Abstract
Background
The vigour and precocity of trees highly influences their efficiency in commercial production. In apple, dwarfing rootstocks allow high-density plantings while their precocious flowering enables earlier fruit production. Currently, there is a lack of pear (Pyrus communis L.) rootstocks that are equivalent to the high yielding apple rootstock ‘M9’. For the efficient breeding of new Pyrus rootstocks it is crucial to understand the genetic determinants of vigour control and precocity. In this study we used quantitative trait loci (QTLs) analysis to identify genetic loci associated with the desired traits, using a segregating population of 405 F1 P. communis seedlings from a cross between ‘Old Home’ and ‘Louise Bonne de Jersey’ (OHxLBJ). The seedlings were grafted as rootstocks with ‘Doyenne du Comice’ scions and comprehensively phenotyped over four growing seasons for traits related to tree architecture and flowering, in order to describe the growth of the scions.
Results
A high density single nucleotide polymorphism (SNP)-based genetic map comprising 597 polymorphic pear and 113 apple markers enabled the detection of QTLs influencing expression of scion vigour and precocity located on linkage groups (LG)5 and LG6 of ‘Old Home’. The LG5 QTL maps to a position that is syntenic to the apple ‘Malling 9’ (‘M9’) Dw1 locus at the upper end of LG5. An allele of a simple sequence repeat (SSR) associated with apple Dw1 segregated with dwarfing and precocity in pear and was identified in other pear germplasm accessions. The orthology of the vigour-controlling LG5 QTL between apple and pear raises the possibility that the dwarfing locus Dw1 arose before the divergence of apple and pear, and might therefore be present in other Rosaceae species.
Conclusion
We report the first QTLs associated with vigour control and flowering traits in pear rootstocks. Orthologous loci were found to control scion growth and precocity in apple and pear rootstocks. The application of our results may assist in the breeding process of a pear rootstock that confers both vigour control and precocity to the grafted scion cultivar.
Similar content being viewed by others
Background
Commercial apple (Malus x domestica Borkh.) production relies on the use of dwarfing rootstocks to reduce scion vigour and promote early flowering in young trees [1, 2]. However, the closely related pear (Pyrus communis L.) lacks comparable dwarfing Pyrus rootstocks, which makes the cultivation of pear currently less profitable than apple. To develop a series of pear rootstocks, it is necessary first to develop an understanding of the mechanisms involved in vigour control and precocity in pear and the genetic determinants of the desired traits.
The physiology of rootstock-induced dwarfing in fruit trees is not fully understood and a number of mechanisms have been suggested to influence dwarfing in perennial fruit tree crops in general. Yonemoto et al. [3] observed that mandarin scions grafted onto rootstocks had a lower sap flow rate and higher soluble solid content than non-grafted trees and Basile et al. [4] found that the daily extension growth of shoots of a peach scion grafted on a semi-dwarfing rootstock was related to the dynamics of stem water potential. In citrus, Lliso et al. [5] found significantly higher concentrations of carbohydrates in fruit and roots of trees on dwarfing rootstocks than on more vigorous ones, suggesting that dwarfing rootstocks promote heavier flowering and crop load and thereby reduce vegetative growth. In apple, research has focused on water and nutrient restriction at the graft union, as well as a reduction of auxin movement from the scion to the rootstock [2, 6–10]. Foster et al. [11] observed that key flowering genes from the Flowering Time (FT) locus family were up-regulated in dwarfing rootstocks, which would promote flowering and reduce shoot extension growth.
They also found several stress response genes were up-regulated and concluded that stress might be a factor in the dwarfing effect. Recently, two major QTLs (Dw1 and Dw2), which control most of the dwarfing effect conferred to the scion, have been identified in the apple rootstock ‘Malling 9’ (‘M9’) on LG5 and LG11 respectively, [11–14]. This ‘M9’ dwarfing effect involves the reduction of the number and length of branches in the first year of growth after grafting and an increase in the proportion of floral buds [11, 15]. However, in pear no QTL has been identified that controls tree productivity traits and no genetic analysis has been carried out on rootstocks, although several QTLs have been identified that control traits such as pest and disease resistance [16–18], leaf morphology [19], and fruit quality traits [20–22].
As pear and apple are closely related species within the Rosaceae family [23], and their genomes exhibit a high degree of synteny [24–27], we hypothesized that orthologous loci might occur in both pear and apple that are responsible for the control of scion growth conferred by rootstocks. In the present study, we tested this hypothesis using a segregating population of 405 seedlings from a P. communis ‘Old Home’ x ‘Louise Bonne de Jersey’ cross grafted with ‘Doyenne du Comice’ (‘Comice’) scions and phenotyped for precocity and scion growth (vigour). We present the results for a QTL analysis of these traits using a high density genetic map based on single nucleotide polymorphism (SNP) markers anchored to the ‘Bartlett’ v1.0 European pear genome assembly [27].
Methods
Segregating population
A cross was made between Pyrus communis L. ‘Old Home’ and ‘Louise Bonne de Jersey’ (OHxLBJ), resulting in a segregating population consisting of 421 F1 seedlings. The seedlings were grown in the glasshouse for three months and planted out into the Plant & Food Research orchard in Motueka, New Zealand (41°6’S; 172°58’E). After 2 months of acclimatisation, the seedlings were summer budded with ‘Comice’ (Pyrus communis L.). In the following spring when the trees were cut down to the bud, grafts from the shoots removed from the OHxLBJ seedlings were inserted onto Pyrus calleryana seedling rootstocks to provide leaf material for DNA extraction. As controls, fifty clonal Cydonia oblonga ‘Quince C’ (QC) rootstocks grafted with ‘Comice’ were systematically distributed throughout the orchard block to give some indication of the variation in growing conditions across the block. The trees were planted into three rows, each containing 157 trees, including the QC controls, with a spacing of 0.8 m within the row and 3.3 m between the rows. Of the original 421 seedlings, propagation of scions failed on 16 trees, leaving 405 for phenotyping. To avoid any horticultural influence on tree shape and vigour, the trees were neither pruned nor trained. Once the trees began to flower and crop, all fruit were removed from the trees each season after first drop to avoid biennial bearing, bending of the branches (to prevent increasing precocity) and a confounding effect of the crop on tree vigour. Drip irrigation, fertilisation and pest and disease control were carried out; woven plastic mat was laid down to repress weed growth.
