Abstract
Background
Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) also known as tobacco caterpillar, is one of the most serious polyphagous pests that cause economic losses to a variety of commercially important agricultural crops. Over the past few years, many conventional insecticides have been used to control this pest. However, the indiscriminate use of these chemicals has led to development of insecticide resistant populations of S. litura in addition to harmful effects on environment. Due to these ill effects, the emphasis is being laid on alternative eco-friendly control measures. Microbial control is one of the important components of integrated pest management. Thus, in search for novel biocontrol agents, the current work was carried out with the aim to evaluate the insecticidal potential of soil bacteria against S. litura.
Results
Among the tested soil bacterial isolates (EN1, EN2, AA5, EN4 and R1), maximum mortality (74%) was exhibited by Pseudomonas sp. (EN4). The larval mortality rate increased in a dose-dependent manner. Bacterial infection also significantly delayed the larval development, reduced adult emergence, and induced morphological deformities in adults of S. litura. Adverse effects were also detected on various nutritional parameters. The infected larvae showed a significant decrease in relative growth and consumption rate as well as efficiency of conversion of ingested and digested food to biomass. Histopathological studies indicated damage to the midgut epithelial layer of larvae due to the consumption of bacteria treated diet. The infected larvae also showed a significantly decreased level of various digestive enzymes. Furthermore, exposure to Pseudomonas sp. also caused DNA damage in the hemocytes of S. litura larvae.
Conclusion
Adverse effects of Pseudomonas sp. EN4 on various biological parameters of S. litura indicate that this soil bacterial strain may be used as an effective biocontrol agent against insect pests.
Similar content being viewed by others
Introduction
Insect pests are a major threat to various agricultural crops throughout the world. Many of the agricultural insect pests belong to the order Lepidoptera, which is the second largest order of class Insecta. Among lepidopterans, the common cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is one of the major polyphagous pests of agricultural crops [1]. It is an economic pest in India, China and Asia–Pacific regions where it causes losses to many economically important cultivated field crops and vegetables [2]. It has been reported to damage 112 species of plants belonging to 44 families that include 40 species from India [3]. In recent years, there has been an increase in the occurrence of S. litura in India causing severe economic losses to commercial and vegetable crops including soybean, cabbage, cauliflower, groundnut etc. [4,5,6]. Approximately, 47% yield losses have been reported in groundnut due to S. litura in India [7]. Recently, Sahu et al. [5] documented 54% infestation of this pest on cabbage crop. S. litura completes a number of generations per year that occasionally overlap [8]. The females exhibit a strong migratory ability and high reproductive potential [9]. Early larval instars are gregarious and mostly scrape the soft part of leaves, while later instars fully defoliate the plants when present in large numbers, and cause significant crop losses. Chemical control is the most commonly used strategy by farmers to manage this pest.
To improve agricultural productivity, pesticides continue to be a significant input in modern agriculture. Synthetic pesticides are effective against a variety of insect species because they are cheap, easily available, fast-acting, and highly reliable. A single application can control a variety of pest species and leaves a persistent residue that kills insects for hours or even days after its application [10]. For the control of Spodoptera spp., a number of insecticides from various chemical classes are used, either individually or in combination. The common conventional and some new chemistry insecticides used against S. litura include lambda-cyhalothrin, chlorpyrifos, quinolphos, deltamethrin, cypermethrin, spinosad, abamectin, indoxacarb, emamectin benzoate, lufenuron etc. [11, 12]. However, these insecticides also result in a direct impact on human health and the environment. Besides contaminating the soil, air and water bodies, they also adversely affect the non-target organisms [13]. One of the most serious problems associated with the use of synthetic insecticides is the development of resistance in insects. Currently, high levels of resistance have been reported in various lepidopteran pests to many insecticides including organochlorines, organophosphates, carbamates and pyrethroids, [14, 15]. Due to its polyphagous nature, S. litura has been exposed to a number of insecticides. There are reports indicating the development of varying levels of resistance in S. litura to many of the conventional insecticides [16,17,18]. In such situations, where chemicals are causing a harmful impact on environment, the use of biopesticides has emerged as a sustainable alternative for the suppression of insect pests. Biopesticides based on pathogenic microorganisms such as fungi, bacteria and viruses are specific to a target pest offering an ecologically sound and effective solution to pest problems [19, 20].
Entomopathogenic bacteria belonging to the genus Bacillus such as Bacillus cereus, Bacillus sphaericus, Bacillus popilliae, Bacillus subtilis, and Bacillus thuringiensis, have been used against various insect pests [21, 22]. Among these, B. thuringiensis (Bt) (Berliner) is one of the most commercially exploited bacteria for insect control. It produces a crystal protein (δ-endotoxin) during bacterial sporulation that is capable of causing lysis of gut cells when consumed by susceptible insects [23, 24]. In comparison to synthetic pesticides, Bt spores and parasporal crystals are thought to be safer and more specific. B. thuringiensis sub-species including B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai, are highly toxic to lepidopteran larval species [25]. A number of Bt formulations including Delfin, Halt, Biosap, Dipel, and Biobit, are commercially available in the market [26]. Shingote et al. [27] found that Vip1/Vip2 toxins of Bt had 60% insecticidal effectiveness against the Coleopteran stored grain pests. However, the most important threat to the continued efficacy of Bt insecticidal proteins (toxins) is the evolution of resistance in target pests. Alteration of toxin binding sites is one of the main mechanisms that cause resistance [28]. Recent reports documented resistance in lepidopteran pests such as Helicoverpa zea (Boddie) and Plutella xylostella (Linnaeus) against Bt formulations under lab and field conditions [29, 30].
Recent reports documented pathogenicity of Burkholderia, Chromobacterium, Pseudomonas, Serratia, Streptomyces, and Yersinia species against various insect pests, primarily against lepidopteran caterpillars [31]. Most entomopathogenic bacteria produce a variety of toxins with similar mechanisms of action to Bt [32]. Therefore, there is a stringent need to explore novel bacterial isolates having insecticidal potential.
In the current studies, we investigated soil bacterial isolates for their insecticidal potential on second instar S. litura larvae. Additionally, we examined the effect of selected bacterial strains on the nutritional, biochemical, histopathological and genotoxic parameters of S. litura.
