (Csa1G085390 and Csa6G303740) expressed highly within 6 d salt treatment comparing to the control. The most remarkable case is the gene Csa2G354820 which was induced by more than 15-fold under salt treatment at 3 d. Then in the case of drought stress, the expression levels of Csa1G085390, Csa6G303740, and Csa7G071440 culminated to the peak with 7.5-fold changes with 6 d, 9 d and 9 d drought treatment, respectively. While the others showed lower expression in response to drought stress. In response to heat stress, eight CsZFPs were up regulated with 3 d or 6 d treatment and then decrease to the lower expression level in 9 d treatment. The highest induction was recorded for Csa6G446540 which showed > 20-fold up-regulation at 3 d comparing to the control. However, Csa7G041440 was progressively inducted along with the time and reached to 15-fold at 9 d comparing to the control. In the case of cold stress, two CsZFPs (Csa1G085390 and Csa4G646130) were significantly induced in 6 d treatment with 7.5 and 14-fold changes, the others were slightly induced. The results showed that all the nine CsZFPs were responsive to these four abiotic stresses.
Relative expression profiles of CsZFPs in response to salt, drought, heat, and cold stresses. The relative expression levels of the representative members of CsZFPs in three independent replications and the error bars represent standard deviation (SD). N indicates none significant difference. The figure was drew by Junliang Yin
Csa4G642460 and Csa6G303740 are cell death inducer
After 5 days transient expression of Csa4G642460 and Csa6G303740 in N. benthamiana leaves, cell death could be observed in agro-infiltration corresponding regions (Fig. 7a and b). To reveal whether the cell death is N. benthamiana specific, Csa4G642460 and Csa6G303740 were transiently expressed in Solanum lycopersicum. It was observed that Csa4G642460 and Csa6G303740 are also cell death inducers in tomato leaves (Additional file 2: Fig. S2). Cell death usually depends on plant immune system and is the outcome of variable receptors and defense signal transduction pathways. In order to verify which signaling transduction pathways involved in Csa4G642460 and Csa6G303740 regulated cell death, a series of genes which are responsible for R (resistance) protein function, such as HSP90, SGT1, and RAR2, as well as the MAPK cascade genes including MEK1 and SIPK, and WRKY3–2 transcription factor were silenced one by one through virus-induced gene silencing (VIGS) method. As shown in Fig. 7a and bs, after silencing of these genes in N. benthamiana, the cell death inducer ability of Csa4G642460 and Csa6G303740 were not abolished. In summary, the Csa4G642460 and Csa6G303740 are cell death inducer and the inducing ability does not require above R genes among the signaling transduction pathways.
Cell death analyses. Those N. benthamiana R genes (HSP90, SGT1, RAR2, MEK1, SIPK and WRKY3–2) were transiently silenced by VIGS approach. Then aCsa4G642460 and bCsa6G303740 were transiently overexpressed in leaves of silencing plants. Whilst GFP, Csa4G642460, and Csa6G303740 were parallel overexpressed in leaves of not silencing plants as control. Similar results were obtained from three independent replicates with 10 plants for each TRV construct. The photos were taken by Junliang Yin and the figure was drawn by Junliang Yin
Csa1G085390 promotes and Csa7G071440 represses pathogen colonization in N. benthamiana
Previous transient expression test showed that Csa1G085390 and Csa7G071440 are not cell death inducer. In order to further analyze the function of Csa1G085390 and Csa7G071440 in response to biotic stress. The pathogen of N. benthamiana, Phytophthora infestans (strain 88,069), was inoculated into tobacco leaves to check the phenotypes of these two transiently transgenic plants. As shown in Fig. 8a and c, the size of disease lesion in Csa7G071440 transiently expressed plants was larger than that in the negative control plant. However, the opposite phenomenon was observed in Csa1G085390 transient expression plants, indicating Csa1G085390 and Csa7G071440 function differently in response to pathogen attack. In addition, the leave lesion diameter statistical analysis supported above results (Fig. 8b and d) that the lesion diameter in Csa7G071440 transiently expressed plants was significantly larger than that of negative control plants, while that in Csa1G085390 transiently transgenic plants was significant smaller.
a Phenotype of transiently transgenic (Csa1G085390 and Csa7G071440) and negative control (pART27:GFP) plants in response to pathogen attack. b Lesion diameter of necrosis in transgenic and control plants. Three independent replicates were observed and 30 leaves were used to do the quantification analysis. ** indicates significant differences at the level of 0.01. Scale bar: 1.5 cm. The photos were taken by Junliang Yin and the figure was drawn by Junliang Yin
Csa1G085390 and Csa7G071440 enhance salt and drought tolerance through initial induction of H2O2 in N. benthamiana
Csa1G085390 and Csa7G071440 are the two typical CsZFPs which highly expressed in response to salt and drought stresses, suggesting they might play critical roles under these two stresses (Fig. 6). In order to verify this hypothesis, the performance of the transiently transgenic plants (Csa1G085390 and Csa7G071440) was analyzed under salt and drought stresses. As shown in Fig. 9a, the detached leaves of N. benthamiana grew well without any stress treatment at the normal condition (CK) within 4 h treatment. However, with 75 mmol NaCl and 5% PEG treatments, the leaves in the negative control plants become wilt and rolling. Comparing to CK condition and negative control plants, the leaves in Csa1G085390 transiently transgenic plants were healthy and did not rolling under drought stress than that in salt stress, but the Csa7G071440 transiently transgenic leaves shown resistance to both stresses. Then water loss rate in the above plants were detected (Fig. 9b), the transiently transgenic leaves exhibited decrease of water loss rate comparing with the negative control under CK condition. However, with NaCl or PEG treatments, water loss rate decreased significantly within the negative control but not in the transiently transgenic leaves, indicating the higher water loss rate under these two stresses in transiently transgenic plants which have more ability to absorb water to improve their stress resistance. As the difference in water loss rate in the leaves of transiently transgenic and control plants could be correlated with the difference in leaf stomatal aperture, thus the status of stomatal aperture was analyzed. Under normal conditions, the transiently transgenic plants exhibited a marked decrease in leaf stomatal openings (Fig. 9c). In addition, the decrescent of stomatal apertures in negative control plant was higher than that of transiently transgenic plants which was consistence with the lower water loss rate trends under both stresses (Fig. 9d). Moreover, the contents of H2O2 were evaluated by DAB staining and accurate measurements (Fig. 9e and f), respectively. Under normal conditions, the brown (indicating H2O2) areas were significantly increased within transiently transgenic plants. Under both stresses, the brown color became deeper in all plants, but the increasement in transiently transgenic plants was lower than that in negative control which is consistence with the accurate contents of H2O2 in Fig. 9e. Furthermore, the MDA contents were also measured and the lower contents of MDA in transiently transgenic plants were observed than that in negative control under both stresses (Fig. 9g). All the results indicated that the transient overexpression of Csa1G085390 and Csa7G071440 could improve both stress resistance with the initial accumulation of H2O2 through triggering the early self-defense system.
