Abstract
Background
The saline-alkali soil area accounts for over 1/4-1/5 of the land area in Gansu Province of China, which are mainly distributed in the north of Hexi corridor and Jingtai basin. The unique ecological environment contains unique and diverse microbial resources. The investigation of microbial diversity in saline environment is vital to comprehend the biological mechanisms of saline adaption, develop and utilize microbial resources.
Results
The Illumina MiSeq sequencing method was practiced to investigate the bacterial diversity and composition in the 5 subtypes and 13 genera of saline-alkali soil in Gansu Province, China. The results from this study show that Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, and Gemmatimonadetes were the dominant bacterial groups in 13 saline soil. Proteobacteria had the greatest abundance in sulfate-type meadow solonchaks and orthic solonchaks, chloride-type orthic solonchaks and bog solonchaks, sulfate-chloride-type, chloride-sulfate-type, and sulfate-type dry solonchaks. Halobacteria was the dominant bacterial class in soil samples except for sulfate-type meadow solonchaks and orthic solonchaks, chloride-type orthic solonchaks and bog solonchaks. The richness estimators of Ace and Chao 1 and the diversity indices of Shannon and Simpson revealed the least diversity in bacterial community in sulfate-chloride-type orthic solonchaks.
Conclusions
The sulfate anion was the most important driving force for bacterial composition (17.7%), and the second most influencing factor was pH value (11.7%).
Similar content being viewed by others
Background
Soil salinization has become a worldwide problem that menaces the growth and yield of crops and impedes the development of modern agricultural sustainability [20]. In Gansu Province of China, about 1.02 × 106 hm2 of cropping land are affected by soil salinity or sodicity [15], the salinity is mainly of sulfate-type saline soil (37.9%) or chloride – sulfate – type saline soil (35.3%). High salt concentrations as well as an uneven temporal and spatial water distribution have a negative impact on sustainable development in agriculture. Soil microorganisms, playing a central role in soil organic matter decomposition, nutrient transformations, enzyme production, and soil quality maintenance [27, 35], can be greatly affected by the salt concentration. For example, moderately halophilic bacteria to extremely halophilic bacteria community can be found in hypersaline environment [21]. Microbes in saline soils varied greatly from the non-saline environment [4], and halophilic and halotolerant bacteria community are essential for the biogeochemical processes in hypersaline soil [24].
Archaea, fungi, bacteria, and viruses are the four major microbial taxa in soil [13]. Archaea can grow in conditions of extreme salinity, temperature and pH, in which bacteria cannot grow [36]. Saline-alkaline soils generally lack fungi [39], and fungi tend to be sensitive to salt stress [33], and long term salt stress reduces fungal diversity [3]. Bacteria are the most abundant microorganisms in the soil [31]. Their ecology, structure, diversity, and population dynamics are driven by soil physicochemical characteristics [12] such as soil organic carbon content [17], pH [11, 34], soil electrical conductivity [38], the plant secretion [32], trophic status [40], geographic distance [41], salinity [22], and types and concentrations of soil ions [19, 43].
Changes in archaeal community diversity has been reported in respond to different types of saline-alkali soil in Gansu Province [25]. However, edaphic factors associated with different types of saline-alkali soil on bacterial diversity and composition have not been sufficiently explored. In this study, we collected 39 soil samples in Gansu Province and sequenced on an high throughput Illumina MiSeq sequencing platform to investigate how bacterial diversity and composition has been affected by types of salinity and sodicity. The aim was to identify key factors in shaping bacterial communities among different types of saline-alkali soil in Gansu Province, which is essential for gaining insight into the biological mechanisms of saline adaption (Figs. 1 and 2).
