Abstract
The Golden2-like (GLK) transcription factors are plant-specific transcription factors (TFs) that perform extensive and significant roles in regulating chloroplast development. Here, genome-wide identification, classification, conserved motifs, cis-elements, chromosomal locations, evolution and expression patterns of the PtGLK genes in the woody model plant Populus trichocarpa were analyzed in detail. In total, 55 putative PtGLKs (PtGLK1-PtGLK55) were identified and divided into 11 distinct subfamilies according to the gene structure, motif composition and phylogenetic analysis. Synteny analysis showed that 22 orthologous pairs and highly conservation between regions of GLK genes across P. trichocarpa and Arabidopsis were identified. Furthermore, analysis of the duplication events and divergence times provided insight into the evolutionary patterns of GLK genes. The previously published transcriptome data indicated that PtGLK genes exhibited distinct expression patterns in various tissues and different stages. Additionally, several PtGLKs were significantly upregulated under the responses of cold stress, osmotic stress, and methyl jasmonate (MeJA) and gibberellic acid (GA) treatments, implying that they might take part in abiotic stress and phytohormone responses. Overall, our results provide comprehensive information on the PtGLK gene family and elucidate the potential functional characterization of PtGLK genes in P. trichocarpa.
Similar content being viewed by others
Introduction
Chloroplasts are responsible for the light energy response of photosynthesis, and contain the green pigment chlorophyll, which is basically relied upon by all plant life [1, 2]. Recent research has shown that chloroplasts originated from primary endosymbiotic events related to these cyanobacteria [3, 4]. Thus, the regulation of photosynthetic organs assembly depends on the synergy of the nucleus and chloroplast. Plastids not only function in photosynthesis but also in the synthesis of amino acids, fatty acids, purines and pyrimidine bases, terpenes and various pigments, and hormones, as well as the key aspects of nitrogen and sulfur assimilation [5,6,7]. Moreover, proplastids in subepidermal meristem cells (or leaf sheaths in dark cotyledons) convert to mesophyll chloroplasts under light [8]. Conversely, members of the Golden 2-like (GLK) family can regulate the appearance of chloroplasts in the transition and maturity stages, and GLK genes are essential in angiosperm chloroplast development [2, 9, 10]
GLK transcription factor was first identified in maize (Zea mays L.), and was proven to be a new transcriptional regulator that functions on cellular differentiation in the leaves of maize [11]. The GLK genes belong to the GARP superfamily of nuclear transcription factors [12], which are defined by GOLDEN2 in maize, RESPONSE REGULATOR-B (ARR-B) proteins in Arabidopsis [13], and the PHOSPHATE STARVATION RESPONSE1 (PSR1) protein in Chlamydomonas [14]. Most GLK proteins contain two domains: a Myb-DNA-binding domain (DBD; containing a helix-loop-helix motif) and a C-terminal box (containing a GCT box) [15, 16].
GLK genes are crucial for the formation and development of chloroplasts, and participating in various biotic and abiotic stress defense processes of organisms [17, 18]. In Arabidopsis, AtGLK1 and AtGLK2 genes were found to be involved in the production of chloroplast redundantly [19, 20]. Overexpression of AtGLK1 can cause resistance to Fusarium graminearum [21, 22] and improve sensitivity on the virulent oomycete pathogen Hyaloperonospora arabidopsidis (Hpa) [1]. In addition, SlGLK2 affects the photosynthesis of developing fruits and contributes to the characteristics of mature fruits in tomato (Solanum lycopersicum) [23]. Moreover, owing to the increased expression of chloroplast development and fruit-photosynthesis-related genes, the carbohydrates and carotenoids in ripe fruit were found to be enhanced in the overexpression of SlGLK2 [24]. ZmGLK1 is considered as a regulator of the development of chloroplasts in mesophyll cells of C4 tissues, while GLK gene pairs plays a redundant role in C3 species and promote the development of chloroplasts in maize [14, 16].
Poplar is an important model plant in the study of woody plants, with the characteristics of rapid growth and easy genetic transformation. The accomplishment of the poplar genome sketch provides potential in gene identification and gene function analysis. The GLK genes have been identified and described in maize [25], Arabidopsis [17], tomato [26], tobacco [27], and moso bamboo [28]. Nevertheless, there has been no comprehensive study on the GLK family genes of P. trichocarpa. In this study, 55 putative PtGLK genes were identified and classified into 11 groups, taking maize GLKs, Arabidopsis GLKs, and their conserved domains as references. A comprehensive bioinformatics analysis was carried out to study gene structure, domain composition, chromosome distribution, syntheses analysis, and expression patterns. Promoter cis-elements and expression level of genes in response to abiotic stress (cold and osmotic) and phytohormone (MeJA and GA) treatments were also examined. The information derived from this study offers a valuable resource for further study on the characterization and function of the poplar GLK gene family.