Phenotypic and physiological analysis of transiently transgenic (Csa1G085390 and Csa7G071440) and negative control (pART27:GFP) plants under salt and drought stresses and normal condition (CK) after 4 h treatment. a Phenotypic comparison of transiently transgenic and negative control plants under salt and drought stresses. Corresponding water treatment was performed as negative control treatment (CK). Measurements of Water loss rate b, Hydrogen peroxide (H2O2) content f and malondialdehyde (MDA) g content in above treated leaves, respectively. Different letter indicates significant differences at the level of 0.05. c Stomata observation in transiently transgenic and negative control plants’ leaves. 30 leaves were observed for each plant and each treatment. d Quantification of stomatal apertures in the leaves of the transiently transgenic and control plants. Different letter indicates significant differences at the level of 0.05. e H2O2 detection by 3,3′-diaminobenzidine (DAB) staining in the leaves of the transiently transgenic and control plants . Scale bar: 5 cm. The photos were taken by Junliang Yin and the figure was drawn by Junliang Yin
Expression analysis of pathogenesis-related (PR) genes in Csa1G085390, Csa7G071440, Csa4G642460, and Csa6G303740 transiently transgenic plants
As discussed above, the CsZFPs could be induced by abiotic and abiotic stresses. Thus, four CsZFPs, including Csa1G085390, Csa7G071440, Csa4G642460, and Csa6G303740, were transiently overexpressed into N. benthamiana leaves. Then three PTI related genes (NbWRKY7, NbWRKY8 and NbACRE31), three SA response genes (NbPR1a, NbPR1b and NbPR2), and five JA-dependent immunity genes (NbPR3, NbPR4, NbLOX, NbTPI and NbPDF1.2) were selected to do the expression analysis in these transgenic plants. As shown in Fig. 10, most of the genes in Csa7G071440 transiently transgenic plant were down regulated within 12 h, such as NbWRKY7, NbWRKY8, NbPR1a, NbPR1b, NbPR2 and NbPDF1.2, which demonstrated overexpression of Csa7G071440 could decrease the PR genes expression to suppress the plant defense. This might be the reason why the larger disease lesion was observed in Csa7G071440 transgenic plant after pathogen infection as shown in Fig. 8a and b. However, the expression levels of these genes were up regulated in Csa1G085390 transiently transgenic plant at specific time point. For example, NbWRKY7 was highly induced at 24 to 48 h. NbTPI was up regulated at 48 h time point. This could give the explanation that Csa1G085390 transiently transgenic plant was resistance to pathogen attack (Fig. 8b and c). Moreover, the expression levels of these genes were mostly up regulated in other two transiently transgenic plants (Csa4G642460 and Csa6G303740) from 12 h to 96 h. As exemplified, the NbWRKY 8 was highly induced in 12 h and the PR genes, such as NbPR1b, NbPR2, NbPR3, were significantly up regulated from 24 h to 72 h in Csa6G303740 transgenic plants. In addition, the same expression changes of NbWRKYs and NbPRs were observed in Csa4G642460 transgenic plants. These results could demonstrate the Csa4G642460 and Csa6G303740 genes could trigger the expression of PR genes to activate the hypersensitive response (leave cell death as shown in Fig. 7).
Relative expression profiles of pathogen response genes in transient overexpression of Csa1G085390, Csa7G071440, Csa4G646130 and Csa6G303740 plants after 12, 24, 48, 72 and 96 h of P. infestans infection. Each bar represents the average of three replicates, and error bar represents standard deviation (SD). Different letter indicates significant differences at the level of 0.05. The photos were taken by Junliang Yin and the figure was drawn by Junliang Yin
Discussion
The C2H2-type of zinc finger proteins that widely existed in eukaryotic kingdom play critical roles in many biological processes, such as hormone signaling, DNA or RNA binding, and stress responses [9, 33]. In addition, the genome wide identification of C2H2-type ZFPs has been conducted in several plant system, whereas not in cucumber. Thus, in order to get insights into the function of C2H2-type ZFPs in cucumber, the comprehensive analysis of CsZFPs was performed in the current study. Finally, 129 full-length CsZFPs were identified (Table 1), which could be classified into four groups according to the variation of the plant-specific conserved amino acid sequence “QALGGH” and distances between metal ligands within C2H2-ZF domains (Fig. 2). The principle classification of C2H2-ZFPs based on the number and structural ZF domains has been firstly studied in yeast and Arabidopsis [5, 34]. In different plant species, the modification of the conserved motif “QALGGH” was widely used to do the classification [35]. In cucumber, majority of CsZFPs contain one or two C2H2 domains with the QALGGH motif were identified as Cs1Q and Cs2Q subgroups (Additional file 1: Table S2). The same typical group has been identified in Brassica rapa L., which suggested that these proteins may be involved in plant-specific life processing [12, 31]. Moreover, the other three groups which do not contain this typical ZF domain have been identified, implies the functional diversity of these C2H2-ZFPs in plant growth and development. With the location analysis, most of the CsZFPs were predicted to be located in the nucleus and these have been confirmed by the subcellular location analysis, suggesting that they indeed function as transcription factors in nucleus, but the Csa7G406990 which comes from Cs1Q group located in cell wall and Csa2G292740 which belongs to Cs1C group located in endoplasmic reticulum (Table 1 and Additional file 1: Table S2), indicating the CsZFPs members in the same phylogenetic group did not necessarily correspond to the same subcellular localization. Moreover, except the DNA or RNA binding function of C2H2-ZFPs in the nucleus [31], the members of CsZFPs which did not locate in the nucleus might have other special function and play important roles in signal transduction.