Results
Soil biochemical characteristics
There were significant differences (P < 0.01) in pH, SOM, TS, SO42−, Cl−, UA, APA, and DHA among soil samples collected from 13 sites (Table 1). TS contents ranged from 3.81 to 24.35%, which far exceeded the thresholds of 0.2% [9], indicating a high hypersaline environment. DS.ST showed the highest SOM (14.56 g/kg), TS (24.35%). DS.CST had the highest SO42−, Cl−, DHA and the lowest APA. OS.CT had the highest UA and the lowest SOM and DHA. The lowest TS (3.81%) and UA (0.05 mg/g) were found in MS.SCT. SO42− was the lowest for OS.ST, while MS.ST had the highest APA and the lowest Cl−.
Alpha diversity patterns
Total effective sequences in all soil samples ranged from 47,376 to 64,521 (Table 2), with 97% coverage indicating that the sequence was sufficient. Analysis of the Chao and ACE indices and Shannon and Simpson indices showed that OS.SCT had the lowest species richness and diversity.
Change in bacterial community compositions in soil samples
Flower diagram revealed the total observed OTUs in soil samples (Fig. 3), and 1857 OTUs were common to all soil samples. Moreover, the distribution of sequences also demonstrated that each subtype had its own microbial population.
Venn diagrams revealed the total observed OTUs in soil samples (Fig. 4). The numbers of unique OTUs for MS.CT, MS.CST, MS.SCT, and MS. ST was 620, 634, 720 and 780, respectively. The number of shared OTUs for 4 soil genera was 1270, accounting for 17.52% of all observed OTUs (Fig. 4A). The number of specific OTUs in OS.CT, OS.CST, OS.SCT, and OS.ST were 979, 542, 642 and 1151, respectively. The total number of OTUs for 4 soil genera were 741(Fig. 4B), accounting for 10.18% of the total OTUs. The number of specific OTUs in DS.CT, DS.CST, and DS.SCT was 612, 785 and 1166, respectively. The total number of OTUs for 3 soil genera was 1706 (Fig. 4C), accounting for 28.13% of the total OTUs. The number of specific OTUs in BS.CT and AS.MgS was 2318 and 1596, respectively. The total OTUs for bacteria was 2073 (Fig. 4D), accounting for 34.63% of the total OTUs.
Venn graph of the numbers of shared and unique operational taxonomic units (OTUs) among 5 subtypes and 13 genera of saline-alkali soil in arid regions of northwest China The overlapping part represents the shared OTUs; non-overlapping part represents the specific OTUs of the given genera; and the number means the corresponding amount of OTUs. Notes: MS, OS, BS, AS, and DS represent meadow solonchaks, orthic solonchaks, bog solonchaks, alkalized solonchaks, and dry solonchaks, respectively. CT, SCT, CST, ST, Mg.S represent chloride-type, sulfate-chloride-type, chloride-sulfate-type, sulphate-type, and magnesium solonetz, respectively
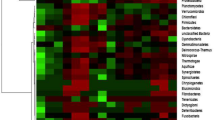
The main bacterial phyla included Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, Gemmatimonadetes, Acidobacteria, TM7, Tenericutes, and Verrucomicrobia (Fig. 5). The relative abundances of these phyla together made up an average of 65.0% for all bacteria. ANOVA revealed that except for Firmicutes and Tenericutes, other bacterial phyla showed significant differences in all samples (P < 0.01 or P < 0.05). Proteobacteria was the most abundant in MS.ST, OS.ST, OS.CT, BS.CT, DS.SCT, DS.CST, and DS.ST.
Relative abundance of soil bacterial community at phylum level among 5 subtypes and 13 genera of saline-alkali soil in arid regions of northwest China. Notes: MS, OS, BS, AS, and DS represent meadow solonchaks, orthic solonchaks, bog solonchaks, alkalized solonchaks, and dry solonchaks, respectively. CT, SCT, CST, ST, Mg.S represent chloride-type, sulfate-chloride-type, chloride-sulfate-type, sulphate-type, and magnesium solonetz, respectively
Average relative abundance of the top 10 microbial dominant classes accounted for 68.44% of all bacterial classes (Fig. 6). There were significant differences in Halobacteria (s.e.m = 0.156**), Gammaproteobacteria (s.e.m = 0.035**), Clostridia (s.e.m = 0.037**), Cytophagia (s.e.m = 0.021*), Alphaproteobacteria (s.e.m = 0.029**), Rhodothermi (s.e.m = 0.025**), Gemm-4 (s.e.m = 0.014**), Acidimicrobiia (s.e.m = 0.013**), and MJK10 (s.e.m = 0.009**) except for Bacilli in soil samples.