Materials and methods
Plant material treatment and gene expression analysis
The material used in this study was poplar 84 K (Populus alba × Populus glandulosa) which is an aspen hybrid poplar from Korea. Populus trichocarpa trees were obtained from Beijing Forestry University poplar nursey planting base, and were grown under the settings of 16 h light and were maintained at 25 °C and 85% relative humidity in a greenhouse in Haidian, Beijing, China (39°56′ N,116°25′ E, 43.5 m above sea level). Three-month-old poplar seedlings were treated with osmotic stress, cold stress, and MeJA and GA treatments. For cold stress, the seedlings were positioned in a 4 °C growth chamber and sampled at 0, 1, 3, 6, 12, and 24 h after stress imposition. For osmotic stress, the seedlings were accumulated after being sprayed with 25% polyethylene glycol (PEG) 6000. For phytohormone treatments, a solution of 200 µM jasmonic acid (JA) and 200 mg/L gibberellic acid (GA) were sprinkled onto poplar plants on the basis of the needs and sampled randomly after the phytohormone treatments were applied. Seedlings irrigated at 28 °C in an artificial growth chamber and sprinkled with MS medium solution were used as controls and were sampled at 0 h.
The primers of the 11 PtGLK genes were designed by the NCBI Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) to amplify 200–250 bp PCR products (Table S4). The heatmap of PtGLK gene expression was generated using the Amazing Heatmap module in TBtools for the poplar FM (female catkins, prior to seed release), F (female catkins, post-fertilization), M (male catkins), ML (mature leaf), REF (washed fibrous roots < 0.5 cm diameter from field-grown trees), RTC (roots from plants in tissue culture), G43h (seedlings were germinated 43 h post-imbibition), ApB (actively growing shoot apex), AxB (axillary bud), YFB (newly initiated female floral buds), YMB (newly initiated male floral buds), Xylem1(developing phloem), Phloem3 (developing phloem/cambium), and PC (phloem, cortex, epidermis) [29, 30].
Identification of PtGLKs
Poplar GLK sequences were acquired from the Phytozome12.1 database (https://phytozome.jgi.doe.gov). The previously reported GLK protein sequences of Arabidopsis [19] were used for the purpose of identifying the poplar GLK proteins for a BLAST alignment of the poplar protein database. More than 30% similarity and an E-values below 0.001 were set as the parameters to determine the poplar candidate GLK proteins. Then the domains of all poplar GLK proteins were investigated using Pfam (http://pfam.xfam.org/) to determine the putative proteins. The gene IDs, physical positions, sequences of the genes and proteins, and the coding sequences (CDS) were downloaded from the P. trichocarpa genome database (https://genome.jgi.doe.gov/portal/Poptr1/Poptr1.home.html). The detailed physical parameters of PtGLK genes, including molecular weight (MW) of amino acids, isoelectric point (pI), and length of the CDS, were predicated using ExPASy (http://www.expasy.ch/tools/pi_tool.html) [31].
Multiple sequence alignment and phylogenetic analysis
The protein sequences of poplar GLK proteins were aligned with the ClustalW tool [32]. The alignment of the PtGLK-domain-containing sequence was displayed by DNAMAN 8 platform (https://www.lynnon.com/dnaman.html). The phylogenetic tree based on the complete PtGLK sequences and the combined phylogenetic tree of GLK protein sequences from P. trichocarpa, Z. mays, and Arabidopsis were constructed with MEGA 7.0.
Gene structure
The exon/intron structures of PtGLK genes were decided by the Gene Structure Display Server (GSDS) platform (http://gsds.cbi.pku.edu.cn/) using the complete genomic sequence and CDS [33]. The conserved motifs presented in PtGLK proteins were analyzed by the online MEME tool (http://meme-suite.org/tools/meme) [34] according to the following rules: optimum width of motifs at 10–50, and maximum number of motifs at 10 residues for PtGLK proteins. Motif annotation was identified using the Pfam tools. The predict protein homology model was analyzed using the Phyre2 website (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index), and alignment of the PtGLK protein sequences was determined via Hidden Markov Models (HMM) [35].
Chromosomal location, synteny analysis and duplication events
Gene location information was acquired from the P. trichocarpa genome database on the basis of the genome annotation file (gff file), and all PtGLKs were mapped onto the poplar chromosomes by MapInspect software (http://www.softsea.com/review/MapInspect.html). The possible gene duplication landscape was identified by the Multiple Collinearity Scan Toolkit (MCScanX) software [36]. Segmental duplication and tandem duplication were determined according to the means covered by Wang et al. (2010) [37]. The syntenic maps were subsequently displayed using the Dual Systeny Plotter software (https://github.com/CJ-Chen/TBtools) [38].
Ka and Ks were computerized by KaKs Calculator 2.0 with Clustalx 2.11, and the Ka/Ks ratios were calculated using DnaSP5 to investigate the gene duplication events [39,40,41].
Putative promoter region analysis of PtGLK genes
The 2000 bp upstream sequences of 55 PtGLK genes were selected as the putative promoter regions to choose the cis-elements. The putative cis-regulatory elements were identified by PlantCARE (http://www.dna.affrc.go.jp/PLACE/), and those that responded to abiotic stresses and phytohormone treatments were screened out [42, 43].
Results
Identification of PtGLK genes in P. trichocarpa
To identify the PtGLK gene family in P. trichocarpa, Arabidopsis AtGLK protein sequences [19] were used as BLASTP sequences in extensive searches and alignment in the poplar genome database. A total of 55 PtGLK genes (PtGLK1-PtGLK55) were identified, all these were used to affirm the existence of the Myb-DNA-binding domain (DBD) through the Pfam database. To further examine the similarity among the PtGLK protein domains, multiple alignments of 55 PtGLK protein domain sequences were conducted (Fig. 1). The result indicated that the PtGLKs were conserved across two regions of the Myb-DNA-binding domain with the HLH structure of the first helix containing the initial sequence PELHRR and the second helix containing NI/VASHLQ, which was coincided with the GLK members in Z. mays [25, 45], Arabidopsis [5], tomato [26, 44], tobacco [27], and moso bamboo [28].