The structural and physicochemical properties of a gene family analysis could indicate the diversity of each member during the evolutionary process [36]. Our results demonstrated a wide range of variations in molecular weight, theoretical pI values, chromosome location and exon-intron number, which elucidates the evolutionary changes occurred in cucumber likely via gene duplications and/or integration into genomic regions after reverse transcription [9]. Duplication modes of genes, including segmental and tandem duplication, are considered to be characteristic features and primary driving forces of the evolution of genomes [37]. Tandem duplicates are defined as paralogous genes that are adjacent to each other on a chromosome [38]. In the current study, gene duplication analysis revealed that no tandem and segmental duplication took place which was consistent with the fact that cucumber genome was absence of recent whole-genome duplication events and tandem duplication [39, 40]. The smaller number of CsZFP genes compared with Arabidopsis, rice and rape, may also be attributed by the absence of recent duplication events in cucumber genome.
CsZFPs are important kind of transcription factors in the regulation of plant growth and development, hormones metabolisms and stress responses. While cis-elements existed in the promoter region play key roles in the transcriptional level of genes in response to multiple stimuli [41]. Our results identified several cis-regulatory elements in related to plant growth and development, phytohormone, biotic and abiotic stresses, including TATA-box, ABRE, G-box, in corresponding promoter region of CsZFPs (Fig. 3 and Additional file 1: Table S3). All these cis-regulating elements have been identified in the study of Arabidopsis [42, 43]. Thus, the prediction of extensive cis-regulatory elements in the promoter regions of CsZFPs suggested that the CsZFPs might play essential roles in response to various stresses and phytohormone regulation. In addition to the function of cis-regulating elements, miRNAs also play essential roles in the development of plant growth, stresses and other morphological processes [44]. For instance, the tae-miR9674a-5p, tae-miR9781 and tae-miR9655-3p which were identified in wheat function importantly in various metabolisms and biological processes [7]. In cucumber, the number of miRNAs target to corresponding CsZFPs transcripts ranges from 1 to 20 (Fig. 3 and Additional file 1: Table S5), indicating that different miRNAs could regulate the translation process of CsZFPs during multiple physiological and stress-induced cellular responses which were consistence with the study in durum wheat [7].
All the prediction of cis-regulating elements and miRNAs in response to CsZFPs suggested they could be involved in cucumber growth and development, and various stresses. Furthermore, multiple studies showed that most of C2H2-ZFPs function as transcription factors are widely employed in plant growth and development [45, 46]. In the current study, with the publicly available RNA-seq data analysis, the expression patterns of CsZFPs demonstrated that these genes are involved in tissue and organ development, especially root and floral development (Fig. 5), illustrating the important function of this gene family in cucumber growth and development. The same conclusion was got from the study of Brassica which showed that the high expression level of most of B. rapa C2H2-ZFP genes was observed in at least one of tested tissues and furthermore one hundred of them were significantly expressed in six tissues [12]. These genes might function importantly in tissue growth and development of cucumber species and deserved to be further studied at their molecular levels. For example, JcZFP8, a C2H2 zinc finger protein gene from Jatropha curcas L., participates the trichome development in transgenic tobacco [47]. The C2H2-typezinc finger protein named ZINC FINGER PROTEIN 1 (AtZP1) negatively regulates Arabidopsis thaliana root hair initiation and elongation [48].
In addition to the function of CsZFPs in cucumber growth and development, transcription profiling analyses have demonstrated that the transcript level of many C2H2-type zinc finger proteins is elevated under different abiotic stress conditions such as low temperature, salt, drought, osmotic stress, and oxidative stress [8]. For example, ZAT12 has been reported to be involved in multiple abiotic stresses in Arabidopsis [49]. In addition, ZAT18 was found to be a positive regulator in response to drought stress in Arabidopsis [19]. In banana, two C2H2-ZFPs, named as MaC2H2–1 and MaC2H2–2, were identified as transcriptional repressors to repress the expression of ethylene biosynthetic genes in fruit ripening [50]. In cucumber, a C2H2 zinc finger transcription factor, named as Zat12 which could be up-regulated under melatonin treatment to alleviate chilling stress by modulation of polyamine and abscisic acid metabolism [51]. Moreover, AtAZF2 play important roles in the regulation of ABA-repressive and auxin-inducible genes under abiotic stress conditions [20, 52]. The expression analysis of three other TFIIIA zinc finger proteins, AZF1, AZF2 and AZF3 show that AZF1 and AZF3 were induced under cold stress but not in ABA treatment [53]. Interestingly, in our study, the orthologous gene of AtAZF2 in cucumber is Csa4G642460 which was up regulated in response to drought, salt and heat stresses, but not response to cold stress (Fig. 6), which was consistence with above results and demonstrated they function essentially under these three abiotic stresses. Furthermore, ZFP245, a C2H2-type zinc finger protein, is highly induced under drought stress and overexpression of ZFP245 increased the tolerance of rice to drought stress [54,55,56]. While Csa6G303740, the orthologous gene of ZFP245, was significantly up regulated in response to drought stress as well as heat stress, indicating it could involve in the regulation of drought and heat stresses in cucumber.