Results
Screening bioassay
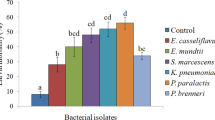
All the bacterial isolates induced significantly higher larval mortality than control (Fig. 1). Among the tested cultures, EN4 induced the highest mortality i.e. 74%. As per the biochemical/microbiological analysis, the EN4 bacterial isolate was observed to be rod shaped, gram-negative, aerobic and non-pigmented bacterium. It was identified as Pseudomonas sp. EN4 (GenBank accession number MW678603) [33]. It was observed that the Pseudomonas sp. was closely related to the members of the genus Pseudomonas and showed 98% nucleotide identity with Pseudomonas citronellolis strain NBRC 103,043 (NR114194).
Concentration response test
The concentration response assay results showed that Pseudomonas sp. induced toxic effects on various biological parameters of S. litura when ingested orally. With respect to 6% in control group, the larval mortality ranged from 42 to 78% in treated larvae (F = 25.25, p ≤ 0.05) (Table 1). It was a dose-dependent effect. At higher concentrations (3.4 × 107 and 4.3 × 109 cfu/ml), larval mortality started after 3 days of treatment and continued till 15 days. Compared to healthy larvae, the infected larvae became lethargic, stopped feeding, and their bodies eventually turned black leading to death (Fig. 2A, B, C). The LC50 and LC90 values of Pseudomonas sp. against S. litura larvae were found to be 1.21 × 109 cfu/ml (95% confidence interval: 0.93–1.41 × 109 cfu/ml) and 5.23 × 109 cfu/ml (95% confidence interval: 3.90–9.18 × 109 cfu/ml) using Probit analysis.
Oral ingestion of Pseudomonas sp. significantly influenced the growth and development of S. litura larvae. Except for the lowest concentration, all the other concentrations of Pseudomonas sp. extended the larval period significantly by 3.33 to 4.39 days with respect to control (F ꞊ 11.06, p ≤ 0.05). A significant effect was also detected on the pupal period. With respect to control the total development period increased significantly by 5.23 days at the highest concentration (F = 5.72, p ≤ 0.05) (Table 1).
The results also revealed a decreasing trend in adult emergence due to bacterial infection with a significant effect at the higher concentrations of Pseudomonas sp. (F ꞊ 12.40, p ≤ 0.05). The bacterial infection also induced morphological deformities such as crumpled and underdeveloped wings of adults (Fig. 2D, E, F). As very few adults emerged at the higher concentrations, no data could be recorded on reproductive potential.
Nutritional assay
Food utilization analysis showed that Pseudomonas sp. negatively affected all the nutritional parameters viz. RGR, RCR, ECI, ECD and AD of S. litura larvae (Table 2). Consumption of higher concentrations of bacterial cell suspension led to a significant decrease in the relative growth and consumption rate of larvae. With respect to control, the values of RGR and RCR dropped by 13.33 to 50% and 12.49 to 22.58%, respectively. The results presented in Table 2, also depicted a significant negative impact on ECI and ECD values especially at higher concentrations (ECI: F ꞊ 43.65, p ≤ 0.05, ECD: F = 58.44, p ≤ 0.05). All the concentrations of Pseudomonas sp. also significantly decreased the approximate digestibility of food (F ꞊ 59.94, p ≤ 0.05).
Histopathological analysis
S. litura larvae from the control group displayed a well-conserved layer of muscles and epithelial cells (Fig. 3A). Infection due to Pseudomonas sp. caused morphological and cellular damage to epithelial, peritrophic, basement membrane and muscle layers of midgut tissue of S. litura. Most of the cells appeared enlarged and disorganized with prominent cytoplasmic cavities. Detachment of the epithelial layer from the basement membrane was also detected (Fig. 3B).
Longitudinal sections of midgut tissue from control and treated S. litura larvae (400x) after 96 h of treatment. A Midgut of control larvae showing intact basement membrane (BM), no disruption in epithelial layer (EL), peritrophic membrane (PM) and muscle layers (ML). B Midgut of larvae treated with Pseudomonas sp. showing degradation in BM, EL, PM, ML, and prominent cytoplasmic vacuoles (CV)
Assessment for the presence of bacteria in larval hemolymph
The growth of Pseudomonas sp. was observed in the hemolymph of treated S. litura larvae while no growth of Pseudomonas sp. was detected in control larvae.
Biochemical analysis
The data presented in Table 3, highlights the inhibitory effect of Pseudomonas sp. on the activities of digestive enzymes of S. litura. When the larvae were allowed to feed on Pseudomonas sp. treated leaves, a significant decrease of 34.64 and 74.28% was detected in α-amylases activity after 48 and 96 h, respectively, compared to the control. Similarly, bacterial treatment suppressed the activity of α-glucosidases with a significant drop of 78.71% after 96 h with respect to control. A similar trend was observed in the case of ß-glucosidases. Pseudomonas sp. also influenced the level of α and ß-galactosidases which showed 20.4 and 19.76 times less activity 96 h post-treatment with respect to control larvae. As is evident from Table 3, significant drop was also observed in activities of lipases and proteases 48 and 96 h post-treatment of Pseudomonas sp.
Comet assay
To determine the extent of DNA damage in S. litura due to infection of Pseudomonas sp., comet parameters viz. tail length, percent tail DNA, tail moment and olive tail moment (OTM) were assessed. The values of all these parameters were significantly higher in larvae fed on bacterial cell suspension for 96 h in comparison to control larvae (Fig. 4). The larvae fed on bacterial-treated leaves showed increased tail length depicting DNA damage in hemocytes as compared to control (Fig. 5). The value of tail length in hemocytes of infected larvae was found to be 20.41 µm compared to 11.23 µm in control (F = 90.34, p ≤ 0.05). Similarly, value of percent tail DNA, tail moment and Olive tail moment were found to be 11.42, 3.64 and 4.23 compared to 3.54, 0.53 and 1.39 in control respectively (F = 95.85, p ≤ 0.05; F = 60.40, p ≤ 0.05; F = 28.12, p ≤ 0.05).
Genotoxic effects of Pseudomonas sp. showing variations in DNA damage parameters viz. Tail length (µm) (L tail), Tail DNA (%), Tail moment (TM) and Olive tail moment (OTM) of hemocytes of treated and control S. litura larvae. Bars represent the Mean ± SE. Different letters above the bars represent significant differences at Tukey’s test P ≤ 0.05
Discussion
There is a growing tendency to identify more pathogenic and effective bacterial biocontrol agents as an effort to develop efficient and eco-friendly methods for controlling insect pests. Thus, in order to search for novel biocontrol agents, different soil bacterial isolates were screened for their insecticidal potential against S. litura larvae. Among the tested bacterial isolates, Pseudomonas sp., closely related to P. citronellolis strain NBRC 103,043 (NR114194) was found to be pathogenic causing 78% larval mortality in S. litura. Larvae infected with Pseudomonas sp. showed typical symptoms of bacterial infection such as sluggishness, decreased movement, cessation of feeding, flaccid body that turned black and ultimately, death of the larvae. Similar symptoms have earlier been reported in many insect pests due to infection of B. thuringiensis and Pseudomonas aeruginosa [34, 35].
