Abstract
Background
Naturally occurring mutations in growth and differentiation factor 9 (GDF9) or bone morphogenetic protein 15 (BMP15) genes are associated with increased ovulation rate (OR) and litter size (LS) but also sterility. Observing the Tunisian Barbarine ewes of the “W” flock selected for improved prolificacy, we found prolific and infertile ewes with streaky ovaries. Blood genomic DNA was extracted from a subset of low-ovulating, prolific and infertile ewes of the “W” flock, and the entire coding sequences of GDF9 and BMP15 were sequenced.
Results
We evidenced a novel polymorphism in the exon 1 of the BMP15 gene associated with increased prolificacy and sterility. This novel mutation called FecX Bar is a composite polymorphism associating a single nucleotide substitution (c.301G > T), a 3 bp deletion (c.302_304delCTA) and a C insertion (c.310insC) in the ovine BMP15 cDNA leading to a frame shift at protein position 101. Calculated in the “W” flock, the FecX Bar allele increased OR by 0.7 ova and LS by 0.3 lambs (p = 0.08). As for already identified mutations, homozygous females carrying FecX Bar exhibited streaky ovaries with a blockade at the primary stage of folliculogenesis as shown by histochemistry.
Conclusions
Our investigation demonstrates a new mutation in the BMP15 gene providing a valuable genetic tool to control fecundity in Tunisian Barbarine, usable for diffusion program into conventional flocks looking for prolificacy improvement.
Similar content being viewed by others
Background
In livestock species, especially in meat sheep, improvement of reproductive traits such as ovulation rate (OR) and embryo survival which are the main components of litter size (LS) or prolificacy is of high economic importance. Sheep husbandry is a main pillar of the red meat value chain in Tunisia and several breeding programs are being implemented to sustain genetic improvement of sheep meat production retaining traits as diverse as meat quality, growth rate, environmental adaptation and prolificacy [1]. Considering the latter trait, most of the sheep breeds and strains in Tunisia are low prolific. Thus, a prolificacy-based selection program was implemented since 1979 by the Tunisian National Institute for Agronomic Research (INRAT) in the experimental center of Oueslatia Kairouan by screening prolific ewes among the fat tailed Barbarine sheep which represents over 60% of the national sheep population. The selection process yielded the prolific “W” strain with LS of 1.6 and a fertility rate ranging between 82 and 91% [2]. LS in conventional flocks of the same breed is about 1.1 [3]. During the 1999–2014 period, repeatability and heritability estimates of prolificacy in the “W” flock were calculated to be 0.62 and 0.13, respectively [3]. Compared to an average OR of 1.1 in the normal Barbarine ewe, the average OR of the “W” females was 1.9 with an upper individual mean OR reaching 3.1 [4]. However, a number of infertile females were recorded in the “W” flock affecting global fertility and examination using laparoscopy of the ovaries of these females revealed their streak aspect similar to what has been reported by [5] and most likely indicative of permanent sterility.
Interestingly, numerous studies in sheep identified point mutations in genes of the bone morphogenetic protein (BMP) family of growth factors associated to increased prolificacy but also to sterility [6] resembling the phenotypes observed in the Barbarine “W” flock. Indeed, up to now, 6 different mutations were evidenced in the bone morphogenetic protein 15 (BMP15) gene and 3 mutations in the growth and differentiation factor 9 (GDF9) gene responsible for this prolific and sterile double phenotype. The ovine BMP15 gene is carried by the X chromosome and is also known as FecX (Fecundity X gene). The 6 FecX/BMP15 mutated alleles were identified in various ovine breeds: FecX I/ BMP15 c893T>A and FecX H/ BMP15 c871C>T in New Zealand Romney [7], FecX G/ BMP15 c718C>T and FecX B/ BMP15 c1100G>T in Irish Cambridge and Belclare, FecX L/ BMP15 c962G>A in French Lacaune [8] and FecX R/ BMP15 c455_471del in Spanish Rasa Aragonesa [9, 10]. Concerning the GDF9 or FecG gene on the ovine chromosome 5, one mutated allele was identified in Cambridge and Belclare sheep and is known as FecG H /GDF9 c1184C>T [11]. A second mutation was evidenced in Icelandic sheep as FecG T /GDF9 c1279A>C [12] and more recently, a third mutation was discovered in Ile-de-France sheep reared in Brazil and named FecG V /GDF9 c943C>T [5]. For both BMP15 and GDF9, all these variants affect the open reading frame. They are considered as loss-of-function mutations increasing OR and thus prolificacy at the heterozygous state, but sterility by blockage or deep alteration of the ovarian follicular development at the homozygous state [6, 13].