In the current study, nine CsZFP genes are significantly induced by drought, cold, heat, and salt stresses, implying the involvement of these genes in response to abiotic stresses (Fig. 6). As an important ROS, H2O2 functions as a signal molecule in plant responses to abiotic stress [28]. Furthermore, transient overexpression of Csa1G085390 and Csa7G071440 into N. benthamiana could enhance salt and drought stresses through the initial induction of H2O2 (Fig. 9). ROS and redox cues could activate stress acclimation process by retrograde signaling to regulate metabolic fluxes [57]. Here, the initial accumulations of H2O2 in these transgenic plants function as signaling molecule to activate the defense mechanism against drought and salt stresses. Besides, Csa1G085390 promotes and Csa7G071440 represses pathogen colonization in N. benthamiana, suggesting that Csa1G085390 could weaken plant defensive immune responses while Csa7G071440 could strengthen the responses. Considering the fact that these two genes are not cell death inducer, they may not involve in the plant hypersensitive defense pathways. Moreover, the exact defense pathway mediated by these two genes are still unclear, which need to be further studied.
Csa4G642460 and Csa6G303740 could induce cell death in their overexpression plants, but the cell death could not be rescued by slicing HSP90, SGT1, RAR2, MEK1, SIPK and WRKY3–2 genes (Fig. 7), indicating there must be other key regulators involved in this signaling transduction. Moreover, the PR genes was highly induced in these two transgenic plants which might illustrating the PR genes might be one potential candidates during Csa4G642460 and Csa6G303740 induced hypersensitive response Since cell death inducer usually play positive roles upon pathogen infection, Csa4G642460 and Csa6G303740 may also play roles in plant resistance to pathogen. However, their roles in pathogen resistance need to be further tested in cucumber. It is interesting that Csa6G303740 was also induced by salt stress, suggesting that it may also participate in salt response of cucumber. In the future study, the stable transformation of these two genes into cucumber in response to biotic and abiotic stresses needs to be further analyzed and confirming the molecular mechanism of CsZFPs under these stresses.
Conclusion
In the current study, 129 full-length CsZFPs were identified, which could be classified into nine groups according to the phylogenetic analysis. The 129 CsZFPs unequally distributed on 7 chromosomes and no tandem duplication events took place during the evolutionary process. Promoter cis-element analysis showed that the CsZFPs might involve in the regulation of phytohormone or abiotic stress response, and different CsZFPs were regulated by several miRNAs. In addition, subcellular localization analysis indicated that most of the CsZFPs located in the nucleus and the RNA-seq analysis of CsZFPs demonstrated that these genes are involved in tissue and organ development. The expression profiling of CsZFPs by qRT-PCR analysis in response to abiotic stresses indicated that all the nine typical CsZFPs are significantly involved in drought, cold, heat and salt stresses. Furthermore, the transient overexpression of Csa1G085390 and Csa7G071440 into N. benthamiana plant revealed that they could decrease and induce leave necrosis in response to pathogen attack, respectively, and they could enhance salt and drought stresses through the initial induction of H2O2. In addition, Csa4G642460 and Csa6G303740 are cell death inducers in tobacco and tomato.
Methods
Identification of ZFP genes in cucumber
To identify all the ZFP genes in cucumber, two searching methods were performed. First, the protein sequences of cucumber (cucumber_ChineseLong_v2_pep.fa) were downloaded from the Cucurbit Genomics Database (http://cucurbitgenomics.org/organism/2). Then, the ZFP protein sequences of the Arabidopsis, rice, and maize were used as queries to identify the potential candidates by local BLASTP searching with a cutoff e-value 1e-10. Second, the keywords “zinc finger” were used as queries to search the Cucurbit Genomics Database (http://cucurbitgenomics.org/search/genome/2). Finally, all the retrieved non-redundant sequences were submitted to InterProScan (http://www.ebi.ac.uk/interpro/) and smart (http://smart.embl-heidelberg.de/) to assess the presences of C2H2 domains (IPR000690, IPR003604, IPR013085, IPR032553, IPR034736, IPR013087, IPR019406, IPR014898).
Protein characterization, amino acid properties, chromosomal localization, gene structure and duplication analysis
Gene locus, chromosomal location, and the exon-intron structure of CsZFP genes were extracted from the genome gff3 annotation file. The approximate positions of CsZFPs were located to 7 chromosomes using software Mapinspect [36]. Gene duplications were classified into tandem duplication and segmental duplication events. Tandem duplication was determined according to Fang et al. [58]. The protein identification and analysis tools in ExPASy Server10 (https://prosite.expasy.org/) were used to predict length, molecular weight (MW), theoretical isoelectric point, instability index, amino acid composition, and atomic composition of CsZFPs.
Multiple sequence alignment and classification of cucumber C2H2-ZFPs into groups
The multiple sequence alignments of the full-length protein sequences were performed by using ClustalW2 (v2.1) with default parameters. An un-rooted phylogenetic tree was constructed using MEGA7 package with the neighbor-joining method based on LG model, and bootstrapping was performed 1000 times [59]. The phylogenetic tree was illustrated using Interactive Tree of Life (IToL, v3.2.317, http://itol.embl.de). The conserved motifs of CsZFPs were identified using MEME motif search tool (http://memesuite.org/tools/meme) [60]. Default parameters were used in this study, except that the maximum number of motifs was set to 20. And the motif patterns were drawn by TBtools software (https://github.com/CJ-Chen/TBtools). Furthermore, SMART database [61] was used to analyze 129 CsZFPs manually to search the numbers, sequences of C2H2-ZF domain, and the space length between C2H2-ZF domains. According to the methods described in soybean and Populus trichocarpa [12, 13], the C2H2-ZF domains in CsZFPs were classified into five main types. Then, based on the types and numbers of C2H2-ZF domains, CsZFPs were further classified into nine groups. The detailed information was showed in results.
