Abstract
Background
APOE ε4 allele is most common genetic risk factor for Alzheimer’s disease (AD) and cognitive decline. However, it remains poorly understood why only some carriers of APOE ε4 develop AD and how ethnic variabilities in APOE locus contribute to AD risk. Here, to address the role of APOE haplotypes, we reassessed the diversity of APOE locus in major ethnic groups and in Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset on patients with AD, and subjects with mild cognitive impairment (MCI), and control non-demented individuals.
Results
We performed APOE gene haplotype analysis for a short block of five SNPs across the gene using the ADNI whole genome sequencing dataset. The compilation of ADNI data with 1000 Genomes identified the APOE ε4 linked haplotypes, which appeared to be distant for the Asian, African and European populations. The common European ε4-bearing haplotype is associated with AD but not with MCI, and the Africans lack this haplotype. Haplotypic inference revealed alleles that may confer protection against AD. By assessing the DNA methylation profile of the APOE haplotypes, we found that the AD-associated haplotype features elevated APOE CpG content, implying that this locus can also be regulated by genetic-epigenetic interactions.
Conclusions
We showed that SNP frequency profiles within APOE locus are highly skewed to population-specific haplotypes, suggesting that the ancestral background within different sites at APOE gene may shape the disease phenotype. We propose that our results can be utilized for more specific risk assessment based on population descent of the individuals and on higher specificity of five site haplotypes associated with AD.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is the most frequent case of dementia worldwide, which is manifested by a progressive decline in cognitive function due to loss of neurons, white matter, and synapses. Although it is thought to be caused by progressive accumulation of diffuse and neuritic extracellular amyloid plaques and intracellular neurofibrillary tangles in the brains of AD patients, the etiological mechanisms underlying the neurodegeneration process remain unclear. Since its conception in 2004, Alzheimer’s Disease Neuroimaging Initiative (ADNI, http://www.adni-info.org/) has been searching for associations between MRI brain profiles, biomarkers and clinical symptoms. To date, the significant progress has been made for neuroimaging of the ADNI subjects and in identifying potentially predictive biomarkers for AD [1,2,3,4,5,6]. Importantly, the whole genome sequencing (WGS) has also been performed for > 800 subjects in ADNI cohort, including AD-patients, individuals with Mild Cognitive Impairment (MCI) and healthy control individuals (CT) (Materials and methods).
Since 1993, the highly significant association of AD with APOE ε4 allele has been demonstrated for various ethnic populations [7]. Two missense nucleotide polymorphisms (SNPs) of APOE, i.e. rs429358 at codon 112 and rs7412 at codon 158, determine the genotype of APOE for ε2, ε3, and ε4 protein isoforms. In particular, APOE ε2 represents the major rs429358 variant and minor rs7412 variant (TT haplotype, correspondingly), while APOE ε3 is presented by (TC) and APOE ε4 by (CC) haplotypes. Notably, it was ascertained that sole rs429358 is the most common AD-associated variant.
APOE gene encodes a plasma apolipoprotein protein E that plays a prominent role in lipid metabolism and cholesterol transport in human tissues [8, 9]. Apolipolipoprotein E maintains affinity for receptors involved in the clearance of remnants of very low density lipoproteins [10]. The biological activity of APOE can be altered by modification of its structure. The APOE isoforms, E2, E3 and E4, are metabolically distinct and differ in their affinity for lipoprotein particles and low-density lipoprotein receptors [11, 12]. Possession of the APOE ε4 allele, the strong genetic factor for AD, facilitates the Aβ deposition from the presymptomatic stage of AD in a gene-dosage-dependent manner. In contrast, the APOE ε2 allele appears to decrease AD risk. [13]. Recently, the APOE isoforms are shown to differentially modulate the cellular uptake of Aβ mediated by sortilin related receptor 1 (LR11/SorLA) [14]. Thus, it is also plausible that APOE isoforms differentially induce the AD pathology through their cooperation with LR11/SorLA, which is involved in the lysosomal targeting of extracellular amyloid-β (Aβ) [15]. However, the exact molecular mechanism underlying the genetic association of AD with APOE [16] remains poorly understood. The presumable DNA methylation shifts for APOE alleles in aging may potentially contribute to differential regulation of APOE alleles [17, 18]. Interestingly, the pattern of AD-association with APOE varies across human populations. For example, APOE ε4 association with AD is lower or even lacking in African-Americans, Hispanic or Yoruban-African populations [19,20,21]. At the same time, the risk of developing AD in APOE ε4 carriers can be modified by other genetic variants, for example, allele G of rs2373115 in gene GAB2 was reported to increase the risk [22].