Base information about the PtGLK genes, such as accession number, gene location, protein length, molecular weight (MW), exon numbers, and physicochemical parameters, is presented in Table 1. The PtGLK genes exhibited an inclusive conservation of amino acid sequence lengths and molecular weights. The encoded amino acid sequences ranged from 282 to 486 aa, and the predicted molecular weight (MW) varied from 28.87 to 53.24 kDa. Moreover, the theoretical isoelectric point (pI) ranged from 5.55 to 9.46.
Phylogenetic analysis of the GLK genes and the determination of gene structures
To analyze the evolutionary relationship of the poplar GLK family, a neighbor-joining phylogenetic tree was produced by aligning 55 PtGLK protein sequences with 59 and 42 protein sequences from Z. mays [25] and Arabidopsis, respectively [5]. The detailed information of ZmGLK genes and AtGLK genes are listed in Table S1. In the phylogenetic tree, the GLK family members were classed into 13 groups according to the evolutionary relationships and motif analysis of PtGLK proteins, and PtGLKs were assigned into 11 groups (G1-G11), but not G12 and G13. The numbers of PtGLK members in different groups was unbalanced, with groups 1 to 11 containing 11, 18, 1, 2, 2, 3, 2, 5, 5, 2, and 6 proteins, respectively (Fig. 2).
A separate phylogenetic tree only with PtGLK proteins was formed to provide additional insight into the structure characteristics of PtGLK genes, and all PtGLK proteins were grouped into 11 subfamilies which is consistent with the phylogenetic tree of P. trichocarpa, Z. mays, and Arabidopsis. Exon/intron organization analysis of the PtGLK genes, which were defined by the arrangement of PtGLK genes, could gain additional insight into the development of poplar GLK family members. The number of exons in the subfamilies ranged from 1 to 9 (Fig. 3). More than half of the PtGLK genes (75%) had six or more exons, and only five genes (9%) contained four or fewer exons. The vast majority of the PtGLK genes that assembled into the same subfamily exhibited similar or identical exon/intron distributions, including the number of exons and their length. In total, phylogenetic analysis and conservative gene structure provide reliable grouping classification results for PtGLK members in the same group. Additionally, the exon/intron structure of each segmentally duplicated pair showed homologous exon/intron distributions.
Analysis of motif distribution and homology modeling in poplar GLK genes
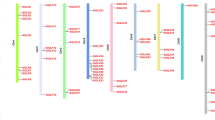
The conserved motifs of 55 PtGLK proteins within each subfamily were analyzed by MEME software. Eight distinct motifs were identified, and detailed sequence information of each motif is displayed in Table S2. With the Conserved Domain Database, six putative motifs were functional comments, being defined as Myb-SHAQKYF for motifs 1, 3, 5, 6, and 8, and Myb-CC-LHEQLE for motif 2 (Fig. 4). Nevertheless, no functional notes were given to the remaining two putative motifs. Members of the protein family gathered in the same subfamily displayed similar or identical motif components and spatial distributions, which revealed the functional similarities of these proteins. For example, all the PtGLK proteins contained a Myb DNA-binding domain (motif 3), which has an HLH structure. Besides the conserved GLK Myb-DNA-binding domain, the members within different subfamilies had specificity motifs that probably represent their variety functions in plant development and in response to abiotic stress (Figure S1). For instance, motif 2 (Myb-CC-LHEQLE) only appeared in subfamilies 3, 4, 5, 6, 7, 8, 9, 10, and 11.
To further investigate the potential structures of the PtGLK proteins, we made use of Phyre2 to predict the homology modeling and aligned the protein sequences [45]. The result in Fig. 5 showed that each PtGLK protein could be modeled with confidence, and 12 PtGLKs (PtGLK17, PtGLK18, PtGLK19, PtGLK22, PtGLK26, PtGLK28, PtGLK29, PtGLK30, PtGLK31, PtGLK35, and PtGLK48) among them had 100% of their predicted lengths modeled with > 40% confidence.
Chromosomal locations and synteny analysis of PtGLK genes in P. trichocarpa
A total of 55 PtGLKs were acquired and were distributed to the 19 poplar chromosomes (Chr1-Chr19) (Fig. 6). The number of PtGLKs per chromosome ranged from one to seven. For example, chromosome 16 contained seven PtGLK genes, with the largest number, followed by chromosome 1, with six, and chromosomes 6, 8, and 10 with five. Conversely, chromosomes 12 and 15 possessed only one PtGLK gene each. In addition, the potential duplication events were analyzed by the MCScanX program to search the mechanism for the PtGLK gene family. A total of 22 duplicated pairs of PtGLK genes were defined as segmental duplication gene pairs, but not tandem duplication gene pairs, in a syntenic map (Fig. 7A, and Table 2). Moreover, the analysis showed that there was an unevenly distribution mode among the 22 segmental duplicated pairs on the 19 chromosomes. These results suggested that segmental duplication events probably play a primary role in the amplification of the poplar GLK gene family.