Other prolific mutations in BMP15 and GDF9 genes segregate in ovine populations but were never associated to sterility; homozygous being more prolific than heterozygous carriers. This is evidenced forFecX Gr /BMP15 c950C>T and FecX O /BMP15 c1009C>T in the French Grivette and Polish Olkuska breeds, respectively [14]; also FecG E /GDF9 c1034T>G in Brasilian Santa Ines and FecG NW /GDF9 c1111G>A in Norwegian White sheep [15, 16]. Such dose-dependent prolific mutations were initially identified in the BMP receptor type 1B gene (BMPR1B, also known as FecB) in the Booroola sheep [17,18,19] and more recently in the beta-1,4-N-acetyl-galactosaminyl transferase 2 (B4GALNT2/FecL) gene in Lacaune sheep [20]. With the identification of several causal mutations affecting prolificacy in sheep, numerous ovine prolific breeds were genotyped for these known polymorphisms. Davis et al. originated the FecB/BMPRIB Booroola mutation (FecB B) in Garole sheep of India [21]. Thereafter, FecB B was found naturally segregating in many Chinese breeds such as Hu, Han, Huyang, Cele, Duolang and Bayanbulak [22,23,24], in Indian Bonpala [25] and in Iranian Kalehkoohi sheep [26]. Some of these known variants in BMP15 and GDF9 were also found in various populations. GDF9 c1111G>A (also known as G7 polymorphism) primarily detected in Belclare is associated to increased prolificacy in Norwegian White sheep [11, 16, 27]. The origin of FecG H /GDF9 c1184C>T and FecX B/ BMP15 c1100G>T was established in the Lleyn breed coming from the Lleyn peninsula of Wales [28]. Moreover, Belclare and Cambridge mutation FecX G/ BMP15 c718C>T is surprisingly present in Chinese small tail Han ewes [29]. The search for these known mutations in BMPR1B, BMP15 and GDF9 by specific genotyping failed in five sheep breeds reared in Tunisia; these are Barbarine, Queue Fine de L’Ouest, Noire de Thibar, Sicilo-Sarde and D’man breeds [30]. However, a second study identified the segregation of the FecX B/ BMP15 c1100G>T allele in prolific Barbarine ewes reared in conventional flocks, with an allele frequency of 21% [31]. Using the same PCR-RFLP technique for the polymorphism FecG H /GDF9 c1184C>T in the GDF9 gene, some reports highlighted the existence of the mutation in Barbarine animals, 10 years ago, both in conventional [32] and in the “W” flocks [33]. The prolific and sterile double phenotype documented in ewes of the Barbarine “W” flock fits very well with the segregation of such mutations in the BMP15 and GDF9 genes, however, by sequencing recently the entire BMP15 and GDF9 coding sequences of selected high-ovulating, low-ovulating and infertile “W” ewes, FecX B and FecG H alleles were absent. The objective was to characterize of what could be a new mutation in the BMP15 gene affecting the ovarian function of Barbarine sheep.
Methods
Animal material
The “W” strain line was originally created using prolific Barbarine ewes purchased from the Bureau of Livestock and Pastures (Office de l’Elevage et des Pâturages (OEP)) flocks present in the Zaghouan governorate, in North of Tunisia under semi-arid conditions. The nucleus of the prolific line was then reared at INRAT Ouesslatia research station. In 1979, the size of the flock reached 65 ewes and 7 rams. Reproduction was managed through 7 families. For many years, one ram and its replacement were assigned for each family. For mating, females born in a given family were systematically moved to another family to limit inbreeding and replacement animals were selected based on their litter size records (LS) [3]. Regarding to particular circumstances that have arisen in the last decade, the genetic monitoring of the flock was disturbed followed by a severe reduction in the flock size.