The presence of bacterial growth in the hemolymph of larvae treated with Pseudomonas sp. suggests that larval death occurs due to breaching the gut epithelial barrier and infiltration of bacterial cells in the hemocoel, resulting in septicemia. Similarly, other workers documented mortality in Spodoptera frugiperda (J.E. Smith), S. litura, Helicoverpa armigera (Hubner) due to the proliferation of Pseudomonas protogens and Photorhabdus akhurstii in hemocoel [36, 37]. The presence of a high bacterial load in the hemolymph causes tissue necrosis as a result of bacterial toxins [38].
The members of genus Pseudomonas are widely distributed in the environment and have been isolated most commonly from insect pests and soil samples. A number of Pseudomonas species such as Pseudomonas chlororaphis, Pseudomonas taiwanensis, Pseudomonas fluorescens, Pseudomonas entomophila, Pseudomonas putida, Pseudomonas cedrina and Pseudomonas paralactis are known to have insecticidal properties against many insect pests [39,40,41,42,43]. Toxins (Fit toxin, Exotoxin A, ExoS, hydrogen cyanide, rhizotoxins) associated with P. protogens, P. aeruginosa, P. taiwanensis etc. contribute to pathogenicity by causing sepsis and eventually death of larvae in various insect pests [44,45,46]. Pathogenicity of Pseudomonas species against insects may also be attributed to hydrolytic enzymes such as proteases, chitinases and phospholipases which are known to be produced by these bacterial strains [46, 47]. Metalloproteinases that degrade the internal peptide bonds of proteins inside the gut play a predominant role as a virulence factor of P. aerugnisosa [48].
Apart from mortality, larval treatment with Pseudomonas sp. extended the overall development period of S. litura, decreased adult emergence and induced morphological deformities in adults. Delayed development and reduced adult emergence have earlier been reported in S. litura, H. armigera and Drosophila melanogaster (Meigen) larvae on exposure to S. marcescens strain SEN, B. thuringiensis and P. fluorescens SBW25 [49,50,51]. The emergence of morphologically deformed adults with underdeveloped and wrinkled wings has been documented previously in S. litura, Delia radicum (Linnaeus) and H. armigera due to Bacillus vallismortis, Enterobacter cloacae, B. thuringiensis and P. paralactis infection [43, 52,53,54].
The delayed larval development may be correlated with the adverse effect of Pseudomonas sp. on nutritional physiology of S. litura larvae. Treatment of larval diet with Pseudomonas sp. led to a decrease in relative consumption rate with concomitant reduction of the relative growth rate of S. litura. There was also a significant reduction in ECI, ECD and AD of S. litura when treated with Pseudomonas sp. A decrease in ECI value specifies that more food is metabolized for energy and less is converted to body mass (i.e. growth). Food digestibility and the relative amount of food converted to body mass and metabolized for energy needs are the important activities that can affect ECI and ECD values [55]. These studies are in line with the previous findings indicating a decrease in all the nutritional parameters when S. litura larvae were treated with bacterial pathogens like E. cloaca, B. subtilis, B. vallismortis, Klebsiella pneumoniae and P. paralactis [43, 52, 53, 56].
Histopathological studies indicated extensive damage in the peritrophic membrane and midgut epithelial cells of S. litura larvae due to infection of Pseudomonas sp. It is well known that the Pseudomonas bacteria produce chitinases, which hydrolyze chitin, a common component of the insect exoskeleton and midgut peritrophic membrane [47, 57]. Chitinases have been shown to damage the peritrophic membrane and impair digestion [58]. Delta endotoxin and Vip3Aa toxin of B. thuringiensis have been known to damage the epithelial and muscle layers of the gut of lepidopteran insects [59,60,61]. Similar histological alterations in the midgut have been documented in S. frugiperda, S. litura, and H. armigera larvae following exposure to P. akhurstii, K. pneumoniae, and P. paralactis [37, 43].
Our studies showed a significant decrease in the activity of digestive enzymes such as α-amylases, glucosidases, galactosidases, lipases and proteases of S. litura larvae due to infection of Pseudomonas sp. The reduction of digestive enzyme activity may be correlated with the histopathological effects produced by this bacterial strain. Zhang et al. [62] reported the significant decrease in protease, amylase and lipase activity due to the destruction of midgut epithelial cells in adult hazelnut weevil due to S. marcescens infection. A similar reduction in digestive enzymes was documented by other workers in various insect pests due to infection with B. thuringiensis and Photorhabdus temperata [63,64,65]. The midgut is the main site for the synthesis and secretion of digestive enzymes. The destruction of peritrophic membrane and midgut epithelial cells observed in the present study may have impaired the digestive enzyme activity. The suppression of enzyme activity in the treated insects may be due to an imbalance in the enzyme–substrate complex and inhibition of peristaltic movement of the gut thus affecting the efficacy of digestion and nutrient absorption [66].
Pseudomonas sp. also caused genotoxicity to S. litura as is evident from DNA damage in the hemocytes of S. litura larvae. There is very little information available on the genotoxic effects of bacterial biocontrol agents, although there are reports of genotoxicity of plant and fungal extracts to lepidopteran insects [67, 68]. Oberholster et al. [69] observed DNA damage in insects such as Periplaneta americana (Linnaeus), Tenebrio molitor (Linnaeus) and Gryllus bimaculatus (De Geer) caused by a cyanobacterial secondary metabolite (microcystin-LR). Bacterial toxins cause DNA single-strand and double-strand breaks, which activate the classical DNA damage response, resulting in cell cycle arrest or cell death [70, 71]. Due to direct adverse impact on the DNA of hemocytes, the cellular immune system of insect pest may get impaired, and thus rendering it more susceptible to pathogenic infections [72].
For future implications of Pseudomonas sp. in integrated pest management (IPM) practices, there is a need to standardize the mass production techniques of bacterial isolate to make it cost effective so that farmers can easily use and further to evaluate its field efficacy. These can also be used in combination with other biological or chemical control agents so as to provide the effective pest control in IPM programs.