To further determine the evolutionary orthologous relationships of PtGLKs, two comparative syntenic maps of P. trichocarpa related to Arabidopsis and Z. mays were also drawn (Fig. 7B). As shown in Table 3, 22 and 11 orthologs of P. trichocarpa between Arabidopsis (Pt-At) and Z. mays (Pt-Zm) were identified, respectively. Moreover, highly conserved microsynteny was found among the regions of PtGLK genes between P. trichocarpa and Arabidopsis, especially in Pt8 and At1 and in Pt10 and At1, with seven and four synteny genes, respectively.
Evolutionary and divergence patterns of the GLK gene family
For each PtGLK gene pair, the Ka/Ks ratios were calculated to evaluate divergence times and selective pressure for the duplicated PtGLK genes. To further search the evolutionary events and divergence profiles of GLK genes between P. trichocarpa and Arabidopsis, statistical analysis of the Ka/Ks ratios and the Ks values were conducted. The average frequency distribution of the calculated Ks values of paralogous pairs (Pt–Pt) was approximately 0.24, suggesting that PtGLK genes went through a large-scale duplication event was approximately 17 million years ago (MYA) (Fig. 8 and Table 2). Compared with a prior study indicating the timing of a whole-genome duplication in P. trichocarpa at 7–12 MYA [46], this result indicated that the large-scale duplication of PtGLK genes occurred earlier [41]. Additionally, the frequency distributions of Ks values for the orthologous pairs from the P. trichocarpa and Arabidopsis genomes averaged ~ 2.25 (Fig. 8, Table 3), suggesting that the divergence time of the GLK genes was 118 MYA. With reference to a previous study, it can be inferred that the divergence times between P. trichocarpa and Arabidopsis were 102–113, this result indicated that the PtGLK genes went through gene evolution before the separation of Z. mays. The Ka/Ks ratios peak in the poplar genome (Pt–Pt) and between the P. trichocarpa and Arabidopsis genomes (Pt-At) were distributed between 0.14–0.50 (Table 2) and 0.06–0.20 (Table 3), respectively, which suggests that PtGLK genes have probably experienced highly positive purifying selection between P. trichocarpa and Arabidopsis genomes, as well as being paralogous in the poplar genome.
Expression profiles of PtGLK genes in various tissues and stages of P. trichocarpa
To characterize the dynamics of PtGLK gene expression, we studied gene expression patterns in several vegetative tissues and stages of poplar reproductive development using high-throughput RNA sequencing (RNA-seq) data from a public database produced in an earlier research [47]. The GLK expression patterns were analyzed in 14 tissues or development stages of P. trichocarpa, including: FM, F, M, ML, PC, G43h, YFB, ApB, AxB, REF, RTC, YMB, Xylem1, and Phloem3. Detailed information about the RNA-seq data for the 55 PtGLK genes are listed in Table S3. Hierarchical clustering of the heatmap showed that PtGLK genes had divergence expressed in a variety of poplar tissues and development stages (Fig. 9). According to the expression profiles in 14 tissues, the poplar GLK family genes were divided into seven clusters (C1-C7). The four genes (PtGLK21, PtGLK43, PtGLK45, PtGLK54) clustered in C2 showed high expression levels in Xylem1 and Phloem3 tissues. A total of 20 genes grouped in C4/C5 were highly expressed in FM, F, and M. Additionally, many genes in C3 (except PtGLK21, PtGLK36, and PtGLK41) displayed high expression levels in Phloem3. In contrast, the majority of the 20 genes (C4, C5) presented lower expression levels in Phloem3. Taken together, the results showed that PtGLKs presented diverse expression profiles in different tissues and senescence stages, providing preliminary insight into further functional exploration.
Analysis of cis-regulatory elements in the promoter regions of PtGLK genes
Analysis of the promoters of PtGLKs in P. trichocarpa revealed that various potential CREs corresponding to defense and stress, light responsiveness, cold responsiveness, osmotic responsiveness, MeJA responsiveness, GA responsiveness, IAA responsiveness, and SA responsiveness were identified[47]. Detailed elements are listed in Fig. 10 and Table S5. The numbers of CREs were also significantly different in the promoters of different poplar GLK gene family members. The promoters of PtGLK30 contained the highest variety of CREs (MBS, G-box, Box4, ARE, ABRE, TCT-motif, TCCC-motif, TCA-element, P-box, GT1-motif, LTR, AE-box, TGACG-motif, MRE, and CGTCA-motif), while PtGLK43 contained only seven kinds of CREs. Moreover, all PtGLKs contained one or more abiotic stress elements, this result revealed that the expression of most PtGLK genes was associated with abiotic stress. Additionally, a total of 36 PtGLKs (65.5%) had two or more phytohormone induction elements, and PtGLK24, PtGLK46 and PtGLK57 included all five phytohormone induction elements (IAA-, ABA, GA-, MeJA- and SA-) (Fig. 10). The analysis of CREs displayed that the type, quantity, and distribution of CREs in different PtGLK genes were dissimilar, suggesting that each PtGLK gene was controlled by differing groups of TFs and that the expression of PtGLKs could respond to different abiotic stresses and phytohormone treatments.