In this study, we investigated the population dynamics of APOE haplotypes and their association with AD development. We also assessed APOE methylation profile and found that some intragenic SNPs can be connected to APOE DNA methylation shift.
Datasets and methods
ADNI data
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI was to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
Whole genome sequencing data for 809 subjects in VCF format have been downloaded from ADNI web site. It comprised 183 AD-affected individuals, 370 Mild Cognitive Impairment (MCI) ones and 256 of healthy individuals (CT) (http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_WGS_Notice_20130917.pdf). The dataset consists mainly of White individuals (94%). The variant data were called using Illumina’s CASAVA SNP Caller (http://support.illumina.com/sequencing/sequencing_software/casava.html) and are available in VCF format.
1000 Genomes data
We prepared a subset of 1000 Genomes data for 3 populations: (a) African (97 individuals from Luhya in Webuye, Kenya (LWK); 88 individuals of Yoruba in Ibadan, Nigeria (YRI); 185 individuals total), (b) East Asian (Han Chinese Southern (CHS0—100 individuals, Han Chinese in Bejing (CSB)—97 individuals, Japanese in Tokyo (JPT)—89 individuals; 286 total); (c) European sample (88 individuals from British in England and Scotland (GBR); 98 individuals from Toscani in Italia (TSI); 94 individuals from Finnish in Finland (FIN); 85 individuals from Utah Residents (CEPH) with Northern and Western European Ancestry; 365 total). The data is available at http://www.internationalgenome.org/data/.
Association analysis
For association analysis in APOE locus we excluded all genetic variants with allele frequency (MAF) less than 1%, call rates less than 98% or not in Hardy–Weinberg equilibrium (P < 10−4 in controls). Then we used R-based GenABEL program [23] for assessing the association with AD, using a AD individuals as cases and excluding MCI individuals from analysis. Using the threshold of 5E−8 to select the statistically significant associations with AD phenotype resulted in 27 SNPs from chromosome 19 in APOE vicinity (Additional file 1: Table S1; Additional file 2: Fig. S1). Two of these 27 SNPs were located within APOE gene (Table 1).
Haplotype analysis
We used Arlequin ver 3.5 [24], Haploview (https://www.broadinstitute.org/haploview/downloads), PLINK [25] software for assessing linkage disequilibrium and haplotype inferences in APOE locus region. In particular, we used ‘haploblocks’ option of Haploview to segregate SNPs into blocks based on ‘tagged’ SNPs [26] algorithm. A block is defined as consequent SNP set over which 95% of informative pairwise comparisons are in strong LD [26].
We inferred the most likely gametic phases of 5 locus genotypes using a pseudo-Bayesian approach (ELB algorithm) [24]. Based on the phased haplotype profiles we inferred the ML haplotype phylogenetic tree for 5 SNPs within the APOE locus. Due to strong linkage disequilibrium at the region [16], the in silico phasing has proved to be non-ambiguous and thus efficient in this particular case. The pairwise comparison of haplotype frequencies has been carried out by Conventional F-test implemented in Arlequin software [24]. In particular, Average number of pairwise differences between populations P(X,Y) has been calculated, then average pairwise differences within populations has been carried out denoted by P(X). Lastly, the corrected pairwise differences between populations was calculated as P’(X,Y) = (P(X,y) − (P(X) + P(Y))/2) [24]. P values have been calculated by Monte-Carlo Method based on 100,000 simulations for each pair.
We used XLStat software for Principal Components analysis (www.xlstat.com).
Haplotype-specific association analysis was performed with Fisher’s exact test against the most common European haplotype (GGATC).
Phylogenetic analysis
PHYLIP DNAML software was used to build an unrooted phylogenetic tree of observed haplotype sequences.