Conclusion
The current work describes the pathogenicity of Pseudomonas sp. against S. litura larvae. Pseudomonas sp. significantly increased the larval mortality rate, delayed the overall development period, decreased adult emergence and induced morphological deformities in adults. The bacterial infection also caused damage to the epithelial membrane and peritrophic matrix of the larval midgut which may further interrupt the digestion ability and nutritional physiology. Insect hemocytes play an essential role throughout the growth and developmental stages of the insects by providing defensive (immune) functions. Genotoxic damage caused by Pseudomonas sp. to larval hemocytes of S. litura larvae thus may have induced detrimental effects on the insect’s growth and development as observed in the bioassays and made them more susceptible to infection. In conclusion, Pseudomonas sp. has the potential to be used as a biocontrol agent against insect pests, however, further research to improve the mass production techniques of bacterial cells and their testing in field conditions is required.
Materials and methods
Insect culture
Egg masses and larvae of S. litura were collected from cabbage and cauliflower fields near Amritsar (Punjab), India. The larvae were reared in the laboratory on fresh castor leaves in plastic jars (15 cm × 10 cm) at 25 ± 2°C temperature and 65 ± 5% humidity, respectively. Until pupation, the larval diet was changed regularly. Pupae were moved to pupation jars and newly emerged adults were transferred to oviposition jars. Honey solution (1 part honey: 4 parts water v/v) soaked on a cotton swab was given to the adults. The newly hatched larvae were transferred to fresh castor leaves.
Bacterial cultures
Five bacterial cultures viz. EN1, EN2, AA5, EN4 and R1, were procured from the Department of Microbiology, Guru Nanak Dev University, Amritsar (Punjab), India. All the cultures were isolated from soil samples collected from different locations.
Maintenance of bacterial cultures
The bacterial cultures (EN1, EN2, AA5, EN4 and R1) were maintained on Luria Bertani (LB) plates. A single colony of each bacterial isolate was inoculated into LB broth and incubated for 48 h at 30°C. To obtain the pellet, each bacterial culture was centrifuged at 10,000 rpm and 4οC for 10 min after incubation. The pellet was resuspended in 1 ml Phosphate Buffer Solution (PBS) (pH 7.0) after being rinsed once with sterile distilled water. The bacterial density was determined at optical density (OD600) and adjusted to 1.89 (1.8 × 109 cfu/ml approximately) before being utilized in bioassays as described by Thakur et al. [52].
Screening assay
Second instar (6 days old) larvae of S. litura were used for testing the insecticidal potential of bacterial isolates. The larvae were randomly selected from the lab culture and placed in rearing vials. Fresh castor leaves were surface sterilized with NaOCl (5% v/v), then washed in distilled water. These leaves (about 10 cm2) were treated by immersing them in 10 ml bacterial cell suspension. After air drying at room temperature, the treated leaves were placed in rearing containers. Only one larva was kept in a container to avoid cannibalism. The experiment was replicated five times with 50 larvae (10 larvae per replicate) for initial screening. The control group was fed on surface sterilized castor leaves soaked in PBS buffer. The experimental conditions were kept at a constant temperature of 25 ± 2°C and relative humidity of 65 ± 5%. The food was changed after every 48 h and the fresh castor leaves treated with freshly made bacterial suspension were provided to larvae. The mortality of the larvae was monitored daily. The bacterial isolate EN4 exhibiting the highest mortality was selected for detailed studies and identified based on 16s RNA.
Concentration response test
Based on the highest larval mortality in S. litura as per screening test, EN4 was selected for the concentration response test. Five different bacterial concentrations i.e. C1 = 1.2 × 103 cfu/ml, C2 = 1.9 × 105 cfu/ml, C3 = 2.6 × 106 cfu/ml, C4 = 3.4 × 107 cfu/ml, and C5 = 4.3 × 109 cfu/ml (based on their OD600 values) were prepared. The castor leaf discs (approximately 10 cm2) were treated with ten ml of each concentration. The PBS-dipped leaves were used as control. Experiments were carried out on 50 larvae (6 days old) with 5 replications (10 larvae per replicate) for each concentration. The diet was changed every 48 h till pupation. Daily observations were taken on larval mortality and development. Data was also collected on adult emergence and morphological deformities. Probit analysis using the SPSS 16.0 statistical program was used to determine the lethal concentration (LC50) value based on larval mortality data.
Nutritional analysis
Second instar larvae of S. litura were starved for 3–4 h to evaluate the effect of bacterial infection on nutritional physiology. The above-mentioned concentrations were used for this analysis. The larvae were weighed individually and released in rearing vials containing treated leaves of known weight. The leaves dipped in PBS only were used as a control. For each concentration, 25 larvae were used in the experiment (five larvae per replicate). The weight of the larvae, residual diet, and faecal matter was measured after 72 h of feeding, and the overall change in each variable was compared to the previous value. At the end of experiment, the dry weight of larva, residual diet and faecal matter was also recorded by incubating at 60°C for 72 h to determine the loss of water. The data were utilized to generate nutritional indices on a dry weight basis by following the procedure of Farrar et al. [73]:
where, RGR = Relative growth rate, RCR = Relative consumption rate, ECI = Efficiency of conversion of ingested food, ECD = Efficiency of conversion of digested food, AD = Approximate digestibility.
Histopathological analysis
The effect of EN4 infection was also investigated on the histology of the midgut tissue of S. litura larvae. The leaves treated with LC50 value of bacterial cell suspension were fed to second instar larvae (6 days old) for 96 h. The larvae that were fed on PBS-treated leaves served as control. The temperature and humidity levels were kept at 25 ± 2οC and 65 ± 5%, respectively. The larvae were dissected aseptically and the larval guts were extracted in distilled water after 96 h. The gut was kept in 10% formalin until the tissue was processed. The sample was washed with distilled water in a tube after fixation, and the process was repeated several times. Dehydration of tissue was achieved by passing it through alcohol concentrations ranging from 30 to 90%. The tissue was fixed in paraffin wax. The microtome was used to make tiny ribbons from wax blocks. These thin ribbons with gut sections were placed on a slide coated with a layer of 1% Mayer's egg albumin and kept warm on a hot plate at 40–45°C to ensure even wax distribution. The slides were rinsed in 100, 90, 80 and 70% ethanol for 1 min each before being dewaxed in xylene for 10 min. The methodology by Dutta et al. [37] was used to stain permanent slides with hematoxylin and eosin stain. Finally, before mounting the samples in Dibutylphthalate Polysterene Xylene (DPX), the slides were rinsed in xylene for 5 min. Images were then captured at 400x magnification using Evos XL core light microscope.