Relative abundance of soil bacterial community at class level among 5 subtypes and 13 genera of saline-alkali soil in arid regions of northwest China. Notes: MS, OS, BS, AS, and DS represent meadow solonchaks, orthic solonchaks, bog solonchaks, alkalized solonchaks, and dry solonchaks, respectively. CT, SCT, CST, ST, Mg.S represent chloride-type, sulfate-chloride-type, chloride-sulfate-type, sulphate-type, and magnesium solonetz, respectively
Relationship between bacterial community compositions and edaphic factors
SOM, pH, TS, SO42−, Cl−, UA, DHA, and APA were closely correlated with the abundance of the dominant bacterial phyla (Table 3). The abundance of Proteobacteria (r = − 0.319, p = 0.047) was correlated negatively with SOM. The abundance of Proteobacteria (r = 0.386, p = 0.015), Bacteroidetes (r = 0.456, p<0.01), Actinobacteria (r = 0.492, p<0.01), Firmicutes (r = 0.332, p = 0.039), and Gemmatimonadetes (r = 0.499, p<0.01) showed positive relationship with pH. The abundance of Proteobacteria (r = − 0.343, p = 0.032), Bacteroidetes (r = − 0.407, p = 0.01), Actinobacteria (r = − 0.325, p = 0.043), and Gemmatimonadetes (r = − 0.561, p<0.01) had a negative correlation with TS.
The abundance of Proteobacteria (r = − 0.560, p<0.01), Gemmatimonadetes (r = − 0.451, p<0.01), and Acidobacteria (r = − 0.495, p<0.01) were correlated negatively with SO42−, then Gemmatimonadetes (r = − 0.387, p = 0.015) had a negative correlation with Cl−, while Tenericutes (r = 0.317, p = 0.049; r = 0.346, p = 0.031, respectively) showed positive relationship with SO42− and Cl−. Additionally, there was a significant positive correlation between Firmicutes (r = 0.483, p<0.01) and UA, Acidobacteria (r = 0.354, p = 0.027) and DHA, Bacteroidetes (r = 0.616, p<0.01) and APA.
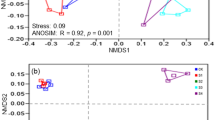
The two redundancy analysis (RDA) axes explained 48.4% of the variation between the soil bacterial communities (Fig. 7). The distinctions of bacterial community structure among 5 subtypes and 13 genera of soil were also supported by the redundancy analysis (RDA). Soil SO42−, pH value, APA, TS, SOM, UA, and DHA had significant effects on bacterial community compositions (P = 0.001 by the Monte Carlo permutation test) (Table 4).
Redundancy analysis (RDA) of the relationships between microbial composition and edaphic factors of 5 subtypes and 13 genera of saline-alkali soil in arid regions of northwest China. Notes: TS, DHA, UA, and APA represent total salt content, dehydrogenase, urease, and alkaline phosphatase, respectively
Discussion
Bacterial community diversity and structure
Bacteria are the most abundant and diverse group of soil microorganisms which play multiple important key roles in soil [23]. We collected 13 saline-alkali soils (5 subtypes and 13 genera) to determine their bacterial diversity and abundance. It was found that the bacterial communities differed greatly among 13 soil samples, and the richness estimators (Ace and Chao 1) and the diversity indices (Shannon and Simpson) revealed a least diversity in bacterial community in OS.SCT.
Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, and Gemmatimonadetes accounted for a large proportion (17.81–89.26%) among different treatments, which was consistent with previous study of bacterial community analysis in saline-alkali soil [24, 38]. Proteobacteria was a ubiquitous and common group in soil [7] and was reported as “salinity related” [42]. Our results further confirmed this finding. Firmicutes which was considered special indicators specifically in high salt soil [38], occurred at a level of 2.51-16.67% in all samples in this study, but Firmicutes was absent in various hyper saline environments in previous studies [8, 29]. Gemmatimonadetes and Bacteroidetes are also important participant in biogeochemical transformations in soils under salinity [14]. The Actinobacteria and Acidobacteria have been depicted as common inhabitants of all soils [18, 44]. Furthermore, Actinomycetes can significantly inhibit the activities of some plant pathogenic fungi, and can also promote the germination of seeds and the growth of plant roots [20]. Halobacteria was the dominant bacterial class in all soils except for MS.ST, OS.CT, OS.ST, and BS.CT. Members of class haloarchaea are obligately halophilic, predominantly aerobic heterotrophs that consistently contribute to the biogeochemical cycling of carbon and nitrogen in saline soils [1, 37].
Effects of soil properties on the dominant bacterial phyla and bacterial community structure
Microorganisms are particularly sensitive to environmental change [27]. The Monte Carlo permutation test showed that pH, TS, SO42−, and UA were significantly correlated with change in bacterial community composition in 13 saline soils. The soil pH account for 32.1% of variance in bacterial community, and acted as the most important driver in bacterial community, in consistent with other finding [11, 20, 34]. In addition, TS was an important factor affecting change in microbial community composition in saline soils in this study, as also reported by Rath et al. [30]. Change in bacterial community is mainly affected by SO42-, a dominant anion in saline-alkali soils of Gansu Province. Enzymatic activity is a sensitive parameter and is influenced by a number of natural and anthropogenic factors [26]. Our soil samples showed a positive relationship between bacterial abundance and enzyme especially UA, suggesting soil halophiles enzymes require salt for maintaining enzyme stability and activity [21].
Conclusions
Our results demonstrated that Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, and Gemmatimonadetes were the main indicator species reflecting changes of the main microbial groups and the richness and diversity of bacterial communities were the lowest in sulfate-chloride-type orthic solonchaks. The sulfate anion as a key factor drives the composition of the bacterial community among 5 subtypes and 13 genera of saline-alkali soil of Gansu Province.
Material and methods
Site description
The study area located in Gansu Province (37°17′-42°48′N, 92°12′-103°48′E), Northwest China, which belonged to a transitional zone between arid and semi-arid regions. It was affected by continental climate and Tibetan Plateau climate with annual mean temperature of 5-10 °C, annual mean evaporation of 1448.4 mm. The altitude is 1100-1500 m above sea level; frost-free days is 160 to 230 days; the sunshine duration is more than 3000-4000 h/a; annual total solar radiation is 120 × 4, 186.8-150 × 4, 186.8 J/cm2 [25].
Saline soils of this region were divided into five subtypes (meadow solonchaks, orthic solonchaks, bog solonchaks, alkalinzed solonchaks, and dry solonchaks, shorting for MS, OS, BS, AS, DS, respectively.). Meadow solonchaks and orthic solonchaks were further divided into four genera, including sulphate type (ST), chloride-sulphate type (CST), sulphate-chloride type (SCT), and chloride type (CT). Bog solonchaks and alkalinzed solonchaks were divided into only one genera, namely bog solonchaks (BS) and magnesium alkalinzed solonchaks (Mg.S), respectively. Dry solonchaks were divided into three genera, including sulphate type (ST), chloride-sulphate type (CST), sulphate-chloride type (SCT) [25]. Most plants in this region are halophyte species. The vegetation pattern consists of Achnatherum splendens (Trin.), Nitraria tangutorum Bobr., Phragmites australis (Cav.), Tamarix ramosissima L., Glycyrrhiza uralensis Fisch., Alhagi sparsifolia Shap., Leymus secalinus (Georgi) Tzvel, Suaeda glauca (Bunge). The vegetation coverage is 0.76, 24.27, 38.50, 19.10, 53.25, 31.37, 37.29, 37.87, 43.68, 29.40, 48.40, 43.67, and 35.32% in MS.CT, MS.SCT, MS.CST, MS.ST, OS.CT, OS.SCT, OS.CST, OS.ST, BS.CT, AS.MgS, DS.SCT, DS.CST, and DS.ST, respectively.