Methylation profile analysis
For methylation analysis of APOE, we used ENCODE epigenetic profiles for 63 cell lines obtained using Illumina Infinium Human Methylation 450 platform (http://www.genome.ucsc.edu/cgi-bin/hgTrackUi?hgsid=612438305_2wY2cahV1lPNoxcvDm5zHm99hpjz&c=chr19&g=wgEncodeHaibMethyl450) and DNA methylation profiles of human fetal brain [27].
Results
Analysis of SNP frequencies in APOE gene revealed that only five of them are common in human population with frequency > 5% (Fig. 1, Table 2). Three of these SNPs are non-coding variants. The other two (rs429358 and rs7412) are missense variations that define APOE ε2, ε3 and ε4 isoforms. We focused on haplotype analysis of these five genetic variants.
In line with previous reports, we found multiple SNPs in APOE locus to have a significant association to AD (Additional file 1: Table S1; Additional file 2: Fig S1). However, only two of the above five SNPs in APOE gene (rs769449 and rs429358) show statistically significant association with AD (Table 1).
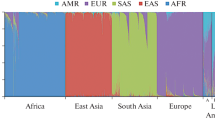
Next, we conducted a haplotype-based analysis of the five SNPs. Pairwise linkage analysis revealed that rs769449 and rs429358 are linked in Asian individuals (r2 = 0.956), but have a lower linkage in European sample (r2 = 0.828), while rs769449 is not polymorphic in Africans. Further, using Arlequin software, we assessed haplotype frequencies (haplotypes with f > 0.01) for six human cohorts (Fig. 2, Table 3).
Haplotype frequencies in human populations and ADNI cohort. X-axis labels represent allelic status of 5 SNPs (rs440446, rs769449, rs769450, rs429358,rs7412; Table 2) in APOE locus. Haplotype associated with APOE ε4 are in bold italic
Analysis of these data demonstrated that haplotype frequency profiles are distinct in human populations (Fig. 3, Table 3). This is supported by statistical analysis, which demonstrated that each pair of populations/cohorts are significantly different (P value < 1e−4), except, for 1000 Genomes European population versus ADNI Control cohort (Table 4). The most common haplotypes (which are presented by APOE ε3-bearing alleles) have different frequencies across populations: GGGTC is almost absent in Europeans (< 1%) and Asians (< 2%), but common in Africans (20%), CGGTC is present in 62% of Asians, but at lower frequencies in Europeans (36%) and Africans (13%). We also observed that two most common ε4—bearing haplotypes have a clear population-specific patterns. GAGCC is present exclusively in Asian and European populations and absent in African population. In contrast GGGCC is the only ε4—bearing haplotype presented in 29% of African individuals, but occurs at low frequencies in Asian and European groups (< 2%). Surprisingly, a protective APOE ε2 allele is presented almost exclusively by a single haplotype GGGTT in all human populations with 6–8% frequency. Thus, this allele has a lower population diversity.
Principal Components analysis based on the haplotype frequencies distribution across 6 populations (Afr, Ori, Eur, AD, MCI, CT). Haplotype encoding corresponds to Table 2. AD—associated haplotypes are marked by the bold italic type
Phylogenetic analysis of APOE haplotypes revealed that APOE ε4 haplotype GGGCC, which is African-specific, is most likely the ancestral variant (Fig. 4). This suggests that a common APOE ε3 allele was distributed in human populations after the split with other archaic hominins.
Phylogenetic tree of APOE haplotypes. GAGCC is the disease haplotype specific to Europeans (Table 3). The AD-associated haplotype subset is encircled
Comparing nucleotide content in the two ε4-bearing haplotypes (GGGCC and GAGCC) we observed that allele G of second SNP (rs769449) separates Africans from individuals of European and Asian ancestry. When we compared these two haplotypes to the most common European haplotype (GGATC), we found that both are significantly associated with AD (Fisher’s exact test P value < 1e−12 and P value < 1e−4), but only GAGCC is associated with MCI. Altogether the data suggests, the state of this SNP might have a modifying effect on ε4-associated AD/MCI risk development with African-specific allele G being potentially protective, in particular, in African populations.