Assessment for the presence of bacteria in larval hemolymph
The presence of bacteria in the larval hemolymph was determined by feeding second instar larvae on LC50 value of EN4. After 96 h of bacterial treatment, 100 µl of hemolymph was collected from ten infected larvae of bacteria-treated groups and ten control larvae. The hemolymph was serially diluted and spread over LB agar plates. Plates were incubated at 30°C for 48 h and the formation of bacterial colonies was observed.
Biochemical analysis
To study the effect of bacterial infection on digestive enzymes third instar larvae (10 days old) of S. litura were fed on leaves treated with LC50 concentration of EN4 for 48 and 96 h. The control group larvae were fed on a diet devoid of the bacterial suspension. For each treatment, ten third instar larvae were obtained from each replicate (3 replicates). The larval guts were collected and homogenized (1% gut homogenate) for enzymatic analysis.
α-Amylases
The activity of α-amylases was determined according to the protocol of Mehrabadi et al. [74]. Enzyme extract (20 µl) was added to tubes containing 100 µl of phosphate buffer (0.02 M) (pH 7.1) and incubated at 35°C for 30 min. The reaction was stopped by adding 100 µl of dinitrosalicylic acid (DNS) reagent by heating it in boiling water for 10 min. The absorbance of the mixture was measured at 540 nm on a microplate reader (Eon BioTek) Winooski, Vermont, USA. Serial dilutions of 0.01 M maltose (100–1000 µM) were used to create the standard curve.
Glucosidases
The estimation of α glucosidases was carried out following the procedure of Zibaee [75]. Enzyme extract (20 µl) was pre-incubated at 37 °C for 10 min with 40 μl of p-nitrophenyl-α-D-glucopyranoside (pNαG) (5 mM) and 100 μl of phosphate buffer (0.02 M, pH 7.1). Sodium carbonate (1 M) (150 µl) was used to terminate the reaction [76]. The absorbance was measured at 450 nm with a microplate reader (Eon BioTek) at Winooski, Vermont, USA. p-Nitrophenol (0.01 M) was used as standard and concentrations ranging from 100–1000 µM were prepared for constructing a standard curve. A similar procedure was applied in the case of ß glucosidases activity estimation, however, the substrate used in this case was p-nitrophenyl- ß-D-glucopyranoside (pNßG) (5 mM).
Galactosidases
The activity of α galactosidases was estimated by incubating 20 μl of gut homogenate with 40 μl of p-nitrophenyl- α-D-galactopyranoside (5 mM) and 100 μl of phosphate buffer (0.02 M, pH 7.1) at 37°C for 10 min [75]. The reaction was stopped by adding 150 μl of sodium carbonate (1 M) [76]. The absorbance was recorded on a microplate reader (Eon BioTek) at 450 nm. The standard curve was prepared using serial dilutions of 0.01 M p-nitrophenol (100–1000 µM). Similar procedure was used for the estimation of ß glucosidases activity except for the substrate used in this case was p-nitrophenyl- ß-D- galactopyranoside (pNßG) (5 mM).
Lipases
The activity of lipases was estimated following the protocol of Tsujita et al. [77]. The enzyme extract (20 µl) and 40 µl of p-nitrophenyl butyrate (27 mM) were added to 100 μl of phosphate buffer (0.02 M, pH 7.1) and incubated at 37°C. After 1 min, 100 μl of NaOH (1 M) was added and absorbance was recorded on a microplate reader (Eon BioTek) at 405 nm. A standard curve was prepared using 100- 1000 µM of p-nitrophenol (0.01 M) and enzyme activity was calculated as µM/mg fresh larval weight.
Proteases
Protease estimation was done using hemoglobin (20 mg/ml) as substrate according to the protocol of Cohen [78] with slight modifications. Hemoglobin solution (40 μl) was added to 100 μl of phosphate buffer (0.02 M, pH 7.1). The reaction was initiated by adding 40 μl of enzyme extract and incubating at 30°C for 120 min. The reaction was terminated by adding 100 μl of 30% TCA and absorbance was recorded on a microplate reader (Eon BioTek) at 410 nm. Bovine serum albumin (0.01 M) was used as standard and concentrations ranging from (100–1000 µM) were prepared for constructing a standard curve.
Comet assay
The level of DNA damage was measured using the comet assay. The comet assay was performed in alkaline conditions, with slight modifications, according to Singh et al. [79]. The larvae were fed on castor leaves treated with LC50 value of selected bacterial isolate (EN4) (pH 7.4) for 96 h. The prolegs of third instar larvae were shrugged off and the hemolymph (from ten larvae) was collected in eppendorf tubes containing phosphate buffer. The slides were coated with 1% normal melting point agarose (NMPA) and hemocytes were layered on coated slides and kept in a refrigerator at 4ºC to settle down. After that, the slides were soaked in the lysing solution (2.5 M NaCl, 100 mM EDTA, 0.25 M Tris aminomethane, 0.25 M NaOH, 1% Triton X-100, 10% DMSO, double distilled water, pH 10.0) and kept in the refrigerator overnight. Electrophoresis was performed using an electrophoretic unit (25 V; 300 mA) containing electrophoretic buffer (1 mM EDTA, 300 mM NaOH, double distilled water, pH > 13) for 20 min. In a neutralization buffer, the slides were neutralized for 15 min (0.4 M Tris amino methane, double distilled water pH 7.5). After drying the slides were stained with ethidium bromide (50 g/ml) and viewed with a Nikon fluorescence microscope. The experiment was replicated thrice. The tail length, tail moment, percent tail DNA and Olive Tail Moment were calculated using Casplab Software (OTM).
Statistical analysis
The experiments on larval mortality, development duration, adult emergence, adult deformities and nutritional analysis parameters were replicated five times, while comet and biochemical assays were replicated thrice. The Mean ± SE of all the values was used to represent them. One way analysis of variance (ANOVA) with Tukey’s test at p ≤ 0.05 was used to compare the differences in means. Statistical analysis was carried out by using SPSS software for windows version 16.0 (SPSS Inc, Chicago) and Microsoft Office Excel 2007 (Microsoft Corp., USA). For biochemical analysis, paired sample t-test was used.
Availability of data and materials
The datasets used and/or analysed during the current study is available from corresponding author on reasonable request.