Analysis of microRNA target sites and cis-acting regulatory elements
The Cucumissativus miRNA sequences were obtained from the miRBase database at http://mirbase.org/ [62] and the mature sequences of cucumber miRNAs were collected from the previously reported publications [26, 63]. To detect potential miRNA target sites within the CsZFP genes, the obtained miRNAs were analyzed with the psRNATarget server (http://plantgrn.noble.org/psRNATarget/) [64]. The up-stream 1500 bp DNA sequences of CsZFP genes were used to do the cis-acting regulatory elements analysis by PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [65].
RNA-seq data analysis of CsZFP genes
To analyze the expression profiles of CsZFP genes, we collected the expression level of each CsZFP represented by FPKM values (Fragments Per Kilobase of transcript per Million fragments mapped values) from the Cucurbit Genomics Database (http://cucurbitgenomics.org/organism/2). The heatmap of cucumber CsZFP genes were generated using R package “pheatmap”.
Plant materials and treatments
Cucumber (Cucumis sativus L. cv. JinYou 1, Xintiandi Co., Yangling, Shannxi, China) seeds were rinsed thoroughly in distilled water and germinated on moist gauze in an incubator at 28 °C in dark for 2 days [66]. The germinated seeds were sown in a mixed substrate (peat: vermiculite: perlite = 2:1:1) in an artificial growth chamber with an average of 12 h/12 h day/night light. The temperature were set to 28 °C/18 °C day/night. At two-leaf stage, the uniform seedlings were transferred to plastic containers filled with 15 L of 1/4 strength of Hoagland nutrient solution. Three days later, the strength of Hoagland solution was increased to 1/2. Seven days after transplanting, seedlings were subjected to four experimental groups: (i) Control, seedlings were incubated in the chamber at 28 °C/18 °C day/night temperature; (ii) Salt stress, 75 mM sodium chloride (NaCl) was added to the nutrient solution; (iii) Heat stress, seedlings were treated with 40 °C/32 °C day/night temperature in another identical growth chamber; (iv) Chilling stress, seedlings were exposed to 18 °C/5 °C day/ night temperature for chilling treatment [67]. The roots were collected after 3, 6, and 9 days of treatment respectively. The solution pH was maintained at 6.0 using 0.2 M H2SO4 or 1 M KOH. All sampled materials were harvested to be frozen with liquid nitrogen and stored at − 80 °C prior to RNA extraction.
RNA extraction and qRT-PCR analysis
Total RNA extraction from the roots of cucumber and following cDNA synthesis were performed according to manufacturer’s instructions (Invitrogen, Carlsbad, CA) as described by Yin et al. (2018) [68]. The qRT-PCR was carried out on a CFX 96 Real-Time PCR system (Bio-Rad) using SYBR Green Master Mix (Vazyme, Nanjing, China) according to manufacturer’s protocols. The 20 μL reaction system contained 10 μL of 2 × SYBR Premix ExTaq™, 0.4 μL each of 10 μM primers, 1 μL diluted cDNA and 8.2 μL ddH2O. The thermal profile was pre-incubation for 3 min at 94 °C, followed by 40 cycles of 5 s at 94 °C, 15 s at 55 ~ 63 °C and 15 s at 72 °C. Relative expression levels of CsZFPs were calculated with the 2-ΔΔCt method [69]. The primer sequences of CsZFPs and reference gene for qRT-PCR were shown in Additional file 1: Table S7.
Subcellular localization analysis of CsZFP proteins
To confirm the sub-cellular localization of CsZFPs, ten CsZFPs from different groups were selected to do the subcellular analysis. Briefly, full lengths of CsZFPs were amplified by Phanta HS Master Mix (Vazyme, Nanjing, China). Then the PCR products were inserted into the XhoI digested vector pART27:GFP (NEB, Beijing, China) by using ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). The CsZFP:GFP fusion constructs were transformed into Agrobacterium tumefaciens strain GV3101 and the pART27:GFP transformation was used as negative control. Tobacco (Nicotiana benthamiana) leaves were used to do the transformation by infiltration method. Two days later, the injected leaves were placed on the glass slides and visualized through fluorescence microscopy (Olympus FV3000, Tokyo, Japan) with 488 nm exciting light wavelength.
Transient overexpression of four typical CsZFPs into N. benthamiana in response to biotic and abiotic stresses
Four typical CsZFPs (Csa1G085390, Csa7G071440, Csa4G642460 and Csa6G303740):GFP fusion vectors were transient transformed into N. benthamiana plants by Agrobacterium tumefaciens strain GV3101 whilst the pART27:GFP transformation was used as negative control. The agroinfiltration leaves were collected after 12, 24, 48, 72, and 96 h treatment and quickly frozen in liquid nitrogen and stored at − 80 °C for RNA extraction. Then the relative expression levels of pathogenesis-related (PR) genes were calculated with the 2-ΔΔCt method and the primer sequences of these genes were shown in Additional file 1: Table S8. Furthermore, the phenotype of Csa1G085390 and Csa7G071440 transgenic plants were assessed in response to pathogen attack (Phytophthora infestans). Briefly, after 2 days of transient expression, the detached leaves were inoculated by zoospores of P. infestans (strain 88,069) [70]. After 5 days of inoculation, the lesion diameter was measured and visualized using a handheld long-wavelength UV lamp (Blak-Ray B-100AP, Ultraviolet Products) and figures were taken using the Carl Zeiss Imaging System.