Architectural measurements and inflorescence assessment
Scions were phenotyped for architectural traits for the first four years of growth after grafting (years 1–4) (Table 1). Detailed architectural measurements were taken after growth cessation (June/July) in years 1–3, including trunk cross-sectional area (TCA) 20 cm above the graft union; length of main axis (length taken for each new growing cycle); and number of branches and spurs (short shoots <2.5 cm) (Table 1). Branches were classified as either sylleptic shoots, which extend in the same year they are initiated, or proleptic shoots, which extend after winter dormancy [28].
In year 3, the tree canopies were visually categorised as being small, moderate or vigorous, using QC controls as models for moderate tree growth. An example for the three vigour classifications can be seen in Fig. 1. The presence or absence of root suckers was recorded in the third year. The first inflorescence assessment was done at the beginning of year 3 and repeated in the following two springs. No ‘Comice’ scions flowered either on the seedlings or on QC control rootstocks in year 2. In year 3, the total number of inflorescences was counted and their positions recorded; this was repeated in year 4. At the beginning of year 5, the proportions of inflorescence production were estimated according to the size of the tree, relative to the tree with the highest number of inflorescences. The trees were ranked into classes from 0–4, with 0 = no flowers, 1 = 1–25 %, 2 = 26–50 %, 3 = 51–75 %, 4 = 76–100 %.
Examples of three vigour classes of trees in the second year of growth after grafting. Trees shown are 1) small, 2) moderate, 3) vigorous ‘Old Home’ x ‘Louise Bonne de Jersey’ pear rootstocks grafted with ‘Comice’. The wires indicate the height of the trees, with the wire being 0.8, 1.3, 1.8, 2.3, 2.75 m from the ground, lowest to the top respectively
Data analysis
Univariate mixed models were fitted to the data with row and a linear effect of tree position in the row as fixed effects, and genotype as the only random effect. Localized spatial trends were modelled by fitting first-order auto-correlations for tree positions [29]. The fixed effects were chosen based on an examination of the variograms when fitting the first-order auto-correlations to both row and tree position, and the auto-correlations to retain were based on likelihood ratio tests. Having determined the optimal univariate model, it was then extended to bivariate models for every pairwise set of variates. These bivariate models allowed for separate fixed and spatial effects for each variate, and also a different genetic variance for each, as well as the genetic correlation. Predicted values from these bivariate analyses were used in the QTL analysis. Data from each year were analysed separately, in order to check whether the putative QTLs were stable across years. Residual plots were examined to check for outliers and assess the validity of the normality assumption. For all variates apart from Branches_year2, a square-root transformation was used to obtain a satisfactory approximation to normality. Basic statistical analysis was carried out using Minitab 16 Statistical Software (2010 Minitab Inc.). All further analysis were conducted using R 3.0.1 [30], and the mixed models were fitted using the asreml package version 3.0-1 [31].
Genetic mapping and QTL analysis
DNA was extracted using a CTAB method [32], followed by purification with NucleoSpin® columns (Macherey-Nagel GmbH & Co. KG). DNA was quantified using a NanoDrop™ 2000c spectrophotometer (Thermo Fisher Scientific Inc.). SNP marker genotyping was performed using the apple and pear Infinium® II IRSC 9 K SNP array [33, 34] on 297 segregating individuals and both parents. Genomic DNA was amplified and hybridized to the apple and pear Infinium® II IRSC 9 K SNP array following the Infinium® HD Assay Ultra protocol (Illumina Inc., San Diego, USA) and scanned with the Illumina HiScan. Data were analysed using Illumina’s GenomeStudio v 1.0 software and genetic mapping carried out using JoinMap 3® [35]. A LOD score of 5 or higher was used for grouping and the genetic distance within the group was calculated using the Kosambi function. The linkage groups (LGs) were identified by aligning the parental maps of OH and LBJ to the map developed by Montanari et al. (2013), which contains apple and pear SSR markers from the ‘Bartlett’ consensus map of Celton et al. [24]. The map was drawn and aligned using MapChart v.2.2 [36]. The parental genetic maps were used with raw and transformed phenotypic data of tree growth, precocity and suckering for QTL analysis employing MapQTL5 [37]. For normally distributed data, Interval Mapping (IM) followed by Multiple QTL Mapping (MQM) was performed and a permutation test (1000 permutations) was used to calculate the LOD threshold for QTL significance. ANOVA was used to calculate the percentage of the phenotypic variance explained by each QTL. When normalisation of the data failed, the Kruskal-Wallis test was used for QTL detection.
Identification of the dwarfing allelotype in a pear germplasm selection
The SSR marker Hi01c04, developed by Silfverberg-Dilworth et al. [38] and identified as the proximal flanking marker for the Dw1 region on LG5 of apple [12] was screened over 96 individuals of the OHxLBJ population to determine the linkage phase between the QTL and the SSR alleles. PCR amplification was carried out using a modified version of the fluorescent M13 universal primer system [39] and a touchdown PCR programme with annealing temperature 60–55 °C (94 °C/2 min 45 s; 10 cycles: 94 °C/55 s, 60 °C/55 s (−0.5 °C per cycle); 72 °C/1 min 30 s; 30 cycles: 94 °C/55 s, 55 °C/55 s, 72 °C/1 min 30 s; 72 °C/10 min). The fragments were separated using the ABI3500 sequencer, and their size analysed with GeneMarker® v 2.2.0 software (© SoftGenetics, LLC.). The marker was then included in the OH map. The allele sizes were compared with those detected by screening the same SSR marker over 92 pear accessions from selections of germplasm from France, New Zealand, Germany and the USA, including OH and LBJ.