PtGLK gene expression profiles in response to abiotic stress and phytohormone treatments
Several GLK genes have been studied to be related to the regulation of abiotic stresses and phytohormone response in maize [25], tobacco [26], tomato [27], and moso bamboo [28]. To explore whether PtGLK genes also had the same function, the dynamic expression of 11 PtGLK genes (PtGLK1, PtGLK3, PtGLK6, PtGLK16, PtGLK17, PtGLK21, PtGLK32, PtGLK36, PtGLK38, PtGLK48, and PtGLK53) as representatives of each subfamily were randomly selected (Table S6). As shown in Fig. 11, there were five, six, five, and three genes, and the change of their expression levels were greater than or equal to fivefold in comparison with 0 h, showing themselves as significantly changed genes in response to cold stress, osmotic stress, MeJA, and GA treatments, respectively. Among them, PtGLK3, PtGLK21, PtGLK32, and PtGLK53 were up-regulated both by cold and osmotic stresses, and PtGLK1, PtGLK21, and PtGLK53 were up-regulated under both MeJA and GA treatments. In addition, PtGLK38 (> 60-fold that of 0 h), PtGLK53 (> 70-fold that of 0 h), PtGLK3 (> 60-fold that of 0 h), and PtGLK53 (> 30-fold that of 0 h) were the most highly expressed after 12 h of cold stress, osmotic stress, MeJA, and GA treatments, respectively. We also found that only the expression of PtGLK53 was strong in response to all the four different treatments.
Discussion
PtGLKs in P. trichocarpa
The GLK genes have only been discovered in photosynthetic eukaryotes, including green algae and higher plants, and they participate in the development of chloroplasts [16, 48]. In earlier research, particular characteristics and functions of GLK genes were identified in Arabidopsis [17], maize [25], tobacco [27], tomato [26] and moso bamboo [28]. Nevertheless, the poplar GLK transcription factor has not yet been described up until now. In the current study, 55 putative PtGLK genes were identified in the poplar genome. The numbers of poplar GLK subfamily members were 13, 1, and 10 more than Arabidopsis (42), tomato (54), and sorghum (45), respectively. The greater number of PtGLK genes contain far more genes than those in these three species, which showed that the poplar genome size is substantially larger and is consistent with the genome duplication event [49, 50].
According to the phylogenetic analysis, the predicted poplar GLK subfamily members were classified into 11 groups (G1-G11), and all 11 groups contained different number of genes from Z. mays and Arabidopsis, suggesting that the PtGLK genes had diversified before these four species evolved. What is more, the absence of orthologous genes in maize G12 and G13, suggesting a divergence among Z. mays and P. trichocarpa. Moreover, PtGLKs belonging to the same subfamilies exhibited highly similar characteristics on the basis of their domain and gene structures, which indicated that the PtGLKs groupings were relatively reliable.
Expansion of the PtGLKs suggests functional diversification
Analysis of the chromosome location showed that PtGLKs were extensive and in-homogeneously distributed in 19 poplar chromosomes, which could be owing to insertion, deletion, duplication, and reversion [51, 52]. Among the 55 PtGLKs, 22 segmental duplication events occurred, but not tandem duplication events. Segmental duplication events were the main pathway for expansion of the poplar GLK gene family. Otherwise, it has been proven that segmental duplication is more common than tandem duplication and plays a crucial role in the long-term evolution in much of the research [53,54,55,56]. The synthesis analysis of P. trichocarpa and Z. mays genome sequences made clear that there was a notable collinearity between P. trichocarpa and monocots maize, which coincided well with the evolutionary relationship between dicotyledons and monocotyledons.
To better explore the profiles of macroevolution and evaluate the evolutionary times in P. trichocarpa, the Ks and Ka for paralogous (Pt–Pt) and orthologous (Pt-At) gene pairs were evaluated. The Ks values indicated that a large-scale duplication event occurred ~ 17 MYA in P. trichocarpa and that the divergence times for Pt-At was approximately 118 MYA. Aggerbeck et al. showed that a whole-genome duplication event in P. trichocarpa occurred 12–18 MYA and the divergence time between P. trichocarpa and Arabidopsis was 102–113 MYA [57, 58]. The results of these comparisons suggest that the poplar GLK gene family went through an earlier large-scale duplication event and diversified before the separation of Arabidopsis. In addition, the Ka/Ks ratio can be used to define the effect of selective pressure selection on coding sequences [54]. Here, the Ka/Ks ratios for the Pt–Pt and Pt-At gene pairs were both < 1, suggesting that the PtGLK genes probably have went through strong purifying selection during evolution [32, 59].