Site selection and soil sampling
The thirteen study sites and basic meteorology data of different counties were shown in Figs. 1 and 2, respectively. At each study site, three sample lines were selected with about 120 degree (angle) between adjacent lines. Five soil cores between adjacent cores spacing 5 m, at a depth of 0–10 cm, were taken from each lines and combined to obtain about 100 g of soil in July, 2015, for a total of 39 samples. Samples were mixed thoroughly, placed in an ice box and brought back to the laboratory immediately. Soil sample was divided into two subsamples: one was air dried, 2 mm sieved, then chemically and physically analysed, while the second one was stored at − 80 °C and later processed for high-throughput sequencing.
Edaphic factors characteristics
Soil organic matter (SOM) was determined using K2Cr2O7-H2SO4 digestion [4]. Potentiometric titration was used to determine Cl− and SO42− with air-dried soil (soil: water, 1:5). Total salt content (TS) was measured in soil suspensions (soil: water = 1:5) with a conductance instrument, and pH was determined with air-dried soil (soil: water, 1:5) by pH meter (PHS-3C, REX, Shanghai). The enzymatic activities of urease (UA), alkaline phosphatase (APA), and dehydrogenase (DHA) were determined according to the methods described by Guan [16].
Soil DNA extraction
DNA was extracted by cetyltrimethyammonium ammonium bromide (CTAB) method. The final DNA concentration and purification were examined using a Nano Drop spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA), and DNA quality was checked by 1% agarose gel electrophoresis.
Amplification and sequencing of 16S rRNA genes
Next generation sequencing was performed in Novogene, Inc. (Beijing, China). The primer pair 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) was used to amplify the V4 region of bacterial 16S rRNA gene [6]. PCR amplification was carried out with Phusion® High-Fidelity PCR Master Mix (New England Biolabs). PCR products were purified with Qiagen Gel Extraction Kit (Qiagen, Germany). The libraries were constructed with the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA). The library quality was quantified by Qubit and quantitative real-time PCR.
Paired-end reads were merged using FLASH [22]. Raw high-throughput sequencing data was processed using QIIME toolkit and the UPARSE pipeline [5, 10]. After filtering DNA sequences using quality files, the remaining sequences were trimmed to remove barcodes and forward primers. The low quality sequences were excluded. The sequencing data were pre-treated to remove the chimeras from the datasets. The sequencing data were pre-treated to remove the chimeras from the datasets. Sequencing results of samples were defined as operational taxonomic units (OTUs) at 97% identity threshold. Species annotation was performed using the GreenGene Database based on the Ribosomal Data Project (RDP) database [10]. All sequencing data were deposited in the NCBI Sequence Read Archive database (BioProject ID PRJNA707428, study accession number SRX10280109 to SRX10280147).
Statistical analysis
Analysis of variance was performed for each measured soil variable, and variance was compared between groups by Duncan’s test (P < 0.05). Chao1, ACE, Shannon and Simpson indexes were calculated by QIIME with normalized data [28]. The flower and Venn diagrams were performed using R software (version: 2.15.3). Pearson’s correlation (r) and significance (p) values were performed using SPSS version 20. Redundancy analysis (RDA) was used to examined the relationships between the environmental factors and bacterial community structure with CANOCO 5.0 for Windows (Monte Carlo permutation test, P = 0.001; Microcomputer Power, USA).
Availability of data and materials
The genome sequences can be accessed after 2021-04-01 at https://www.ncbi.nlm. nih.gov/bioproject/browse using Bioproject PRJNA707428. Until then, the sequences are available from the corresponding author upon reasonable request.