After transient expression pART27:GFP, Csa1G085390:GFP, and Csa7G071440:GFP fusions in N. benthamiana for 2 days, leaves were excised from the plants and the phenotype of these leaves in response to drought (1% PEG) and salt (400 mM NaCl) stresses were evaluated. In addition, water loss rate was conducted as described by Zhu et al. [36]. Stomatal apertures were examined in the abaxial epidermis of leaves by Olympus BX51 (Shinjuku-ku, Tokyo, Japan) microscope after 4 h of PEG and salt treatment. The quantification of stomatal apertures was measured by ImageJ. Finally, H2O2 and MDA concentration were determined as described by Yin et al. [26]. Moreover, the H2O2 accumulation was detected by histochemical staining diaminobenzidine (DAB) method [71].
VIGS assay in N. benthamiana
The cell death in Csa4G642460 and Csa6G303740 transgenic plants were observed after 5 days transformation. The same phenotype of Csa4G642460 and Csa6G303740 transiently expressed in Solanum lycopersicum leaves were observed after 6 days transformation. Cell death inducing signaling transduction pathway related genes (HSP90, SGT1, RAR2, MEK1, SIPK, and WRKY3–2) were amplified and constructed to pTRV2 vector [72], which was mixed with pTRV1 in equal ratios to a final OD600 of 0.25. The pTRV2:GFP was used as a control. These constructed vectors were transformed into the lower leaf of four-leaf stage N. benthamiana by Agrobacterium tumefaciens strain GV3101, and 3 weeks later, the constructed vectors of Csa4G642460:GFP, and Csa6G303740:GFP were transiently expressed in the upper leaves. The degree of cell death was analyzed in the upper leaves after 5 days transformation.
Availability of data and materials
The genome data and sequences and expression profiles of CsZFP genes used in the current study are available in the Cucurbit Genomics Database (http://cucurbitgenomics.org/search/genome/2). All data generated or analyzed during this study are included in this published article and its Additional files. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ZFPs:
-
Zinc-finger proteins
- CsZFPs:
-
C2H2 ZFPs in cucumber
- TFs:
-
Transcription factor
- Cys:
-
Cysteine
- His:
-
Histidine
- ABA:
-
Abscisic acid
- NaCl:
-
Sodium chloride
- AtZP1:
-
Zinc Finger Protein 1
References
Wang L, Sadeghnezhad E, Nick P. Upstream of gene expression-what is the role of microtubules in cold signalling? J Exp Bot. 2019;71(1):36–48.
Tian Y, Che Z, Sun D, Yang Y, Lin X, Liu S, Liu X, Gao J. Resistance identification of tree peony cultivars of different flowering time to gray mold pathogen Botrytis cinerea. HortScience. 2019;54(2):328–30.
Fang ZW, Jiang WQ, He YQ, Ma DF, Liu YK, Wang SP, Zhang YX, Yin JL. Genome-wide identification, structure characterization, and expression profiling of Dof transcription factor gene family in wheat (Triticum aestivum L.). Agronomy. 2020;10(2):294.
Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004;136(1):2734–46.
Englbrecht CC, Schoof H, Bohm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics. 2004;5(1):39.
Agarwal P, Arora R, Ray S, Singh AK, Singh VP, Takatsuji H, Kapoor S, Tyagi AK. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol Biol. 2007;65(4):467–85.
Faraji S, Rasouli SH, Kazemitabar SK. Genome-wide exploration of C2H2 zinc finger family in durum wheat (Triticum turgidum ssp. Durum): insights into the roles in biological processes especially stress response. Biometals. 2018;31(6):1019–42.
Kielbowicz-Matuk A. Involvement of plant C(2)H(2)-type zinc finger transcription factors in stress responses. Plant Sci. 2012;185–186:78–85.
Mishra AK, Muthamilarasan M, Khan Y, Parida SK, Prasad M. Genome-wide investigation and expression analyses of WD40 protein family in the model plant foxtail millet (Setaria italica L.). PloS One. 2014;9(1):e86852.
Wang K, Ding Y, Cai C, Chen Z, Zhu C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol Plantarum. 2019;165(4):690–700.
Iuchi S. Three classes of C2H2 zinc finger proteins. Cell Mol Life Sci. 2001;58(4):625–35.
Alam I, Batool K, Cui DL, Yang YQ, Lu YH. Comprehensive genomic survey, structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS One. 2019;14(5):e0216071.
Liu Q, Wang Z, Xu X, Zhang H, Li C. Genome-wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in poplar (Populus trichocarpa). PLoS One. 2015;10(8):e0134753.
Yuan S, Li X, Li R, Wang L, Zhang C, Chen L, Hao Q, Zhang X, Chen H, Shan Z, Yang Z, Chen S, Qiu D, Ke D, Zhou X. Genome-wide identification and classification of soybean C2H2 zinc finger proteins and their expression analysis in legume-Rhizobium symbiosis. Front Microbiol. 2018;9:126.
Wei K, Pan S, Li Y. Functional characterization of maize C2H2 zinc-finger gene family. Plant Mol Biol Rep. 2016;34(4):761–76.
Wang F, Tong W, Zhu H, Kong W, Peng R, Liu Q, Yao Q. A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta. 2016;243(3):783–97.
Tao Z, Huang Y, Zhang L, Wang X, Liu G, Wang H. BnLATE, a Cys2/His2-type zinc-finger protein, enhances silique shattering resistance by negatively regulating lignin accumulation in the silique walls of Brassica napus. PLoS One. 2017;12(1):e0168046.
Kam J, Gresshoff PM, Shorter R, Xue GP. The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol Biol. 2008;67(3):305–22.
Yin M, Wang Y, Zhang L, Li J, Quan W, Yang L, Wang Q, Chan Z. The Arabidopsis Cys2/His2 zinc finger transcription factor ZAT18 is a positive regulator of plant tolerance to drought stress. J Exp Bot. 2017;68(11):2991–3005.