This SNP is non-coding and therefore might have a regulatory effect on APOE. Potentially, A vs G allele in rs769449 can modify the epigenetic state in the APOE gene region. Supporting this hypothesis, we observed a robust H3K4Me3 signal using ChIP-seq data in this rs769449-containing region (Fig. 5; encircled) that is common mark of open chromatin. We assessed methylation profile of APOE locus based on ENCODE HAIB methylation data performed using Illumina Human Methylation 450 K Bead Arrays (Fig. 5) [18, 27]. While the methylation profile is U-shaped, the region from TSS down to exon 4 is highly sensitive to methylation [18], and comprises a range of transcription factor binding sites (Additional file 1: Table S2). The methylation rate of this region, which includes the SNP rs769449, is anticorrelated with APOE expression rate and is significantly associated with aging [18]. It is also located 78 bp downsteam to second APOE exon. The methylation state in this region is changed in aging and associated with APOE dysfunction [18]. The rs769449 context is (gGc) and, when turning to A, one of the methylation sites drops out, thus possibly altering intragenic methylation profile. A set of transcription factor binding sites in the areas of SNPs rs769449 also implies its possible regulatory effect (Additional file 1: Table S2).
Methylation profile of the APOE locus. a Genomic location of Illumina Methyl 450 bead array probes; b methylation profile of APOE gene based on two methylome projects. 63 HAIB cell lines (HAIB ENCODE methylation data), and 179 fetal brain samples [27] were used. Vertical dotted bars correspond to standard deviation of methylation score. Arrows indicate age related methylation drive [18]
It is worth noting that at least three out of five SNPs affect the CG dinucleotide content in APOE gene. APOE ε4 bears two CG dinucleotides mediated by rs429358 (minor allele) and rs7412 (major allele) that reside in the CpG island of exon 4 (Fig. 5) [18, 28]. rs769450 does not affect CG content (Table 2), while rs440446, the first target haplotype SNP meditates the CG dinucleotide arisen by minor allele, similar to the last ones,. Thus, the APOE ε4-bearing haplotypes maintain the largest number of CG dinucleotides within APOE (Table 3). Notably, rs769449 mediates CG dinucleotide in the inverse strand. It is resided within hotspot of H3K3me3 region (Fig. 6; encircled), and its C→A transition might affect the binding site of the transcription factor (Additional file 1: Table S1). Notably, the target Illumina Methylation 450 array CpG site cg06750524 located close to rs769449 (Fig. 6; encircled) methylation status is highly associated with APOE ε4 allele: it was reported that its methylation rate is higher for the minor “disease” allele [18].
UCSC genome browser screenshot depicting active H3K4me3 spot in the vicinity of rs769449 (encircled) along with previously associated with APOE ε4 cg06750524 methylation status [18]
Discussion
APOE gene maintains the highest genetic association with AD reported to date. However, the association is ethnic- dependent, e.g., the evidence for AD-association with APOE is lower for African-Americans, Hispanic or Yoruban-African populatuion [19,20,21]. We have demonstrated that frequencies of APOE haplotypes a significantly different in human populations (Fig. 2, Table 4). Specifically, the context of APOE ε4, which is the AD risk allele, drastically differs in populations (Fig. 3, Table 3). In particular, the two haplotypes for AD-associated APOE ε4 variant are GGGCC for African, and CAGCC for European and Asian individuals.
Sequence analysis of the chimpanzee APOE gene showed that it is most closely related to human ε4-type haplotypes, differing from the human consensus sequence at 67 synonymous (54 substitutions and 13 indels) and 9 nonsynonymous fixed positions [29]. Our analysis showed further that haplotypes defining the ε3 and ε2 alleles are derived from the ancestral ε4 s and that the ε3 group of haplotypes have increased in European and Asian populations.
The issue of ancestry of APOE ε4 allelotype has been widely discussed [30], and it has been established that the C→ T variant for ε3 allele arose after primate radiation [30]. Its relatively rapid expansion could be attributed to converging to meat diet in ancient human populations [30]. The data suggest also that specific APOE haplotypes might have protective effect against AD development potentially via epigenetic reprogramming of APOE due to CpG emergence/dropout [18, 28]. Altogether, our data demonstrated that ethnic genetic background defines significant differences in haplotypes for AD- risk alleles in human populations that may potentially be additional factor modifying risk for AD.
Abbreviations
- AD:
-
Alzheimer’s disease
- LOD:
-
Logarithm of odds
- SNPs:
-
Single nucleotide polymorphisms
References
Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kimura H, Kakita A, Takahashi H, Tsuji S, Kanazawa I, Ihara Y, Odani S, Kuwano R. Japanese genetic study consortium for Alzheimer Disease. Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93(5):441–8. https://doi.org/10.1016/j.ygeno.2009.01.003.