References
Shu Y, Du Y, Chen J, Wei J, Wang J. Responses of the cutworm Spodoptera litura (Lepidoptera: Noctuidae) to two Bt corn hybrids expressing Cry1Ab. Sci Rep. 2017;7:41577.
Chattopadhyay N, Balasubramaniam R, Attri SD, Ray K, John G, Khedikar S, Karmakar C. Forewarning of incidence of Spodoptera litura (Tobacco caterpillar) in soybean and cotton using statistical and synoptic approach. J Agrimet. 2019;21(1):68–75. https://doi.org/10.54386/jam.v21i1.208.
Chari MS, Patel SN. Cotton leaf worm Spodoptera litura Fabricius, its biology and integrated control measures. Cotton Dev. 1983;13:7–8.
Sundar B, Chouhan RS, Khandwe N, Venkateshwar J. Determination of yield losses of soybean entries/varieties caused by Spodoptera litura. Int J Res Appl Nat Soc Sci. 2017;5(9):137–42.
Sahu B, Pachori R, Navya RN, Patidar S. Extent of damage by Spodoptera litura on cabbage. J Entomol Zool Stud. 2020;8:1153–6.
Babu SR, Singh B. Population Dynamics of Spodoptera exigua (F.) and S. litura (F.) in Soybean. Indian J Entomol. 2022;84(4):819–23.
Baskaran RK, Rajavel DS. Yield loss by major insect pests in groundnut. Ann Plant Protection Sci. 2013;21(1):189–90.
Xue M, Pang YH, Wang HT, Li QL, Liu TX. Effects of four host plants on biology and food utilization of the cutworm, Spodoptera litura. J Insect Sci. 2010;10(1):22. https://doi.org/10.1673/031.010.2201.
Fu XW, Zhao XY, Xie BT, Ali A, Wu KM. Seasonal pattern of Spodoptera litura (Lepidoptera: Noctuidae) migration across the Bohai strait in Northern China. J Econ Entomol. 2015;108:525–38.
Sinha SN, Rao MVV, Vasudev K. Distribution of pesticides in different commonly used vegetables from Hyderabad. India Food Res Int. 2012;45(1):161–9. https://doi.org/10.1016/J.FOODRES.2011.09.028.
Saleem M, Hussain D, Ghouse G, Abbas M, Fisher SW. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2016;79:177–84. https://doi.org/10.1016/j.cropro.2015.08.024.
Gandhi K, Patil RH, Srujana Y. Field resistance of Spodoptera litura to conventional insecticides in India. Crop Prot. 2016;88:103–8.
Guimarães-Cestaro L, Martins MF, Martínez LC, Alves MLTMF, Guidugli-Lazzarini KR, Nocelli RCF, Malaspina O, Serrão JE, Teixeira ÉW. Occurrence of virus, microsporidia, and pesticide residues in three species of stingless bees (Apidae: Meliponini) in the field. Sci Nat. 2020;107:1–14. https://doi.org/10.1007/S00114-020-1670-5/FIGURES/5.
Ahmad M, Arif MI, Ahmad M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007;26:809e817.
Saleem MA, Ahmad M, Ahmad M, Aslam M, Sayyed AH. Resistance to selected organochlorin, organophosphate, carbamate and pyrethroid, in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. J Econ Entomol. 2008;101(5):1667–75. https://doi.org/10.1093/JEE/101.5.1667.
Shad SA, Sayyed AH, Fazal S, Saleem MA, Zaka SM, Ali M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J Pest Sci. 2012;85:153e162.
Abbas N, Shad SA, Razaq M, Waheed A, Aslam M. Resistance of Spodoptera litura (Lepidoptera: Noctuidae) to profenofos: Relative fitness and cross resistance. Crop Prot. 2014;58:49–54.
Wang X, Huang Q, Hao Q, Ran S, Wu Y, Cui P, Yang J, Jiang C, Yang Q. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan China. Crop Prot. 2018;106:110–6.
Kumar S, Singh A. Biopesticides: present status and the future prospects. J Fertil Pestic. 2015;6(2):100–29.
Majeed A, Muhammad Z, Islam S, Ullah Z, Ullah R. Cyanobacterial application as biofertilizers in rice fields: role in growth promotion and crop productivity. PSM Microbiol. 2017;2:47–50.
Priest FG, Barker M, Baillie LW, Holmes EC, Maiden MC. Population structure and evolution of the Bacillus cereus group. J Bacteriol. 2004;186(23):7959–70. https://doi.org/10.1128/JB.186.23.7959-7970.2004.20.
Stahly DP, Andrews RE, Yousten AA. The genus Bacillus-insect pathogens Prokaryotes. 2006;4:563–608.
Jisha VN, Smitha RB, Benjamin S. An Overview on the Crystal Toxins from Bacillus thuringiensis. Adv Microbiol. 2013;03:462–72. https://doi.org/10.4236/AIM.2013.35062.
Olson S. An analysis of the biopesticide market now and where it is going. Outlooks Pest Manag. 2015;26(5):203–6.
Copping LG, Menn JJ. Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci: Formerly Pestic Sci. 2000;56(8):651–76.
Kumari D, John S. Health risk assessment of pesticide residues in fruits and vegetables from farms and markets of Western Indian Himalayan region. Chemosphere. 2019;224:162–7. https://doi.org/10.1016/j.chemosphere.2019.02.091.
Shingote PR, Moharil MP, Dhumale DR, Jadhav PV, Satpute NS, Dudhare MS. Screening of vip1/vip2 binary toxin gene and its isolation and cloning from local Bacillus thuringiensis isolates. Sci Asia. 2013;39:620–4.
Jurat-Fuentes JL, Heckel DG, Ferré J. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu Rev Entomol. 2021;66:121–40.
Welch KL, Unnithan GC, Degain BA, Wei J, Zhang J, Li X, Tabashnik BE, Carriere Y. Cross-resistance to toxins used in pyramided Bt crops and resistance to Bt sprays in Helicoverpa zea. J Invertebr Pathol. 2015;132:149–56. https://doi.org/10.1016/j.jip.2015.10.003.
Tabashnik BE, Carrière Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol. 2017;35(10):926–35.
Yadav A, Rastegari A, Yadav N. Microbiomes of extreme environments: biodiversity and biotechnological applications. 1st ed. USA: CRC Press, Taylor and Francis Group; 2020.
Castagnola A, Stock SP. Common virulence factors and tissue targets of entomopathogenic bacteria for biological control of lepidopteran pests. Insects. 2014;5(1):139–66. https://doi.org/10.3390/insects5010139.