Kodaira KS, Qin F, Tran LS, Maruyama K, Kidokoro S, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011;157(2):742–56.
Ciftci-Yilmaz S, Morsy MR, Song L, Coutu A, Krizek BA, Lewis MW, Warren D, Cushman J, Connolly EL, Mittler R. The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J Biol Chem. 2007;282(12):9260–8.
Isernia C, Bucci E, Leone M, Zaccaro L, Di Lello P, Digilio G, Esposito S, Saviano M, Di Blasio B, Pedone C, et al. NMR structure of the single QALGGH zinc finger domain from the Arabidopsis thaliana SUPERMAN protein. Chembiochem. 2003;4(2–3):171–80.
Zhao J, Liu M, Jiang L, Ding L, Yan SS, Zhang J, Dong Z, Ren H, Zhang X. Cucumber SUPERMAN has conserved function in stamen and fruit development and a distinct role in floral patterning. PLoS One. 2014;9(1):e86192.
Liu D, Yang L, Luo M, Wu Q, Liu S, Liu Y. Molecular cloning and characterization of PtrZPT2-1, a ZPT2 family gene encoding a Cys2/His2-type zinc finger protein from trifoliate orange (Poncirus trifoliata (L.) Raf.) that enhances plant tolerance to multiple abiotic stresses. Plant Sci. 2017;263:66–78.
Kim JC, Lee SH, Cheong YH, Yoo CM, Lee SI, Chun HJ, Yun DJ, Hong JC, Lee SY, Lim CO, et al. A novel cold-inducible zinc finger protein from soybean, SCOF-1, enhances cold tolerance in transgenic plants. Plant J. 2001;25(3):247–59.
Yin J, Jia J, Lian Z, Hu Y, Guo J, Huo H, Zhu Y, Gong H. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotox Environ Safe. 2019;169:8–17.
Zhu YX, Jia JH, Yang L, Xia YC, Zhang HL, Jia JB, Zhou R, Nie PY, Yin JL, Ma DF, Liu LC. Identification of cucumber circular RNAs responsive to salt stress. BMC Plant Biol. 2019;19(1):164.
Zhu Y, Gong H, Yin J. Role of silicon in mediating salt tolerance in plants: a review. Plants. 2019;8(6).
Hu R, Qi G, Kong Y, Kong D, Gao Q, Zhou G. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010;10:145.
Kumar R. Role of microRNAs in biotic and abiotic stress responses in crop plants. Appl Biochem Biotech. 2014;174(1):93–115.
Takatsuji H. Zinc-finger proteins: the classical zinc finger emerges in contemporary plant science. Plant Mol Biol. 1999;39(6):1073–8.
Han G, Lu C, Guo J, Qiao Z, Sui N, Qiu N, Wang B. C2H2 zinc finger proteins: master regulators of abiotic stress responses in plants. Front Plant Sci. 2020;11:115.
Liu Y, Liu D, Hu R, Hua C, Ali I, Zhang A, Liu B, Wu M, Huang L, Gan Y. AtGIS, a C2H2 zinc-finger transcription factor from Arabidopsis regulates glandular trichome development through GA signaling in tobacco. Biochem Bioph Res Co. 2017;483(1):209–15.
Bohm S, Frishman D, Mewes HW. Variations of the C2H2 zinc finger motif in the yeast genome and classification of yeast zinc finger proteins. Nucleic Acids Res. 1997;25(12):2464–9.
Kubo K, Sakamoto A, Kobayashi A, Rybka Z, Kanno Y, Nakagawa H, Takatsuji H. Cys2/His2 zinc-finger protein family of petunia: evolution and general mechanism of target-sequence recognition. Nucleic Acids Res. 1998;26(2):608–15.
Zhu YX, Yang L, Liu N, Yang J, Zhou XK, Xia YC, He Y, He YQ, Gong HJ, Ma DF, Yin JL. Genome-wide identification, structure characterization, and expression pattern profiling of aquaporin gene family in cucumber. BMC Plant Biol. 2019;19:345.
Moore RC, Purugganan MD. The early stages of duplicate gene evolution. P Natl Acad Sci USA. 2003;100(26):15682–7.
Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10.
Huang S, Li R, Zhang Z, Li L, Gu X, Fan W, Lucas WJ, Wang X, Xie B, Ni P, et al. The genome of the cucumber, Cucumis sativus L. Nat Genet. 2009;41(12):1275–81.
Ling J, Jiang W, Zhang Y, Yu H, Mao Z, Gu X, Huang S, Xie B. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics. 2011;12:471.
Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci. 2014;5:170.
Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006;580(28–29):6537–42.
Ciftci-Yilmaz S, Mittler R. The zinc finger network of plants. Cell Mol Life Sci. 2008;65(7–8):1150–60.
Lu XY, Huang XL. Plant miRNAs and abiotic stress responses. Biochem Biophysical Res Commun. 2008;368(3):458–62.
Morita MT, Sakaguchi K, Kiyose SI, Taira K, Kato T, Nakamura M, Tasaka M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006;47(4):619–28.
Shi H, Chan Z. The cysteine2/histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J Pineal Res. 2014;57(2):185–91.
Shi X, Gu Y, Dai T, Wu Y, Wu P, Xu Y, Chen F. Regulation of trichome development in tobacco by JcZFP8, a C2H2 zinc finger protein gene from Jatropha curcas L. Gene. 2018;658:47–53.
Han GL, Wei X, Dong X, Wang C, Sui N, Guo JR, Yuan F, Gong Z, Li X, Zhang Y, et al. Arabidopsis ZINC FINGER PROTEIN1 acts downstream of GL2 to repress root hair initiation and elongation by directly suppressing bHLH genes. Plant Cell. 2020;32:206–25.