PtGLKs play an important role in poplar development
To predict possible functions of PtGLK genes in the growth and development of P. trichocarpa, we examined the expression patterns of 55 PtGLK genes in view of a previous reported transcriptome data. Most PtGLK genes showed high expression levels in xylem, which implied that they may have a function in the development of xylem. Generally speaking, compared with genes located in different subfamilies, genes in the same subfamily often have the same domains and similar functions. Previous studies show that two Arabidopsis genes (AT5G44190.1 and AT2G20570.2) were identified as functioning in leaf senescence [60]. In the current results, a total of 10 PtGLK genes (PtGLK4, PtGLK14, PtGLK15, PtGLK17, PtGLK19, PtGLK22, PtGLK26, PtGLK31, PtGLK45, and PtGLK52) were classified with the Arabidopsis GLK genes (AT5G44190.1 and AT2G20570.2) in G1 (Fig. 2), which suggested that these genes in different species were alike functionally and structurally. Therefore, it is speculated that these 10 putative PtGLK genes were involved in poplar Phloem3 senescence. The RNA-seq data showed that the transcript abundance of 17 PtGLK genes in group three decreased, which was closely related to the increase of leaf senescence level, indicating that these 17 PtGLK genes may play an essential role in the process of poplar leaf senescence. In addition, previous reports showed that the expression of ZmGLK47 was high in all maize tissues and played a significant role in the formation and evolution of chloroplasts [25]. As the ortholog pair of ZmGLK47 in P. trichocarpa, the PtGLK4, PtGLK14, PtGLK15, PtGLK17, PtGLK19, PtGLK22, PtGLK26, PtGLK29, PtGLK31, PtGLK45, and PtGLK52 shared the same protein structure and conserved domains and also exhibited the same expression patterns.
Potential functions of PtGLKs in abiotic stress and phytohormone signaling responses
Plant genomes have a diversity of stress-related genes, allowing plants to respond to diverse living environments [61]. The GLK family has been reported to play a significant role in abiotic stress and phytohormone treatment response, such as cold stress, osmotic stress, salinity stress, ABA, MeJA, GA, and SA [25, 26]. Additionally, the cis-elements of the promoter, to a large extent, decide the stress-responsive gene expression profiles which contribute to plants adaption to disadvantages, and are associated with a variety of stimuli-responsive genes [62, 63]. Therefore, we investigated the expression of 11 selected PtGLK genes under the two stress treatments and two phytohormone treatments. Preliminary research showed that orthologous genes of different species were conservative in gene functions, while paralogous genes presented different functions, because of gene duplication [64]. For instance, we found that the expression of ZmGLK1 and PtGLK32 (the ortholog of ZmGLK1 in P. trichocarpa) displayed similar patterns in response to cold and osmotic stress [25]. However, the expression of PtGLK17 and its ortholog in maize, ZmGLK50, exhibited opposite patterns, which suggested that PtGLKs could have lost or obtained new functions during evolution (Fig. 10). These results revealed that paralogous pairs probably contribute similarly in the course of plant growth and development. In the present study, PtGLK1, PtGLK21, and PtGLK53 were significantly induced in response to MeJA and GA treatments, implying that they may play important roles in the jasmonic acid and gibberellic acid signaling pathways. The expression of PtGLK1 was induced under MeJA and GA treatments and changed only slightly under cold and osmotic treatments. In addition, there were three PtGLK genes (PtGLK6, PtGLK16, and PtGLK48) showed slight (< fivefold that at 0 h) changes in response to cold stress, osmotic stress, MeJA, and GA treatments.
Conclusions
In this study, 55 members of the poplar GLK family were identified, which could be classified into 11 subfamilies on the basis of gene structures and conserved domains. Furthermore, the systematic analysis of chromosomal locations, synteny analysis, and evolutionary pattern offered valuable insight into the biological functions of the poplar GLK family members. The expression profiles of poplar GLK family genes indicated that PtGLKs were involved in various tissues and stages of poplar growth and development. The expression levels of PtGLKs under different abiotic and phytohormone treatments provides a basis for understanding the role of PtGLKs in the stress and phytohormone response. On the whole, these results will provide valuable resources to further explore the potential functional characteristics of PtGLKs in P. trichocarpa.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The protein sequencing data of Populus trichocarpa for this study have been downloaded from the Phytozome12.1 database (https://phytozome.jgi.doe.gov). The putative PtGLK genes were obtained by a extensive search and alignment of previously reported Arabidopsis AtGLK1 (AT2G20570) and AtGLK2 (AT5G44190) protein sequences in the poplar genome bank. The general feature format (GFF) sequence file of Populus trichocarpa used in this study is available at Populus trichocarpa genome database (https://genome.jgi.doe.gov/portal/Poptr1/Poptr1.home.html). The raw RNA-Seq data in different tissues (FM, F, M, ML, REF, RTC, G43h, ApB, AxB, YFB, YMB, Xylem1, Phloem3, and PC) of Populus trichocarpa are available in the NCBI database under the Bioproject accession number GSE21481 and GSE21485 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA477910).
Abbreviations
- CDD:
-
Conserved domain database
- DBD:
-
Myb-DNA-binding domain
- DRE:
-
Osmotic-responsive element
- GA:
-
Gibberellic acid
- GLK:
-
Golden2-like
- LTRE:
-
Cold-responsive element
- MeJA:
-
Methyl jasmonate
- MW:
-
Molecular weight
- MYA:
-
Million years ago
- PEG:
-
Polyethylene glycol 6000
- pI:
-
Isoelectric point
- Ptr:
-
Populus trichocarpa
- SA:
-
Silicylic acid
- FM:
-
Female catkin prior to seed release
- F:
-
Female catkins post-fertilization
- M:
-
Male catkins
- ML:
-
Mature leaf
- REF:
-
Washed fibrous roots
- RTC:
-
Roots from plants in tissue culture
- AxB:
-
Axillary bud
- YFB :
-
Newly initiated female floral buds
- YMB:
-
Newly initiated male floral buds
- Xylem1:
-
Developing xylem
- PC:
-
Phloem, Cortex, Epidermis
- Phloem3:
-
Developing phloem/cambium
References
Eberhard S, Finazzi G, Wollman FA. The dynamics of photosynthesis. Annu Rev Genet. 2008;42:463–515.