OR of females of the “W” flock was observed by laparoscopy during one or several breeding seasons. For the gene sequencing study and initial polymorphism discovery, 25 ewes were selected based on OR records (records between 2010 and 2014) and then classified as low-ovulating (n = 7), high-ovulating (n = 11) and infertile ewes exhibiting streak ovaries (n = 7). The low-high ovulating cut-off classification was set at individual mean OR = 1.66 to distinguish between individuals with typically one ovulation per estrus cycle (occasionally two) and individuals with typically two or more ovulations per estrus cycle but allowing an occasional lower value. Servicing rams of the “W” flock (n = 17) were analyzed by genotyping and sequencing. Thereafter, all females and males of the “W” flock present in 2015 were specifically genotyped for the identified mutation (n = 101).
A second genotyping analysis was conducted on 137 Barbarine animals, non-selected for prolificacy, originating from conventional flocks belonging to several state and research farms. All procedures were approved by the Agricultural and Scientific Research Government Committees (CPERA-Tunisia) in accordance with the guidelines for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.
Blood samples and DNA extraction
Approximately 5 ml blood was collected aseptically by venipuncture from the jugular vein using vacutainer collection tubes containing EDTA. All samples were then immediately refrigerated and transported to the laboratory under low temperature. The genomic DNA was extracted from white blood cells following a salt-based DNA extraction [34]. Genomic DNA samples were stored at −20 °C for further analysis.
DNA sequencing analysis
Entire ovine BMP15 and GDF9 genes (from exon 1 to exon 2) were amplified for the 25 selected ewes by long-range PCR (Fermentas, Life Technologies) using primers designed by extracting reference genomic sequences (Oar_v3.1) from ovine chromosome X (NC_019484) and chromosome 5 (NC_019462), respectively. As a first step, forward 5′-TTCCTTGCCCTATCCTTTGTG-3′ and reverse 5′- TCTTCACCCCAAACCGTCTA-3′ primers were used to amplify the ovine BMP15 gene, and forward 5′-TCGGACGGACTAAGAGTAGAAGA-3′ and reverse 5′-GGTTTGCCAGGTAAGAACAC-3′ primers were used to amplify the ovine GDF9 gene. Long-range amplification primers and internal primers (Table 1) were used for Sanger sequencing reaction realized via the Big Dye Terminator v3.1 Cycle Sequencing Kit and analyzed on an ABI3730 sequencing machine (Applied Biosystems).
Polymorphism genotyping
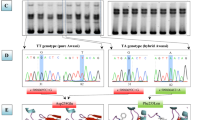
FecX B and FecG H allele genotyping was performed through Restriction Fragment Length Polymorphism (RFLP) approach as previously described [11]. Restriction enzyme digestion by DdeI (New England Biolabs) of each specific PCR product was performed overnight at 37 °C to avoid for partial restriction. A genotyping test by allele specific PCR amplification was established for the detection of the FecX Bar mutation in the W flock and other non-selected Barbarine flocks (conventional). For each individual, a fragment of 445 (or 440) bp from BMP15 exon 1 is amplified by two independent PCR using the same forward primer 5′-TTCCTTGCCCTATCCTTTGTG-3′ and one of the two allele specific reverse primer, 5′-GAGGCCTTGCTACACTAGCC-3′ for the FecX + wild-type allele and 5′-TGAGAGGCCTTGGCTACACA-3′ for the FecX Bar mutated allele. After 35 cycles of amplification (94 °C/30s, 62 °C/30s, 72 °C/30s), the PCR products were analyzed on 1% agarose gel. Heterozygous ewes were positive for the two PCR while homozygous ewes were positive only for one PCR depending on the carried allele (Fig. 1). The BMP15 gene is located on the X chromosome, thus rams were expected to be positive only for one of the two PCR’s.