Finding orthologous loci in pear and apple
Apple and European pear regions were compared to identify orthologous genes using OrthoMcl2.0.3. [40]. Synteny gene blocks were detected with OrthoCluster [41]. Pyrus scaffolds were aligned to Malus scaffolds using the MUMmer 3.3 package [42]. Pear scaffolds were further filtered based on having at least two alignments, each alignment longer than 2kbp or total alignment length not shorter than 3kbp.
Results
Architectural measurements
Architectural measurements were taken on ‘Comice’ scions grafted on both the OHxLBJ segregating population and QC controls from the first to the fourth years of growth (Table 1). The phenotypic variability of the raw data is illustrated in Table 2. A wide range of vigour was observed in the grafted scions as early as in the first year of growth. In total, 343 trees (89 %) of the OHxLBJ population developed sylleptic shoots in year 1, of which 87 trees (25 %) grew more than 10 sylleptic shoots.
After proleptic shoots developed in the second year of growth, a large variability was observed in the total number of branches, with a range of zero to 107 branches per tree. After the third year, third-order branches and spurs grew off the second-order sylleptic and proleptic branches. This branching habit was repeated in the following growing cycle, resulting in a very complex tree structure which could be ranked into three vigour classes based on overall tree size, with 55 small, 200 moderate and 148 vigorous phenotypes (Fig. 1).
Flowering first occurred at the beginning of the third year of growth after grafting for 257 individuals (63 %) of the OHxLBJ population. The following spring (year 4), 398 trees flowered. In year 5, 56 of the trees (14 %) did not flower, of which only five (1 %) had never flowered before. Flowering occurred mainly on spurs and terminal buds, with an average of 10.5 flower clusters per tree in year 3 and 113.6 in year 4 for the OHxLBJ population. High numbers of axillary (1-year-old lateral bud) flower clusters were found on the scions grafted onto the QC controls in year 3, with an average of 22.5 axillary buds and 116 spurs and terminal buds per tree. The trees grafted onto OHxLBJ showed only minimal axillary flowering in year 3 and year 4, with averages of 2.2 and 4.3 respectively. In total, 247 rootstocks exhibited root suckering, while 161 did not. Root suckering was detected for 38 (69 %) out of 55 of the trees classified as small, 128 (64 %) out of 199 moderate trees, and 77 (52 %) out of 148 vigorous trees. Trees with root suckering had a significantly smaller average TCA than those without (3.65 cm2 and 4.28 cm2, respectively; p = 0.002).
Correlation between traits
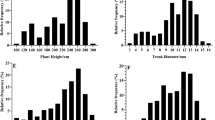
The raw phenotypic data were used to look at relationships between traits. Figure 2 shows some selected correlation graphs, while the correlation matrix for all traits, with Pearson correlations (r) and their significance values, can be found in Additional file 1: Table S1. A significant positive correlation (r = 0.81) was observed between Branches_year3 and the TCAtrunk_year4 and between the Height_year3 and the TCAtrunk_year4 (r = 0.71). As expected, the highest correlation (r = 0.9) was found between the TCAtrunk_year4 and the TCAroot_year4, showing the consistency in the measurements.
Scatterplots between different pear architectural and flower traits designed with RStudio. TCA: trunk cross-sectional area. Black circles represent ‘Old Home’ x ‘Louise Bonne de Jersey’ (OHxLBJ) seedlings and blue dots ‘Quince C’ controls. The purple line shows a “Friedman's super smoother” (span = 0.2). The correlation coefficients (shown at the top left of each plot) were calculated for the OHxLBJ values only
No strong positive correlation between flowering and architectural traits was found. However, trees that flowered early (year 3) had significantly more sylleptic branches than those that did not (Chi-square = 31.49, p-value = <0.0005) (Fig. 3). The TCA showed the strongest correlation with other traits and was therefore a representative measurement for tree vigour, becoming a stronger indicator for overall tree size with each annual growth cycle (Fig. 4). The variation in vigour of the scions budded onto the QC rootstocks indicates the environmental influence within the orchard.
Box-plots of each year’s trunk cross-sectional area (TCA) comparing three vigour classes. 1) small, 2) moderate, 3) vigorous within the ‘Old Home’ x ‘Louise Bonne de Jersey’ (OHxLBJ) pear population and the ‘Quince C’ (QC) controls. Box-plot symbols show the median, Q1 and Q3, and the highest and lowest values
Analysis of the phenotypic variability within the orchard and among genotypes
Positional effects within the orchard were accounted for by using three different linear mixed models: Model 1: first-order autocorrelation for both row and plant position within the row; Model 2: first-order autocorrelation for only the plant position; Model 3: no autocorrelation for both row and plant position. For Branches_year1-3, Height_year1 + 3, Inflorescence_year2 + 3, Spurs_year2 + 3, TCA_year3 + 4, TCAroot_year3 + 4 and TCAsec_year3, the row and plant position auto-correlation did not improve the fit. For the Height_year4, LNG_year2-4, Nodes_year2, Spurs_year1, TCA_year2 and the TCAtert_year3, the plant position improved the fit and Model 2 was used for bivariate models for QTL detection. The clonal QC controls should arguably be fitted as fixed effects. This was tested with a few key variates and the breeding values obtained were very similar to those obtained from the model described. Square root transformation was necessary to normalise the data for all traits recorded, except for Branches_year2. However, some variables (LNG_year2 + 3, Height_year1 + 2 + 3, Nodes_year2, Spurs_year1 + 3, TCA_year2 and the Inflorescence_year2 + 4) showed marked deviations from normality, even after transformation.