Liu M, Bian C, Zhang J, Wen F. Apolipoprotein E gene polymorphism and Alzheimer’s disease in Chinese population: a meta-analysis. Sci Rep. 2014;4:4383. https://doi.org/10.1038/srep04383.
Lutz MW, Sundseth SS, Burns DK, Saunders AM, Hayden KM, Burke JR, Welsh-Bohmer KA, Roses AD. A Genetics-based biomarker risk algorithm for predicting risk of Alzheimer’s Disease. Alzheimers Dement (N Y). 2016;2(1):30–44.
Anderson ED, Wahoske M, Huber M, Norton D, Li Z, Koscik RL, Umucu E, Johnson SC, Jones J, Asthana S, Gleason CE. Alzheimer’s disease neuroimaging initiative. Cognitive variability-a marker for incident MCI and AD: an analysis for the Alzheimer’s Disease neuroimaging initiative. Alzheimers Dement (Amst). 2016;4:47–55. https://doi.org/10.1016/j.dadm.2016.05.003.
Luo X, Qiu T, Jia Y, Huang P, Xu X, Yu X, Shen Z, Jiaerken Y, Guan X, Zhou J, Zhang M. ADNI. Intrinsic functional connectivity alterations in cognitively intact elderly APOE ε4 carriers measured by eigenvector centrality mapping are related to cognition and CSF biomarkers: a preliminary study. Brain Imaging Behav. 2017;11(5):1290–301. https://doi.org/10.1007/s11682-016-9600-z.
Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, Foroud T, Pankratz N, Moore JH, Sloan CD, Huentelman MJ, Craig DW, Dechairo BM, Potkin SG, Jack CR Jr, Weiner MW, Saykin AJ. Alzheimer’s Disease neuroimaging initiative. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: a study of the ADNI cohort. Neuroimage. 2010;53(3):1051–63.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3.
Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8(1):1–21.
Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50(Suppl):S183–8. https://doi.org/10.1194/jlr.R800069-JLR200.
Blum CB, Type III. Hyperlipoproteinemia: still Worth Considering? Prog Cardiovasc Dis. 2016;59(2):119–24. https://doi.org/10.1016/j.pcad.2016.07.007.
Hui DY, Innerarity TL, Mahley RW. Defective hepatic lipoprotein receptor binding of beta-very low density lipoproteins from type III hyperlipoproteinemic patients. Importance of apolipoprotein E. J Biol Chem. 1984;259(2):860–9.
Mooijaart SP, Berbée JF, van Heemst D, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG. APOE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3(6):e176.
Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human APOE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3(89):89ra57. https://doi.org/10.1126/scitranslmed.3002156.
Yajima R, Tokutake T, Koyama A, Kasuga K, Tezuka T, Nishizawa M, Ikeuchi T. APOE-isoform-dependent cellular uptake of amyloid-β is mediated by lipoprotein receptor LR11/SorLA. Biochem Biophys Res Commun. 2015;456(1):482–8. https://doi.org/10.1016/j.bbrc.2014.11.111.
Caglayan S, Takagi-Niidome S, Liao F, Carlo AS, Schmidt V, Burgert T, Kitago Y, Füchtbauer EM, Füchtbauer A, Holtzman DM, Takagi J, Willnow TE. Lysosomal sorting of amyloid-β by the SORLA receptor is impaired by a familial Alzheimer’s disease mutation. Sci Transl Med. 2014;6(223):223ra20. https://doi.org/10.1126/scitranslmed.3007747.
Jun G, Vardarajan BN, Buros J, Yu CE, Hawk MV, Dombroski BA, Crane PK, Larson EB; Alzheimer’s Disease Genetics Consortium., Mayeux R, Haines JL, Lunetta KL, Pericak-Vance MA, Schellenberg GD, Farrer LA. Comprehensive search for Alzheimer disease susceptibility loci in the APOE region. Arch Neurol. 2012;69(10):1270-1279. https://doi.org/10.1001/archneurol.2012.2052.
Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS ONE. 2008;3(7):e2698. https://doi.org/10.1371/journal.pone.0002698.