Mahajan R, Sharma G, Koundal S, Chadha P, Kumar S, Saini HS. Co-metabolism of 4-bromophenol by Pseudomonas sp. EN-4 and toxicity evaluation of biotransformed samples. J Environ Chemical Eng. 2022;10(5):108223.
Eski A, Demir I, Güllü M, Demirbağ Z. Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb Pathog. 2018;121:350–8.
Maciel-Vergara G, Jensen AB, Eilenberg J. Cannibalism as a possible entry route for opportunistic pathogenic bacteria to insect hosts, exemplified by Pseudomonas aeruginosa, a pathogen of the giant mealworm Zophobas morio. Insects. 2018;9(3):88. https://doi.org/10.3390/insects9030088.
Flury P, Vesga P, Dominguez-Ferreras A, Tinguely C, Ullrich CI, Klesspies RG, Keel C, Maurhofer M. Persistence of root-colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J. 2019;13:860–72. https://doi.org/10.1038/s41396-018-0317-4.
Dutta TK, Santhoshkumar K, Mathur C, Mandal A, Sagar D. A Photorhabdus akhurstii toxin altered gut homeostasis prior conferring cytotoxicity in Spodoptera frugiperda. S litura and Helicoverpa armigera Phytoparasitica. 2021;49(5):943–58.
Jurat-Fuentes JL, Jackson TA. Bacterial Entomopathogens. In: Vega FE, Kaya HK, editors. Insect Pathology. Elsevier; 2012. p. 265–349. https://doi.org/10.1016/B978-0-12-384984-7.00008-7.
Rufner B, Péchy-Tarr M, Ryfel F, Hoegger P, Obrist C, Rindlisbacher A, Keel C, Maurhofer M. Oral insecticidal activity of plant-associated pseudomonads. Environ Microbiol. 2013;15(3):751–63.
Chen WJ, Hsieh FC, Hsu FC, Tasy YF, Liu JR, Shih MC. Characterization of an insecticidal toxin and pathogenicity of Pseudomonas taiwanensis against insects. PLoS Pathog. 2014;10(8):e1004288.
Dieppois G, Opota O, Lalucat J, Lemaitre B. Pseudomonas entomophila: a versatile bacterium with entomopathogenic properties. In: Pseudomonas. Dordrecht: Springer; 2015. p. 25–49.
Liu FH, Lin XL, Kang ZW, Tian HG, Liu TX. Isolation and characterization of Pseudomonas cedrina infecting Plutella xylostella (Lepidoptera: Plutellidae). Arch Insect Biochem Physiol. 2019;102(3):e21593. https://doi.org/10.1002/arch.21593.
Devi S, Saini HS, Kaur S. Insecticidal and growth inhibitory activity of gut microbes isolated from adults of Spodoptera litura (Fab.). BMC Microbiol. 2022;22(1):1–14. https://doi.org/10.1186/S12866-022-02476-3.
Okuda J, Hayashi N, Okamoto M, Sawada S, Minagawa S, Yano Y, Gotoh N. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na, K-ATPase regulator, FXYD3. Infect Immun. 2010;78:4511–22. https://doi.org/10.1128/IAI.00428-10.
Chieda Y, Iiyama K, Lee JM, Kusakabe T, Yasunaga-Aoki C, Shimizu S. Virulence of an exotoxin A-defcient strain of Pseudomonas aeruginosa toward the silkworm. Bombyx mori Microb Pathog. 2011;51:407–14. https://doi.org/10.1016/j.micpath.2011.09.002.
Flury P, Aellen N, Ruffner B, Péchy-Tarr M, Fataar S, Metla Z, Dominguez-Ferreras A, Bloemberg G, Frey J, Goesmann A, Raajimakers JM, Dufy B, Höfte M, Blom J, Smits THM, Keel C, Maurhofer M. Insect pathogenicity in plant-beneficial pseudomonads: phylogenetic distribution and comparative genomics. ISME J. 2016;10(10):2527–42. https://doi.org/10.1038/ismej.2016.5.
Loper JE, Henkels MD, Rangel LI, Olcott MH, Walker FL, Bond KL, Kidarsa TA, Hesse CN, Sneh B, Stockwell VO, Taylor BJ. Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf-5 to Drosophila melanogaster. Environ Microbiol. 2016;18(10):3509–21.
Andrejko M, Zdybicka-Barabas A, Cytryńska M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J Invertebr Pathol. 2014;115:14–25.
Mohan M, Sushil SN, Bhatt JC, Gujar GT, Gupta HS. Synergistic interaction between sublethal doses of Bacillus thuringiensis and Campoletis chlorideae in managing Helicoverpa armigera. Biocontrol. 2008;53(2):375–86.
Olcott MH, Henkels MD, Rosen KL, Walker FL, Sneh B, Loper JE, Taylor BJ. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fuorescens strains. PloS One. 2010;5(9):e12504.
Aggarwal C, Paul S, Tripathi V, Paul B, Khan M. Chitinolytic activity in Serratia marcescens (strain SEN) and potency against different larval instars of Spodoptera litura with effect of sublethal doses on insect development. Biocontrol. 2015;60(5):631–40.
Thakur A, Dhammi P, Saini HS, Kaur S. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J Invertebr Pathol. 2015;127:38–46. https://doi.org/10.1016/j.jip.2015.02.007.
Kaur PK, Thakur A, Saini HS, Kaur S. Evaluation of Bacillus vallismortis (Bacillales: Bacillaceae) R2 as insecticidal agent against polyphagous pest Spodoptera litura (Lepidoptera: Noctuidae). 3 Biotech. 2017;7(5):1–8.
Fite T, Tefera T, Negeri M, Damte T, Sori W. Evaluation of Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis for the management of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) under laboratory and field conditions. Biocontrol Sci Technol. 2020;30(3):278–95. https://doi.org/10.1080/09583157.2019.1707481.
Abdel-Rahman HR, Al-Mozini RN. Antifeedant and toxic activity of some plant extracts against larvae of cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Pakistan J Biol Sci. 2007;10(24):4467–72.
Chandrasekaran R, Revathi K, Nisha S, Kirubakaran SA, Sathish-Narayanan S, Senthil-Nathan S. Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pestic Biochem Physiol. 2012;104(1):65–71.
Chen L, Jiang H, Cheng Q, Chen J, Wu G, Kumar A, Sun M, Liu Z. Enhanced nematicidal potential of the chitinase pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci Rep. 2015;5(1):1–1.