Genetic Map construction
High density genetic maps were constructed for both parents using 597 and 113 polymorphic pear and apple SNP markers [33] respectively (Table 3). The OH map consists of 17 linkage groups (LG) representing the 17 chromosomes of the pear genome. Only 16 linkage groups were obtained for LBJ, with LG17 being absent.
The genetic maps of OH and LBJ were aligned with parental maps of ‘Moonglow’ (Moon) and PEAR1 [33] which contain SSR markers derived from apple (Additional file 2: Figure S1).
QTL detection
QTLs were detected using the OH and LBJ parental genetic maps for the tree architecture and flowering traits across four years (Tables 4 and 5, Additional file 2: Figure S1). Significant QTLs for the control of the number of branches were detected in three successive years on LG5 and LG6 of OH. A small-effect QTL controlling Branches_year1 was located on LG6 of LBJ and was also detected in year 3. In the first year of growth after grafting, significant QTLs were detected for the TCAtrunk_year1 on LG16 and 6 of OH. The LG6 QTL was confirmed in years 2, 3 and 4, whereas the LG16 QTL was not reproducible. A QTL influencing TCAtrunk was detected on LG5 of OH in both years 3 and 4. Additional smaller-effect QTLs controlling TCAtrunk, inherited from LBJ, were detected on LG13 and LG6. QTLs influencing LNG were detected on OH LG5 in years 2–4 and these co-located with the TCAtrunk QTL. Smaller-effect QTLs controlling the LNG from LBJ were located on LG6 and LG7; however, only the LG6 QTL could be replicated across two years. QTLs controlling the TCAsec_year3 and TCAtert_year3 (only measured in year 3), Height and the Spurs per tree were detected on LG5 and LG6 of OH and LG6 and LG1 of LBJ. A QTL controlling Size_year3 was detected on LG5 and LG6 of OH, co-locating with the TCAtrunk and Height QTLs. The architectural OH LG5 QTLs explained between 5.44 % and 16.6 % of the variability for Spurs_year2 and TCAsec_year3, respectively. The variance explained for the OH LG6 QTLs ranked from 3.98 % for TCAtrunk_year3 to 16.42 % for Height_year3. The highest variance explained by any LBJ LG6 QTL was 7.72 % for the QTL controlling the TCAtert_year3, and the lowest was 4.25 % for the QTL influencing Branches_year1. A QTL controlling Inflorescence phenotyped at the beginning of the third year after grafting was detected on LG5 of OH, co-locating with the tree architecture QTLs. No flowering-related QTLs were detected segregating from LBJ. A QTL controlling Suckers_year3 was detected on LG5 of OH.
Synteny between apple and pear dwarfing QTLs
Alignment of the top of LG5 of the apple and pear genomes (Fig. 5) showed that the OH LG5 QTL for rootstock control of architecture and flowering traits is syntenic to the dwarfing and precocity Dw1 QTL detected in apple ‘M9’ rootstocks (Foster et al. 2015). The pear LG5 QTL markers with the highest LOD scores are located on scaffolds 3 and 4 on LG5 of the ’Golden Delicious’ v1.0 genome [43]. After filtering, 20 Pyrus scaffolds mapped to three Malus scaffolds (Scaffold3, 4 and 5) on LG5. Only alignments longer than 2kbp and with >90 % identity are drawn on Fig. 5. Three of the markers with the highest LOD scores for the total number of flowers (year 3) and the TCA of the trunk (year 3) were located on ‘Bartlett’ v1.0 Scaffold00014, and could be aligned with loci on Scaffold3 and Scaffold4 of the ‘Golden Delicious’ v1.0 LG5. Two other markers mapped to ‘Bartlett’ v1.0 Scaffold00214 and Scaffold00116, and were both aligned to Scaffold4 in ‘Golden Delicious’ v1.0 LG5. ‘Golden Delicious’ Scaffold3, 4 and 5 span approximately 1.33Mbp of the apple genome and the 20 ‘Bartlett’ v1.0 scaffolds cover 3.45Mbp of the European pear genome in total.
Alignment of the pear (‘Bartlett’ v1.0) and apple (‘Golden Delicious’ v1.0p) genomes in the orthologous QTL region. The Dot-plot shows the alignments (longer than 2kbp and >90 % identity) of the apple (X-axis) and European pear (Y-axis) genomes in the LG5 QTL region controlling vigour and precocity in apple and pear. “*” in front of the scaffold name represents the scaffold is aligned in the opposite direction
Dwarfing and precocity
Architectural QTLs were mainly detected on LG5 and LG6 of OH. QTLs for the control of the total number of inflorescences co-located with the architectural QTLs on LG5 of OH. The effects of the QTLs indicate that smaller trees tended to have delayed flowering. Analysis of the genotype of the marker with the highest LOD score (ss475878191) of the LG5 QTL (Table 6) demonstrated that individuals carrying the high vigour genotype (AA) had a higher tendency for precocity, with 74 % being precocious, while 50 % of the individuals with the low vigour genotype (AB) were precocious. However, only 14 % of the total population had the desired low vigour and precocious phenotype, with more individuals carrying the AB allelotype.
Detection of the LG5 precocious allele in a pear germplasm set
The microsatellite marker Hi01c04 that was located within the QTL region on LG5 (Additional file 2: Figure S1) was heterozygous in both OH (116 bp and 121 bp alleles) and LBJ (113 bp and 117 bp alleles). The 121 bp fragment was more frequent in precocious OHxLBJ segregating individuals (Table 7).