Jarvis P, López-Juez E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat Rev Mol Cell Biol. 2013;14:787–802.
Becker SFS, Mayor R, Kashef J. Cadherin-11 mediates contact inhibition of locomotion during xenopus neural crest cell migration. PLoS ONE. 2013;8:e85717.
Parente DJ, Swint-Kruse L. Multiple co-evolutionary networks are supported by the common tertiary scaffold of the LacI/GalR proteins. PLoS ONE. 2013;8:e84398.
Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell. 2009;21:1109–28.
Moreira D, Le Guyader H, Philippe H. The origin of red algae and the evolution of chloroplasts. Nature. 2000;405:69–72.
Lopez-Juez E, Pyke KA. Plastids unleashed: their development and their integration in plant development. Int J Dev Biol. 2005;49:557–77.
Park J, Werley CA, Venkatachalam V, Kralj JM, Dib-Hajj SD, Waxman SG, Cohen AE. Screening fluorescent voltage indicators with spontaneously spiking HEK cells. PLoS ONE. 2013;8:e85221.
Neuhaus HE, Emes MJ. Nonphotosynthetic metabsolism in plastid. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–40.
Han XY, Li PX, Zou LJ, Tan WR, Zheng T, Zhang DW. GOLDEN2-LIKE transcription factors coordinate the tolerance to Cucumber mosaic virus in Arabidopsis. Biochem Biophys Res Commun. 2016;477:626–32.
Hall LN, Rossini L, Langdale C. GOLDEN 2: a novel transcriptional regulator of cellular differentiation in the maize leaf. Plant Cell. 1998;10:925–36.
Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–10.
Imamura A, Hanaki N, Nakamura A, Suzuki T, Taniguchi M, Kiba T, Ueguchi C, Sugiyama T, Mizuno T. Compilation and characterization of Arabiopsis Thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol. 1999;40:733–42.
Dennis DW, Arthur RG, Donald PW, Hideaki U, Kosuke S. Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc Natl Acad Sci U S A. 1999;96:15336–41.
Hosoda K, Imamura A, Katoh E, Hatta T, Tachiki M, Yamada H, Mizuno T, Yamazaki T. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell. 2002;14:2015–29.
Rossini L, Cribb L, Martin DJ, Langdale JA. The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell. 2001;13:1231–44.
Waters MT, Moylan EC, Langdale JA. GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 2008;56:432–44.
Xiao Y, You S, Kong W, Tang Q, Bai W, Cai Y, Zheng H, Wang C, Jiang L, Wang C, et al. A GARP transcription factor anther dehiscence defected 1 (OsADD1) regulates rice anther dehiscence. Plant Mol Biol. 2019;101:403–14.
Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002;31:713–27.
Yasumura Y, Moylan EC, Langdale JA. A conserved transcription factor mediates nuclear control of organelle biogenesis in anciently diverged land plants. Plant Cell. 2005;17:1894–907.
Savitch LV, Subramaniam R, Allard GC, Singh J. The GLK1 ‘regulon’ encodes disease defense related proteins and confers resistance to Fusarium graminearum in Arabidopsis. Biochem Biophy Res Co. 2007;359:234–8.
Schreiber KJ, Nasmith CG, Allard G, Singh J, Subramaniam R, Desveaux D. Found in translation: high-throughput chemical screening in Arabidopsis thaliana identifes small molecules that reduce fusarium head blight disease in wheat. Mol Plant-microbe interact. 2011;24:640–8.
Murmu J, Wilton M, Allard G, Pandeya R, Desveaux D, Singh J, Subramaniam R. Arabidopsis GOLDEN2-LIKE (GLK) transcription factors activate jasmonic acid (JA)-dependent disease susceptibility to the biotrophic pathogen hyaloperonospora Arabidopsidis, as well as JA-independent plant immunity against the necrotrophic pathogen botrytis cinerea. Mol Plant Pathol. 2013;15:174–84.
Powell ALT, Nguyen CV, Hill T, Cheng KL, Figueroa-Balderas R, Aktas H, Ashrafi H, Pons C, Fernández-Muñoz R, Vicente A, et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science. 2012;336:1711–5.
Liu F, Xu Y, Han G, Zhou L, Ali A, Zhu S, Li X. Molecular evolution and genetic variation of G2-like transcription factor genes in maize. PLoS One. 2016;11:e0161763.
Wang Z, Liu J, Zhao H, Sun X, Wu T, Pei T, Wang Y, Liu Q, Yang H, Zhang H, et al. Genome-wide identification of tomato Golden 2-like transcription factors and abiotic stress related members screening. BMC Plant Biol. 2022;82:22.
Qin M, Zhang B, Gu G, Yuan J, Yang X, Yang J, Xie X. Genome-wide analysis of the G2-like transcription factor genes and their expression in different senescence stages of tobacco (Nicotiana Tabacum L.). Front Genet. 2021;12:787.
Wu R, Guo L, Wang R, Zhang Q, Yao H. Genome-Wide Identification and Characterization of G2-Like Transcription Factor Genes in Moso Bamboo (Phyllostachys edulis). Molecules. 2022;27:5491.