Ma Y, Smith CE, Lai CQ, Irvin MR, Parnell LD, Lee YC, Pham L, Aslibekyan S, Claas SA, Tsai MY, Borecki IB, Kabagambe EK, Berciano S, Ordovás JM, Absher DM, Arnett DK. Genetic variants modify the effect of age on APOE methylation in the genetics of lipid lowering drugs and diet network study. Aging Cell. 2015;14(1):49–59. https://doi.org/10.1111/acel.12293.
Barnes LL, Bennett DA. Alzheimer’s disease In African Americans: risk factors and challenges for the future. Health Aff (Project Hope). 2014;33(4):580–6. https://doi.org/10.1377/hlthaff.2013.1353.
Hendrie HC, Murrell J, Baiyewu O, Lane KA, Purnell C, Ogunniyi A, Gao S. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr. 2014;26(6):977–85. https://doi.org/10.1017/S1041610214000167.
Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–5.
Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Huentelman MJ, Craig DW, Coon KD, Liang WS, Herbert RH, Beach T, Rohrer KC, Zhao AS, Leung D, Bryden L, Marlowe L, Kaleem M, Mastroeni D, Grover A, Heward CB, Ravid R, Rogers J, Hutton ML, Melquist S, Petersen RC, Alexander GE, Caselli RJ, Kukull W, Papassotiropoulos A, Stephan DA. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54(5):713–20.
Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23(10):1294–6.
Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10(3):564–7. https://doi.org/10.1111/j.1755-0998.2010.02847.x.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75.
Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9.
Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CC, O’Donovan MC, Bray NJ, Mill J. Methylomic trajectories across human fetal brain development. Genome Res. 2015;25(3):338–52. https://doi.org/10.1101/gr.180273.114.
Yu CE, Foraker J. Epigenetic considerations of the APOE gene. Biomol Concepts. 2015;6(1):77–84. https://doi.org/10.1515/bmc-2014-0039.
Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengârd JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet. 2000;67(4):881–900.
Finch CE. Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1718–24. https://doi.org/10.1073/pnas.0909606106.
About this supplement
This article has been published as part of BMC Neuroscience Volume 19 Supplement 1, 2018: Selected articles from Belyaev Conference 2017: neuroscience. The full contents of the supplement are available online at https://bmcneurosci.biomedcentral.com/articles/supplements/volume-19-supplement-1.
Authors’ contributions
E IR, DAA and VNB participated in project conception and in the study design. EIR and FEG coordinated downloading of genotype data from the ADNI web site. VNB and FEG assessed linkage disequilibrium and haplotype inferences in APOE locus region; VNB performed PCA analysis and analyzed methylation profiles. DAA and AVK performed statistical analysis with GenABEL program. EVI contributed to data interpretation. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to anonymous reviewers for their valuable comments on our manuscript. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense Award Number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Evgeny I. Rogaev: For the Alzheimer’s Disease Neuroimaging Initiative (ADNI): ADNI data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The ADNI dataset analyzed in this study is available from ADNI consortium upon request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The present study was supported in part by the Government of the Russian Federation, Grant No. 14.B25.31.0033, Resolution No. 220 (assessing linkage disequilibrium and haplotype inferences in APOE locus region, haplotype and PCA analysis), in part, E.I.R. supported by R01AG54712-02 and ADDF. Dmitry A. Afonnikov and Anton V, Klimov acknowledge the support of the statistical analysis with GenABEL program by the Federal Agency of Scientific Organizations, Project 0324-2018-0017. This work was also supported in part by Russian Science Foundation #14-44-00077 (ADNI data analysis) and #14-50-00029 (population analysis). The publication costs for this article were funded by the project 0324-2018-0017.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
27 significant SNPs in the APOE region. Table S2. Transcription factor binding sites annotation related to SNPs analysed.
Additional file 2: Fig. S1.
Output of GenABEL [23] program underlining chromosome 19 APOE region significance on ADNI sample.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Babenko, V.N., Afonnikov, D.A., Ignatieva, E.V. et al. Haplotype analysis of APOE intragenic SNPs. BMC Neurosci 19 (Suppl 1), 16 (2018). https://doi.org/10.1186/s12868-018-0413-4
Published:
DOI: https://doi.org/10.1186/s12868-018-0413-4