This allele was also detected in 17 European pear cultivars (P. communis and P. syriaca), including Pyrus rootstocks such as ‘Pyriam’, ‘Fox’ and some rootstocks of the ‘Old Home’ x ‘Farmingdale’ (OHxF) series. The 116 bp fragment was more frequent in OHxLBJ individuals, conferring low vigour to the scion. This allele was also detected in the dwarfing rootstock ‘Pyrodwarf’, also derived from an OHxLBJ population.
Discussion
Tree architecture and productivity are complex traits that are expressed only after several years of growth following grafting and are influenced by both genetics and environmental factors [44, 45]. Knowledge about genetic factors that confer rootstock-induced dwarfing of the scion in fruit trees is limited because of the difficulty in generating large segregating rootstock populations, as well as the requirement for robust and time-consuming phenotyping across multiple years, and the construction of dense genetic maps. Our systematic attention to these factors has enabled us to identify QTLs that control vigour and precocity in the grafted scion in pear rootstocks for the first time.
TCA is a strong indicator of tree vigour
TCA was found to be a strong indicator for overall tree size in ‘Comice’ scions grafted to our seedling rootstocks, first becoming evident in year 2 and becoming stronger with each successive annual cycle, as previously reported for apple [46]. High correlations between the TCA and other vegetative growth traits, such as the height of the tree and the number of branches, substantiate our finding.
QTLs controlling precocity and vigour
QTLs controlling both precocity and architectural traits were located on LG5 and LG6 of OH and LG6 for architectural traits segregating from LBJ. These QTLs are considered robust, as they were detected for successive years in the same position and often with the same marker having the highest LOD score. The co-location of all QTLs on LG5 and LG6, respectively, of OH indicates that the overall dwarfing effect is controlled by at least two loci. However, the variance explained by each QTL is low (4–18 %) indicating that the traits under investigation may be controlled by more loci than identified in this study. Furthermore, a strong environmental influence and a lack of replicates hampered the detection of major effect loci controlling precocity and architectural traits.
A small effect QTL for root suckering was detected on LG5 in the same genomic region as the architecture QTLs. Rootstocks of 69 % of the trees classified as dwarf produced suckers, indicating a strong correlation between dwarfing and suckering, and suggesting that the same physiological mechanisms might control both traits. We hypothesise that a reduced auxin transport from the scion to the roots, induced by Pyrus rootstocks, may promote root suckering and reduce scion vigour. This hypothesis is consistent with the findings that polar auxin transport in the xylem parenchyma inhibits suckering [47, 48], and that a reduced auxin transport in the rootstock stem occurs in dwarfed apple trees [6, 10].
Synteny between apple and pear QTLs controlling scion vigour
The QTL controlling tree architecture and flowering on pear LG5 is in a genomic region orthologous to that of Dw1, which is the major locus for dwarfing conferred on apple scions by the ‘M9’ rootstock [12, 13]. These results are consistent with our hypothesis that orthologous loci in apple and pear control scion growth and precocity conferred by the rootstock. We found that the proximal marker flanking the Dw1 locus in apple also segregates for dwarfing and precocity in pear, and that the 116 bp pear allele linked to low vigour in the rootstock mapping population is carried by the dwarfing pear rootstock ‘Pyrodwarf’. Our findings raise the possibility that the apple dwarfing locus Dw1 and the OH LG5 QTL are derived from the same source, and therefore probably existed before the divergence of apple and pear. The conservation of synteny for QTLs involved in tree architecture has enabled us to align the genomic regions of interest. This will facilitate the identification of candidate genes for dwarfing in both species and enable the testing of our hypothesis of a common origin for this locus, either before the Pyrus-Malus speciation or due to hybridization.
Indeed, Pyrus and Malus are known to infrequently cross-hybridize [49, 50] and P. communis is sympatric with M. sylvestris, a related species that contributed genetically to the ancestry of modern M. x domestica [43, 51–53], including the dwarfing rootstock ‘M9’. It is interesting to note that a second pear QTL corresponding to apple Dw2 was not detected on LG11 in the present study. Phenotypic analysis of scions grafted to segregating rootstock populations has demonstrated that the combination of Dw1 and Dw2 confers the greatest degree of rootstock-induced dwarfing in apple rootstocks [12, 14].
Tree size and precocity
We found that precocious and non-precocious trees differed significantly in the number of sylleptic branches grown in the first year, with a lower sylleptic branch number correlating with a delay in flowering. Although studies in pear indicate that the scion cultivar has a greater influence on sylleptic shoot formation than the rootstock [54, 55], Watson et al. [56] found that both the rootstock and the scion influence the number of first-year sylleptic branches. In this study we have confirmed the influence of the rootstock on early branch development in pear; however, we could not evaluate the effect of the scion. Watson et al. [56] also found that increased flowering did not result in early growth reduction in pear, and they suggested that the difference between the apple and pear rootstock dwarfing effects might lie in the length of the juvenile period.
The QTL conferring reduced sylleptic branching, tree size and TCA in pear co-locates with the QTL on LG5 conferring precocity. However, the effects of the detected QTLs are in trans, unlike the situation in apple, meaning that a smaller tree takes longer to flower. In terms of breeding, this means that it is difficult to breed for rootstocks conferring both reduced tree size and precocity to the scion cultivar. However, 14 % of the trees in our segregating rootstock population did exhibit low vigour and precocity in their grafted scions.