Davletova S, Schlauch K, Coutu J, Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005;139(2):847–56.
Han YC, Fu CC, Kuang JF, Chen JY, Lu WJ. Two banana fruit ripening-related C2H2 zinc finger proteins are transcriptional repressors of ethylene biosynthetic genes. Postharvest Biol Tech. 2016;116:8–15.
Zhao H, Zhang K, Zhou X, Xi L, Wang Y, Xu H, Pan T, Zou Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci Rep. 2017;7(1):4998.
Drechsel G, Raab S, Hoth S. Arabidopsis zinc-finger protein 2 is a negative regulator of ABA signaling during seed germination. J Plant Physiol. 2010;167(16):1418–21.
Sakamoto H, Araki T, Meshi T, Iwabuchi M. Expression of a subset of the Arabidopsis Cys2/His2-type zinc-finger protein gene family under water stress. Gene. 2000;248(1–2):23–32.
Huang J, Wang JF, Wang QH, Zhang HS. Identification of a rice zinc finger protein whose expression is transiently induced by drought, cold but not by salinity and abscisic acid. DNA Seq. 2005;16(2):130–6.
Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009;23(15):1805–17.
Huang J, Sun SJ, Xu DQ, Yang X, Bao YM, Wang ZF, Tang HJ, Zhang H. Increased tolerance of rice to cold, drought and oxidative stresses mediated by the overexpression of a gene that encodes the zinc finger protein ZFP245. Biochem Biophysical Res Commun. 2009;389(3):556–61.
Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35(2):259–70.
Jiang W, Yang L, He Y, Zhang H, Li W, Chen H, Ma D, Yin J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). Peer J. 2019;7:e8062.
He YQ, Huang WD, Yang L, Li YT, Lu C, Zhu YX, Ma DF, Yin JL. Genome-wide analysis of ethylene-insensitive3 (EIN3/EIL) in Triticum aestivum. Crop Sci. 2020. https://doi.org/10.1002/csc2.20115.
Jiang WQ, Yin JL, Zhang HT, He YQ, Shuai SM, Chen SH, Cao SL, Li W, Ma DF, Chen HG. Genome-wide identification, characterization analysis and expression profiling of auxin-responsive GH3 family genes in wheat (Triticum aestivum L.). Mol Biol Rep. 2020. https://doi.org/10.1007/s11033-020-05477-5.
Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43(Database issue):D257–60.
Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2013;42(D1):D68–73.
Jin W, Wu F. Identification and characterization of cucumber microRNAs in response to Pseudoperonospora cubensis infection. Gene. 2015;569(2):225–32.
Dai X, Zhao PX. PsRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 2011;39(Web Server issue):W155–9.
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Zhu YX, Xu XB, Hu YH, Han WH, Yin JL, Li HL, Gong HJ. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015;34(9):1629–46.
Zhu Y, Yin J, Liang Y, Liu J, Jia J, Huo H, Wu Z, Yang R, Gong H. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediatedalleviation of salt stress in cucumber plants. Ecotox Environ Safe. 2019;174:245–54.
Yin J, Liu M, Ma D, Wu J, Li S, Zhu Y, Han B. Identification of circular RNAs and their targets during tomato fruit ripening. Postharvest Biol Tec. 2018;136:90–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–8.
Yin J, Gu B, Huang G, Tian Y, Quan J, Lindqvist-Kreuze H, Shan W. Conserved RXLR effector genes of Phytophthora infestans expressed at the early stage of potato infection are suppressive to host defense. Front Plant Sci. 2017;8:2155.
Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012;196(1):125–38.
Huang GY, Liu ZR, Gu B, Zhao H, Jia JB, Fan GJ, Meng YL, Du Y, Shan WX. An RXLR effector secreted by Phytophthora parasitica is a virulence factor and triggers cell death in various plants. Mol Plant Pathol. 2019;20(3):356–71.
Acknowledgements
We thank Prof. Yongli Qiao for beneficial comments on the initial project design and data analysis. We thank Dr. Hua Zhao (State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University, Yangling, China) for confocal experimental assistance.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31701911), the Funds for Young Talent Project of Hebei Agricultural University Foundation (grant number YJ201853), the Green Channel Fund of Hebei Province Natural Science Foundation (C2019204308), the Open Project Program of Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education (No. KF201909). These funding bodies took part in the design of the study and collection, analysis, and interpretation of data, and the writing of the manuscript, as well as in the open access payment.
Author information
Authors and Affiliations
Contributions
YJL and ZYX: designed this work; WLX and YJL: wrote the manuscript; ZYX, ZJ, HR, LYT, ZXK, HY, HYQ: performed most of the experiments and analysis; ZXM and JXC: contributed substantially to the completion of this work; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1 Table S1.
The types and sub-types of C2H2-ZF domains and their characteristics. Table S2. Detailed information of C2H2 motifs in the classified C2H2-ZFPs. Table S3. The cis-regulatory elements analysis of the promoter region of CsZFPs. Table S4. The detailed information of cis-regulatory elements in the promoter region of CsZFPs. Table S5. The detailed information of miRNAs targeted to CsZFPs. Table S6. RNA-seq analysis of CsZFP genes in cucumber different growth, developmental stages and in response to biotic and abiotic stresses. Table S7. The primer sequences of CsZFP genes for qRT-PCR. Table S8. The primer sequences of pathogen response genes for qRT-PCR.
Additional file 2 Figure S1.
The motif sequences and the conserved residuals in the motifs. Figure S2. Cell death analysis in tomato leaves. pART27:GFP, Csa4G642460 and Csa6G303740 were transiently expressed in Solanum lycopersicum.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yin, J., Wang, L., Zhao, J. et al. Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol 20, 359 (2020). https://doi.org/10.1186/s12870-020-02575-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-020-02575-1