Rodgers-Melnick E, Mane SP, Dharmawardhana P, et al. Contrasting patterns of evolution following whole genome versus tandem duplication events in Populus. Genome Res. 2012;22:95–105.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202.
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;3784:3788–91.
Walther D, Brunnemann R, Selbig J. The regulatory code for transcriptional response diversity and its relation to genome structural properties in A. Thaliana PLoS Genet. 2007;3: e11.
Hu B, Jin J, Guo A, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;1296:1297–311.
Cannon S, Mitra A, Baumgarten A, Young N, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis Thaliana. BMC Plant Biol. 2004;10:4.
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–58.
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, Lee T, Jin H, Marler B, Guo H, et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49.
Gabaldón T, Koonin EV. Functional and evolutionary implications of gene orthology. Nat Rev Genet. 2013;14:360–6.
Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–2.
Rozas J. DNA sequence polymorphism analysis using DnaSP. Methods Mol Biol. 2009;537:337–50.
Peng Z, Lu Y, Li L, Zhao Q, Feng Q, Gao Z, Lu H, Hu T, Yao N, Liu K, et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys Heterocycla). Nat Genet. 2013;45:456–61.
Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300.
Lescot M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7.
Liu HL, Wu M, Li F, Gao YM, Chen F, Xiang Y. TCP transcription factors in moso bamboo (Phyllostachys Edulis): genome-wide identification and expression analysis. Front Plant Sci. 2018;9:1263.
Jiao YL, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–30.
Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2004;21:951–60.
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462.
Rodgers M, Mane SP. Contrasting patterns of evolution following whole genome versus tandem duplication events in Populus. Genome Res. 2012;22:95–105.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Lugli GA, Tarracchini C, Alessandri G, Milani C, Mancabelli L, Turroni F, Neuzil-Bunesova V, Ruiz L, Margolles A, Ventura M. Decoding the genomic variability among members of the bifidobacterium dentium species. Microorganisms. 2020;8:1720.
Opanowicz M, Vain P, Draper J, Parker D, Doonan JH. Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci. 2008;13:172–7.
Kirchhoff H. Chloroplast ultrastructure in plants. New Phytol. 2019;223:565–74.
Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–8.
Gu Z, Steinmetz LM, Gu X, Scharfe C, Davis RW, Li WH. Role of duplicate genes in genetic robustness against null mutations. Nature. 2003;421:63–6.
He Y, Mao S, Gao Y, Zhu L, Wu D, Cui Y, Li J, Qian W. Genome-wide identification and expression analysis of WRKY transcription factors under multiple stresses in Brassica Napus. PLoS One. 2016;11:e0157558.
Wu S, Wu M, Dong Q, Jiang H, Cai R, Xiang Y. Genome-wide identification, classification and expression analysis of the PHD-finger protein family in Populus Trichocarpa. Gene. 2016;575:75–89.
Song H, Wang P, Lin JY, Zhao C, Bi Y, Wang X. Genome-wide identification and characterization of WRKY gene family in peanut. Front Plant Sci. 2016;7:9.
Nasim J, Malviya N, Kumar R, Yadav D. Genome-wide bioinformatics analysis of Dof transcription factor gene family of chickpea and its comparative phylogenetic assessment with Arabidopsis and rice. Plant Syst Evol. 2016;302:1009–26.
Aggerbeck M, Fjelds J, Christidis L. Resolving deep lineage divergences in core corvoid passerine birds supports a proto-Papuan island origin. Mol Phylogenet Evol. 2014;70:272–85.
Julien M, Thomas L, Couvreur P, Hervé S. Five major shifts of diversification through the long evolutionary history of Magnoliidae (angiosperms). BMC Evol Biol. 2015;15:49.
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KFX, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–34.
Rauf M, Arif M, Dortay H, Matallana-Ramírez LP, Waters MT, GilNam H, Lim PO, Mueller-Roeber B, Balazadeh S. ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. Embo Rep. 2013;14:382–8.
Ahuja I, de Vos RCH, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci. 2010;15:664–74.
Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. BBA-Mol Cell Res. 2004;1695:189–207.
Nakamura H, Muramatsu M, Hakata M, Ueno O, Nagamura Y, Hirochika H, Takano M, Ichikawa H. Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol. 2009;50:1933–49.
Acknowledgements
Not applicable
Sample availability
Samples of the compounds MeJA, GA, PEG are available from the authors.
Funding
This research was supported by Beijing Forestry University Excellent experimenter cultivation project (BJFUSY20210908), and National Undergraduate Training Programs for Innovation and Entrepreneurship 202210022022. These funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.W.; methodology, R.W.; software, L.G.; validation, Y.G.; formal analysis, B.Z. and L.M.; investigation, R.W.; resources, L.G.; data curation, K.X.; writing—original draft preparation, R.W.; writing—review and editing, R.W.; visualization, L.D.; supervision, L.D.; project administration, R.W.; funding acquisition, L.D.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declared that a permission to collect Populus trichocarpa material has been obtained, and experimental research works on the plants described in this paper comply with institutional, national and international guidelines.
Consent for publication.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, R., Guo, L., Guo, Y. et al. The G2-Like gene family in Populus trichocarpa: identification, evolution and expression profiles. BMC Genom Data 24, 37 (2023). https://doi.org/10.1186/s12863-023-01138-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-023-01138-1