Assuming the application of marker assisted selection (MAS) on the basis of the ss475878191 AA allelotype, only 11 % of the individuals would have the desired low vigour and precocious phenotype. However, selection for the AB allelotype would result in a slightly higher percentage (16 %) of individuals with both desired traits in the progeny. Hence, selecting for the ss475878191 AB allele would increase the proportion of rootstocks in a breeding population that confer both precocity and a reduced vigour to the scion. However, the percentage of rootstocks conferring low vigour and precocity was generally low (14 %). It is noteworthy that in this respect, our findings for the pear rootstock QTLs differ from the effects of the apple QTL on LG5 which confers both precocity and a reduced tree size. The dwarfing effect of ‘M9’ apple rootstocks is correlated with an increase in the proportion of floral buds relative to vegetative buds and sylleptic shoots that develop within the first year of growth [11, 15]. The up-regulation of key flowering genes in ‘M9’ rootstocks may be responsible for this shift from vegetative to floral development. As a high proportion of axillary floral shoots leads to reduced vegetative growth in the next growth cycle, the tree becomes more dwarfed over time. A substantial difference between flowering in apple scions grafted onto ‘M9’ and the pear scions in this study was the position of the floral buds on the developing tree. In apple, flowering occurs in axillary and terminal buds, and, as the tree ages, is more common on 2-year-old spurs. In pear, flowering mostly occurs on two-year-old spurs and terminal buds. This biological difference has profound implications to the subsequent development of tree architecture. Apple scions grafted onto ‘M9’ flower at the beginning of the second year of growth after grafting, while pear scions on pear rootstocks do not flower until the third year of growth and thus have a longer period of vegetative growth before flowering. In pear, an increase in branching in years 1 and 2 would generate the potential for more spurs for flowering in year 3, and hence the relationship between early vegetative vigour and flowering would be opposite to that observed in apple. No QTL was detected for the percentage of axillary inflorescence in this study. It might be predicted that crossing a rootstock that increases the number of axillary buds on the scion cultivar with OH might result in a dwarfing pear rootstock, similar to ‘M9’, conferring both low vigour and precocity to the scion.
Conclusion
In this study we detected the first pear rootstock QTLs associated with control of architectural and flowering traits in scions. Furthermore, we found that these orthologous loci control scion growth and precocity in apple and pear rootstocks. These findings will facilitate the identification of candidate genes for control of scion traits by rootstocks in both species. Future research may focus on finding the common origin for the dwarfing locus in apple and pear. The application of our results in pear rootstock breeding may assist in developing a marker for MAS selection for breeding a pear rootstock that confers both vigour control and precocity to the grafted scion cultivar.
Availability of supporting data
Genetic map data used in this study can be found in Montanari et al. [33]. Phenotypic data is available on request.
Abbreviations
- ANOVA:
-
Analysis of variance
- bp:
-
Base pair
- cM:
-
CentiMorgan
- CTAB:
-
Cetyltrimethyl ammonium bromide
- DNA:
-
Deoxyribonucleic acid
- Dw1 :
-
Dwarfing locus 1
- Dw2 :
-
Dwarfing locus 2
- FT :
-
FLOWERING LOCUS
- IM:
-
Interval mapping
- INRA:
-
Institut National de Recherche Agronomique
- IRSC:
-
International RosBREED SNP Consortium
- kbp:
-
Kilo base pairs
- LBJ:
-
‘Louise Bonne de Jersey’
- LG:
-
Linkage group
- LNG:
-
Length of the new main axis growth
- LOD:
-
Logarithm of odds
- M9:
-
‘Malling 9’
- MAS:
-
Marker assisted selection
- Mbp:
-
Mega base pairs
- MQM:
-
Multiple QTL mapping
- NCBI:
-
National Centre for Biotechnology Information
- OH:
-
‘Old Home’
- OHxF:
-
‘Old Home’ x ‘Farmingdale’
- PCR:
-
Polymerase chain reaction
- PFR:
-
Plant and Food Research
- QC:
-
‘Quince C’
- QTL:
-
Quantitative trait locus
- SNP:
-
Single nucleotide polymorphism
- SSR:
-
Simple sequence repeat
- TCA:
-
Trunk cross-sectional area
References
Webster T. Dwarfing rootstocks: past, present and future. The compact fruit tree. 2002;35:67–72.
Lockard RG, Schneider GW. Stock and scion growth relationships and the dwarfing mechanism in apple. Horticultural Reviews. 1981;3:315–75.
Yonemoto Y, Matsumoto K, Furukawa T, Asakawa M, Okuda H, Takahara T. Effects of rootstock and crop load on sap flow rate in branches of ‘Shirakawa Satsuma’ mandarin (Citrus unshiu Marc.). Scientia Horticulturae. 2004;102:295–300.
Basile B, Marsal J, Dejong TM. Daily shoot extension growth of peach trees growing on rootstocks that reduce scion growth is related to daily dynamics of stem water potential. Tree Physiology. 2003;23:695–704.
Lliso I, Forner JB, Talón M. The dwarfing mechanism of citrus rootstocks F&A 418 and #23 is related to competition between vegetative and reproductive growth. Tree Physiology. 2004;24:225–32.
Soumelidou K, Battey NH, John P, Barnett JR. The Anatomy of the developing bud union and its relationship to dwarfing in apple. Annals of Botany. 1994;74:605–11.
Webster AD. Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. New Zealand Journal of Crop and Horticultural Science. 1995;23:373–82.
Jones OP. Mode-of-action of rootstock/scion interactions in apple and cherry trees. Acta Horticulturae. 1984;146:175–82.
Atkinson CJ, Else MA, Taylor L, Dover CJ. Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). Journal of Experimental Botany. 2003;54:1221–9.
van Hooijdonk BM, Woolley DJ, Warrington IJ, Tustin DS. Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot-root-shoot signalling by auxin, gibberellin, and cytokinin. Journal of Horticulural Science and Biotechnology. 2010;85:59–65.
Foster TM, Watson AE, van Hooijdonk BM, Schaffer RJ. Key flowering genes including FT-like genes are upregulated in the vasculature of apple dwarfing rootstocks. Tree Genetics and Genomes. 2014;10:189–202.
Foster TM, Celton J-M, Chagné D, Tustin DS, Gardiner SE. Two quantitative trait loci, Dw1 and Dw2, are primarily responsible for rootstock-induced dwarfing in apple. Horticulture Research. 2015. doi:10.1038/hortres.2015.1031.