Suganthi M, Senthilkumar P, Arvinth S, Chandrashekara KN. Chitinase from Pseudomonas fluorescens and its insecticidal activity against Helopeltis theivora. J Gen Appl Microbiol. 2017;63(4):222–7.
Pandey S, Joshi B, Tiwari LD. Histopathological changes in the midgut of Spodoptera litura larvae on ingestion of Bacillus thuringiensis delta endotoxin. Arch Phytopathol Plant Prot. 2009;42(4):376–83. https://doi.org/10.1080/03235400601121497.
Song F, Lin Y, Chen C, Shao E, Guan X, Huang Z. Insecticidal activity and histopathological effects of Vip3Aa protein from Bacillus thuringiensis on Spodoptera litura. J Microbiol Biotechnol. 2016;26(10):1774–80.
Castro BMDCE, Martinez LC, Barbosa SG, Serrão JE, Wilcke CF, Soares MA, da Silva AA, de Carvalho AG, Zanuncio JC. Toxicity and cytopathology mediated by Bacillus thuringiensis in the midgut of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Sci Rep. 2019;9(1):6667.
Zhang P, Zhao Q, Ma X, Ma L. Pathogenicity of Serratia marcescens to hazelnut weevil (Curculio dieckmanni). J For Res (Harbin). 2021;32:409–17. https://doi.org/10.1007/S11676-020-01096-9.
Nathan SS, Chung PG, Murugan K. Combined effect of biopesticides on the digestive enzymatic profiles of Cnaphalocrocis medinalis (Guenée) (the rice leaffolder) (Insecta: Lepidoptera: Pyralidae). Ecotoxicol Environ Saf. 2006;64(3):382–9.
Carneiro CNB, DaMatta RA, Samuels RI, Silva CP. Effects of Entomopathogenic Bacterium Photorhabdus temperata Infection on the Digestive Enzymes of Diatraea saccharalis (Lepidoptera: Crambidae) Larvae. Protein Pept Lett. 2008;15:658–62. https://doi.org/10.2174/092986608785133618.
Zibaee I, Bandani AR, Sendi JJ, Talaei-Hassanloei R, Kouchaki B. Effects of Bacillus thuringiensis var. kurstaki and medicinal plants on Hyphantria cunea Drury (Lepidoptera: Arctiidae). Res Rep. 2010;7:251–61.
Kilani-Morakchi S, Bezzar-Bendjazia R, Ferdenache M, Aribi N. Preimaginal exposure to azadirachtin affects food selection and digestive enzymes in adults of Drosophila melanogaster (Diptera: Drosophilidae). Pestic Biochem Physiol. 2017;140:58–64.
Kaur M, Chadha P, Kaur S, Kaur A, Kaur R, Yadav AK. Kaur R Schizophyllum commune induced genotoxic and cytotoxic effects in Spodoptera litura. Sci Rep. 2018;8(1):1–12.
Datta R, Kaur A, Saraf I, Kaur M, Singh IP, Chadha P, Kaur S. Assessment of genotoxic and biochemical effects of purified compounds of Alpinia galanga on a polyphagous lepidopteran pest Spodoptera litura (Fabricius). Phytoparasitica. 2020;48(3):501–11.
Oberholster PJ, Mthethwa B, Botha AM. Development of a rapid and sensitive battery of bioassays for risk assessment of cyanobacterial microcystin-LR in drinking water of rural water treatment plants. South Africa African J Biotechnol. 2009;8(18):4562–71.
Cortes-Bratti X, Frisan T, Thelestam M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon. 2001;39(11):1729–36. https://doi.org/10.1016/S0041-0101(01)00159-3.
Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins. 2011;3(3):172–90. https://doi.org/10.3390/toxins3030172.
Kalita MK, Haloi K, Devi D. Larval exposure to chlorpyrifos affects nutritional physiology and induces genotoxicity in silkworm Philosamia ricini (Lepidoptera: Saturniidae). Front Physiol. 2016;7:535. https://doi.org/10.3389/fphys.2016.00535.
Farrar RR, Barbour JD, Kennedy GG. Quantifying food consumption and growth in insects. Ann Entomol Soc Am. 1989;82(5):593–8. https://doi.org/10.1093/aesa/82.5.593.
Mehrabadi M, Bandani A, Saadati F, Mahmudvand M. α-Amylase activity of stored products insects and its inhibition by medicinal plant extracts. J Agr Sci Tech. 2011;13:1173–82.
Zibaee A. Digestive enzymes of large cabbage white butterfly, Pieris brassicae L. (Lepidoptera: Pieridae) from developmental and site of activity perspectives. Italian J Zool. 2012;79(1):13–26.
Terra WR, Ferreira C. Further evidence that enzymes involved in the final stages of digestion by Rhynchosciara do not enter the endoperitrophic space. Insect Biochem. 1983;13(2):143–50.
Tsujita T, Muderhwa JM, Brockman HL. Lipid-lipid interactions as regulators of carboxylester lipase activity. J Biological Chem. 1989;264(15):8612–8.
Cohen AC. Organization of digestion and preliminary characterization of salivary trypsin like enzymes in a predaceous Heteropteran, Zelus renadii. J Insect Physiol. 1993;39(10):823–9.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Res. 1988;175(1):184–91. https://doi.org/10.1016/0014-4827(88)90265-0.
Acknowledgements
The authors are thankful to the Department of Zoology and Department of Microbiology, Guru Nanak Dev University, Amritsar (Punjab), India for providing infrastructural facilities. Financial assistance received from University Grants Commission, New Delhi, India, under Special Assistance Programme (UGC–SAP) is also duly acknowledged. Financial support to Meena Devi from CSIR, New Delhi, India, is also duly acknowledged.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Sanehdeep Kaur*, Harvinder Singh Saini and Pooja Chadha conceived and designed the experiments. Sunaina Sarkhandia performed the experiments, maintained the insect culture, analyzed the data and prepared the manuscript with the help of Sanehdeep Kaur*. Meena Devi provided assistance and visualization. Geetika Sharma assisted in comet assay. Rohit Mahajan isolated and identified bacterial culture. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarkhandia, S., Devi, M., Sharma, G. et al. Larvicidal, growth inhibitory and biochemical effects of soil bacterium, Pseudomonas sp. EN4 against Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). BMC Microbiol 23, 95 (2023). https://doi.org/10.1186/s12866-023-02841-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02841-w