Abbreviations
- MS:
-
Meadow solonchaks
- OS:
-
Orthic solonchaks
- BS:
-
Bog solonchaks
- AS:
-
Alkalized solonchaks
- DS:
-
Dry solonchaks
- CT:
-
Chloride-type
- SCT:
-
Sulfate-chloride-type
- CST:
-
Chloride-sulfate-type
- ST:
-
Sulphate-type
- Mg.S:
-
Magnesium solonetz
References
Andrei AS, Banciu HL, Oren A. Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett. 2012;330:1–9.
Bao SD. Soil agricultural chemistry analysis. 3rd ed. Beijing: China Agriculture Press; 2000.
Bruggen AHCV, Semenov AM, Zeiss MR. In search of biological indicators for soil health and disease suppression. Appl Soil Ecol. 2000;15:13–24.
Canfora L, Bacci G, Pinzari F, Papa GL, Dazzi C, Benedetti A. Salinity and bacterial diversity: to what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS One. 2014;9:e106662.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010. https://doi.org/10.1038/nmeth.f.303.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(SUPPL):4516–22.
Chen LX, Li JT, Chen YT, Huang LN, Hua ZS, Hu M, et al. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ Microbiol. 2013;15:2431–44.
Demergasso C, Casamayor EO, Chong G, Galleguillos P, Escudero L, Pedrós-Alió C. Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol. 2004;48:57–69.
Dion P, Nautiyal CS. [Soil Biology] Microbiology of Extreme Soils. Springer Berlin Heidelberg, 2008; (Chapter 6):117–32.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200.
Eichorst SA, Breznak JA, Schmidt TM. Isolation and characterization of soil bacteria that define gen. Nov in the phylum Tetrahedron Letters. 2007;48:9144–7.
Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci. 2006;103:626–31.
Fierer N, Breitbart M, Nulton J, Salamon P, Lozupone C, Jones RT, et al. Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of bacteria, archaea, fungi, and viruses in soil. Appl Environ Microbiol 2007;73(21):7059-66.
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber$ CL, et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci USA, 2012;109:21390.
Gansu Province Soil Survey Office. Gansu soil. Beijing:China Agricultural Press. 1993.
Guan SY. Soil enzyme and its research method. Beijing: China Agricultural Press; 1986.
Hansel CM, Fendorf S, Jardine PM, Francis CA. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl Environ Microbiol. 2008;74:1620–33.
Karolina F, Jarosław G, Anna G, Jacek N. Analysis of soil properties, bacterial community composition, and metabolic diversity in Fluvisols of a floodplain area. Sustainability. 2019;11. https://doi.org/10.3390/su11143929.
Li M, Bi JT, Wang J. Bacterial community structure and key influence factors in saline soil of different sites in Ningxia. Acta Ecol Sin. 2020;40:1316–30.
Liu Z, Shang HG, Han F, Zhang MR, Li Q, Zhou WZ. Improvement of nitrogen and phosphorus availability by Pseudoalteromonas sp during salt-washing in saline-alkali soil. Appl Soil Ecol. 2021;168:104117. https://doi.org/10.1016/j.apsoil.2021.104117.
Loredana C, Giuseppe LP, Livia VA, Giuseppe B, Carmelo D, Anna B. Spatial microbial community structure and biodiversity analysis in “extreme” hypersaline soils of a semiarid Mediterranean area. Appl Soil Ecol. 2015;93:120–9.
Lozupone CA, Knight R. Global patterns in bacterial diversity. PNAS. 2007;104:11436–40.
Lynn TM, Liu Q, Hu YJ, Yuan HZ, Wu XH, Khai AA, et al. Influence of land use on bacterial and archaeal diversity and community structures in three natural ecosystems and one agricultural soil. Arch Microbiol. 2017;199:711–21.
Ma B, Gong J. A meta-analysis of the publicly available bacterial and archaeal sequence diversity in saline soils. World J Microb Biot. 2013;29:2325–34.
Nan LL, Guo QE, Cao SY. Archaeal community diversity in different types of saline-alkali soil in arid regions of Northwest China. J Biosci Bioeng. 2020;130:382–9.