Rusholme Pilcher RL, Celton JM, Gardiner SE, Tustin DS. Genetic markers linked to the dwarfing trait of apple rootstock ‘Malling 9’. Journal of the American Society for Horticultural Science. 2008;133:100–6.
Fazio G, Wan Y, Kviklys D, Romero L, Adams R, Strickland D, et al. Dw2, a new dwarfing locus in apple rootstocks and its relationship to induction of early bearing in apple scions. Journal of the American Society for Horticultural Science. 2014;139:87–98.
Seleznyova AN, Tustin DS, Thorp TG. Apple dwarfing rootstocks and interstocks affect the type of growth units produced during the annual growth cycle: precocious transition to flowering affects the composition and vigour of annual shoots. Annals of botany. 2008;101:679–87.
Dondini L, Pierantoni L, Gaiotti F, Chiodini R, Tartarini S, Bazzi C, et al. Identifying QTLs for fire-blight resistance via a European pear (Pyrus communis L.) genetic linkage map. Molecular Breeding. 2004;14:407–18.
Pierantoni L, Dondini L, Cho KH, Shin IS, Gennari F, Chiodini R, et al. Pear scab resistance QTLs via a European pear (Pyrus communis) linkage map. Tree Genetics & Genomes. 2007;3:311–7.
Won K, Bastiaanse H, Kim YK, Song JH, Kang SS, Lee HC, et al. Genetic mapping of polygenic scab (Venturia pirina) resistance in an interspecific pear family. Molecular Breeding. 2014;34:2179–89.
Sun W, Zhang Y, Le W, Zhang H. Construction of a genetic linkage map and QTL analysis for some leaf traits in pear (Pyrus L.). Frontiers of Agriculture in China. 2009;3:67–74.
Zhang R, Wu J, Li X, Khan MA, Chen H, Korban SS, et al. An AFLP, SRAP, and SSR genetic linkage map and identification of QTLs for fruit traits in pear (Pyrus L.). Plant Molecular Biology Reporter. 2012;31:678–87.
Wu J, Li LT, Li M, Khan MA, Li XG, Chen H, et al. High-density genetic linkage map construction and identification of fruit-related QTLs in pear using SNP and SSR markers. Journal of Experimental Botany. 2014;65:5771–81.
Saeed M, Brewer L, Johnston J, McGhie TK, Gardiner SE, Heyes JA, et al. Genetic metabolite and developmental determinism of fruit friction discolouration in pear. BMC Plant Biology. 2014;14:1–18.
Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, et al. Phylogeny and classification of Rosaceae. Plant Systematics and Evolution. 2007;266:5–43.
Celton JM, Tustin DS, Chagné D, Gardiner SE. Construction of a dense genetic linkage map for apple rootstocks using SSRs developed from Malus ESTs and Pyrus genomic sequences. Tree Genetics and Genomes. 2009;5:93–107.
Celton JM, Chagné D, Tustin DS, Terakami S, Nishitani C, Yamamoto T, et al. Update on comparative genome mapping between Malus and Pyrus. BMC Research Notes. 2009;2.
Yamamoto T, Kimura T, Saito T, Kotobuki K, Matsuta N, Liebhard R, et al. Genetic linkage maps of Japanese and European pears aligned to the apple consensus map. Acta Horticulturae (ISHS). 2004;663:51–6.
Chagné D, Crowhurst RN, Pindo M, Thrimawithana A, Deng C, Ireland H, et al. The draft genome sequence of European pear (Pyrus communis L. ‘Bartlett’). PLoS One. 2014;9:e92644.
Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests: an architectural analysis. Berlin: Springer Verlag; 1978.
Gilmour AR, Cullis BR, Verbyla A. Accounting for natural and extraneous variation in the analysis of field experiments. Journal of Agricultural, Biological, and Environmental Statistics. 1997;2:269–93.
R Core Development Team. R: A language and environment for statistical computing, R Foundation for Statistical Computing. Vienna: R Core Development Team; 2013.
Butler DG, Cullis BR, Gilmour AR, Gogel BJ. ASReml-R reference manual, release 2. Queensland: Department of Primary Industries and Fisheries, Brisbane, Australia; 2007.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 1987;19:11–5.
Montanari S, Saeed M, Knäbel M, Kim YK, Troggio M, Malnoy M, et al. Identification of Pyrus single nucleotide polymorphisms (SNPs) and evaluation for genetic mapping in European pear and inter-specific Pyrus hybrids. PLoS One. 2013;8, e77022.
Chagné D, Crowhurst RN, Troggio M, Davey MW, Gilmore B, Lawley C, et al. Genome-wide SNP detection, validation, and development of an 8K SNP array for apple. PLoS One. 2012;7, e31745.
van Ooijen JW, Voorrips RE. JoinMap (Version 3.0). Wageningen: University and Research Center; 2001.
Voorrips RE. MapChart: Software for the graphical presentation of linkage maps and QTLs. Journal of Heredity. 2002;93:77–8.
van Ooijen JW. MapQTL® 5, Software for the mapping of quantitative trait loci in experimental populations. Wageningen: Kyazma, B.V.; 2004.
Silfverberg-Dilworth E, Matasci CL, van de Weg WE, van Kaauwen MPW, Walser M, Kodde LP, et al. Microsatellite markers spanning the apple (Malus x domestica Borkh.) genome. Tree Genetics and Genomes. 2006;2:202–24.
Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology. 2000;18:233–4.
Li L, Stoeckert Jr CJ, Roos DS. OrthoMCL: Identification of ortholog groups for Eukaryotic genomes. Genome Research. 2003;13:2178–89.
Zeng X, Pei J, Vergara IA, Nesbitt MJ, Wang K, Chen N. Proceedings of the 11th Conference of Extending Database Technology EDBT’08. 2008. Orthocluster: A new tool for mining synteny blocks and applications in comparative genomics. New York; ACM Press. pp. 656–667.
Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, et al. Versatile and open software for comparing large genomes. Genome Biology. 2004;5.
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, et al. The genome of the domesticated apple (Malus x domestica Borkh.). Nature Genetics. 2010;42:833–41.
Segura V, Durel C-E, Costes E. Dissecting apple tree architecture into genetic, ontogenetic and environmental effects: QTL mapping. Tree Genetics and Genomes. 2009;5:165–79.
Barthélémy D, Caraglio Y. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany. 2007;99:375–407.
Westwood MN, Roberts AN. The relationship between trunk cross-sectional area and weight of apple trees. Journal of the American Society for Horticultural Science. 1970;95:28–30.
Farmer Jr RE. Aspen sucker formation and apical dominance. Forest Science. 1962;8:403–12.
Wan X, Landhäusser SM, Lieffers VJ, Zwiazek JJ. Signals controlling root suckering and adventitious shoot formation in aspen (Populus tremuloides). Tree Physiology. 2006;26:681–7.
Crane MB, Marks E. Pear-apple hybrids. Nature. 1952;170:1017.
Fischer TC, Malnoy M, Hofmann T, Schwab W, Palmieri L, Wehrens R, et al. F1 hybrid of cultivated apple (Malus × domestica) and European pear (Pyrus communis) with fertile F2 offspring. Molecular Breeding. 2014;34:817–28.
Cornille A, Giraud T, Bellard C, Tellier A, Le Cam B, Smulders MJ, et al. Postglacial recolonization history of the European crabapple (Malus sylvestris Mill.), a wild contributor to the domesticated apple. Molecular Ecology. 2013;22:2249–63.
Cornille A, Gladieux P, Smulders MJ, Roldan-Ruiz I, Laurens F, Le Cam B, et al. New insight into the history of domesticated apple: secondary contribution of the European wild apple to the genome of cultivated varieties. PLoS Genet. 2012;8, e1002703.
Yamamoto T, Chevreau E. Pear genomics. In: Folta K, Gardiner S, editors. Genetics and Genomics of Rosaceae. New York: Springer; 2009. p. 163–86.
Milošević T, Milošević N. Growth and branching of pear trees (Pyrus domestica, Rosaceae) in nursery. Acta Scientiarum Polonorum-Hortorum Cultus. 2010;9:193–205.
Tadeusz J. The role of cultivar and rootstock insylleptic shoot formation in maiden pear trees. Journal of Fruit and Ornamental Plant Research. 2004;12:41–7.
Watson AE, Seleznyova AN, Dayatilake GA, Tustin DS. Rootstocks affect pear (Pyrus communis) tree growth through extent of node neoformation and flowering with key differences to apple. Functional Plant Biology. 2012;39:493–502.
Postman J, Kim D, Bassil N. OHxF paternity perplexes pear producers. Journal of the American Pomological Society. 2013;67:157–67.
Acknowledgements
This work was funded by the New Zealand Ministry of Business Innovation and Employment grant “Pipfruit: a Juicy Future” [Contract number: 27744] and “Future Orchard Planting Systems” [Contract number: 30467]. We want to thank Lester Brewer for making the cross, Sara Montanari for providing the reference map, Shona Seymour and Angela Shirtliff for help with phenotyping the trees, and Robert Lamberts for providing the photographs of the trees. We also want to thank several people for providing leaves and DNA from their pear germplasm: Nahla Bassil and Bruce R Bartlett from the USDA-ARS (USA), Marie-Helene Simard from INRA (France), Peter Braun from Hochschule Geisenheim University (Germany), Erik Schulte and Andreas Zschammer from Bundessortenamt (Germany), Kate M Evans from East Malling Research (UK), and Lester Brewer from Plant & Food Research (NZ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: DC DST RS TF JWP. Performed the experiments: MK. Phenotyping: CW MK RD APF. Analysed the data: MK CD PA. Contributed reagents/materials/analysis tools: CW JWP PA SEG. Wrote the paper: MK DC SEG. All authors read and approved the final manuscript.
Authors' information
Not applicable.
Availability of data and materials
Not applicable.
Additional files
Additional file 1: Table S1.
Pearson correlation (first cell) and P-value (second cell) of all the traits measured over four years in the ‘Old Home’ x ‘Louise Bonne de Jersey’ OHxLBJ segregating pear population. Branches: branches per tree; Height: total tree height; Inflorescence: inflorescences per tree; Nodes: nodes per tree; Spurs: spurs per tree; TCAtrunk: trunk cross-sectional area 20 cm above graft unit; TCAroot: TCA of rootstock; TCAsec: TCA secondary growth of the main axis; TCAtert: TCA tertiary growth of the main axis. (PDF 164 kb)
Additional file 2: Figure S1.
Alignment of linkage groups from ‘Louise Bonne de Jersey’ (LBJ) and ‘Old Home’ (OH) pears with the maps of ‘Moonglow’ (Moon) and PEAR1 (Montanari et al., 2013). The markers are named using the NCBI dbSNP accessions and their positions are indicated in centiMorgan. Microsatellite markers mapped in the ‘Moonglow’ x PEAR1 population are underlined. The linkage group (LG) numbering system is consistent with the apple LG numbering. Identified QTLs are shown with blue symbols coming from OH and brown symbols from LBJ. The Dw1 flanking marker Hi01c04 (underlined and red) mapped to LG5 of OH. (PDF 306 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Knäbel, M., Friend, A.P., Palmer, J.W. et al. Genetic control of pear rootstock-induced dwarfing and precocity is linked to a chromosomal region syntenic to the apple Dw1 loci. BMC Plant Biol 15, 230 (2015). https://doi.org/10.1186/s12870-015-0620-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-015-0620-4