Nannipieri P, Grego S, Ceccanti B. Ecological significance of the biological activity in soils. Soil Biochemistry. 1990.
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G. Microbial diversity and soil functions. Eur J Soil Sci. 2003;54:655–70.
Petrova SN, Andronov EE, Pinaev AG, Pershina EV. Prospects for using the methods of molecular genetic analysis in soil ecology. Vestn Orlovsk Gos Agrarn Univ. 2010;26:45–8.
Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62:142–60.
Rath KM, Fierer N, Murphy DV, Rousk J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019;13:836–46.
Roesch LF. Pyrose-quencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1:283–90.
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010;4:1340–51.
Sardinha M, Müller T, Schmeisky H, Joergensen RG. Microbial performance in soils along a salinity gradient under acidic conditions. Appl Soil Ecol. 2003;23:237–44.
Shen CC, Xiong JB, Zhang HY, Feng YZ, Lin XG, Li XY, et al. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem. 2013;57:204–11.
Sleator RD, Shortall C, Hill C. Metagenomics. Lett Appl Microbiol. 2008;47:361–6.
Valentine DL. Adaptations to energy stress dictate the ecology and evolution of the archaea. Nat Rev Microbiol. 2007;5:316–23.
Ventosa A, Fernandez AB, Leon MJ, Sanchez-Porro C, Rodriguez-Valera F. The Santa Pola saltern as a model for studying the microbiota of hypersaline environments. Extremophiles. 2014;18:811–24.
Wang S, Sun L, Ling N, Zhu C, Chi FQ, Li WQ, et al. Exploring soil factors determining composition and structure of the bacterial communities in saline-alkali soils of Songnen plain. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2019.02902.
Wei Y, Zhang SH. Abiostress resistance and cellulose degradation abilities of haloalkaliphilic fungi: applications for saline–alkaline remediation. Extremophiles. 2018;22:155–64.
Xiong J, Wu L, Tu S, Van Nostrand JD, He Z, Zhou J, et al. Microbial communities and functional genes associated with soil arsenic contamination and rhizosphere of the arsenic hyper-accumulating plant Pterisvittata L. Appl Environ Microbiol. 2010;76:7277–84.
Xiong JB, Liu YQ, Lin XG, Zhang HY, Zeng J, Hou JZ, et al. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan plateau. Environ Microbiol. 2012;14:2457–66.
Yang J, Zhan C, Li Y, Zhou D, Yu Y, Yu J. Effect of salinity on soil respiration in relation to dissolved organic carbon and microbial characteristics of a wetland in the Liaohe River estuary, Northeast China. Sci Total Environ. 2018;642:946–53.
Yu BQ, Yang S, Zhu L, Gao F, WeiW. Diversity characteristic of archaeal community responding to soil anion in saline-alkali soil. China Environ Sci. 2018;38:2731–9.
Zhang MM, Wang N, Hu YB, Sun GY. Changes in soil physicochemical properties and soil bacterial community in mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. Microbiologyopen, 2018. doi:https://doi.org/10.1002/mbo3.555.
Acknowledgements
We are thankful to the anonymous reviewers who helped improve this paper. We are also thankful to Dr. Xiaojuan Wang from La Trobe University, Australia, to revise the entire article.
Funding
Authors are grateful to the National Natural Science Foundation of China (Nos. 41363004), and the Department of Science and Technology of Gansu Province for funding this research (20YF3FA011) and the Key Research and Development Program of Gansu Academy of Agricultural Sciences (2019GAAS24).
Author information
Authors and Affiliations
Contributions
Lili Nan and Quanen Guo designed research; Lili Nan, Quanen Guo, Shiyu Cao and Zongbing Zhan performed research; Lili Nan and Quanen Guo analyzed data; and Lili Nan and Quanen Guo wrote the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nan, L., Guo, Q., Cao, S. et al. Diversity of bacterium communities in saline-alkali soil in arid regions of Northwest China. BMC Microbiol 22, 11 (2022). https://doi.org/10.1186/s12866-021-02424-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-021-02424-7