Allele-specific PCR amplification at the FecX Bar locus.FecX BarandFecX +allele- specific PCR amplification of genomic DNA from Barbarinenon-carrier ewes (+/+), heterozygous (+/Bar) and homozygous carrier (Bar/Bar) of the FecX Barallele. Amplified bands were resolved on a 1% agarose gel (MW: molecular weight marker). H20 was used instead of genomic DNA as a contamination PCR control
Histological analysis
Four adult ewes from the “W” flock genotyped as non-carriers (n = 2) or FecX Bar homozygous carriers (n = 2) were unilaterally ovariectomized under local anesthesia with parenteral subcutaneous infiltration of 2% lidocaine. After recovery, ovaries from the two cyclic+/+ ewes (presence of corpora lutea) and the two sterile Bar/Bar ewes bearing streak ovaries were fixed in 4% formaldehyde solution for 48 h and embedded in paraffin. The paraffin-embedded tissues were sectioned serially at 3 μm and these sections were stained with hematoxylin and eosin solution for light microscopy studies as described [35]. Different types of follicles, primordial follicles, primary, secondary or pre-antral, antral or tertiary and ovulatory follicles were identified as described [36].
Statistical analysis
For the genotype-OR association among the 25 selected ewes, the genotype effect at GDF9 or BMP15 locus was tested by one-way ANOVA followed by a Tukey multiple comparison test. For the population study, LS at birth was defined as the total number of lambs born dead or alive per ewe lambing. Statistical analysis using least-squares techniques was performed to assess non genetic effects to be included in the final model [37]. The model applied included fixed effects of combined season-year of lambing [S*Y j ], lambing interval nested within parity [Hiera kl ] that corresponds to the age of the ewe when the ewe lambs for the first time, and to the interval between two consecutive parturitions for subsequent lambing.
Only significant fixed effects at the probability level of 5% were then considered to adjust the litter size performance (Yjklm) and it was for Hiera effect. The litter size performance was adjusted to the Hiera factor using the following equation [38]:
Where \( {y_{ikl}}^{*} \) is the litter size performance (LS) of the ith ewe in the klth Hiera factor, δ kl is the standard deviation of the error by lambing interval nested within parity, σb is the global residual deviation, and \( {\overset{-}{\mathrm{Y}}}_{\mathrm{kl}} \) corresponds to the litter size average. The GLM procedure and Duncan’s multiple range test were used for LS and OR comparison within genotype groups.
Results
BMP15 and GDF9 sequence analysis
Following laparoscopic observation of Tunisian Barbarine ewes of the “W” flock selected for increased LS, ewes were classified in 3 phenotypic groups regarding OR: low-ovulating (n = 7), high-ovulating (n = 11) and “streaky-bearing ovaries” females (n = 7). Animals in the latter class were kept in the flock for experimental purposes (Table 2). Surprisingly, among these 25 selected ewes, the FecX B allele in BMP15 and the FecG H allele in GDF9 were evidenced in any of the sequences. In line with what has been reported for Cambridge and Belclare sheep [11], we found the already described G3, G5 and G6 polymorphisms within the GDF9 gene with no significant genotype association with the prolific or sterile phenotypes. In striking contrast, apart from the c.28_30delCTT already known as a non-causal polymorphism [11; 14], we discovered an original haplotype of 3 polymorphisms lying on 10 bp within the exon 1 of BMP15 (Fig. 2a). This local haplotype associated the single nucleotide substitution c.301G > T, the 3 bp deletion c.302_304delCTA and the C nucleotide insertion c.310insC. The polymorphism nomenclature [301G > T; 302_304delCTA; 310insC] was given according to the ovine BMP15 cDNA [NM_001114767]. As shown in Table 2, the mutated haplotype appeared homozygous in all “streaky ovaries” ewes, heterozygous in 9 of the 11 high-ovulating ewes, but absent from low-ovulating ewes. When grouped by genotype, the mean OR of heterozygous ewes was significantly higher than for non-carrier or homozygous carrier ewes (2.42 ± 0.54, n = 9 vs. 1.34 ± 0.49, n = 9 vs. 0 ± 0, n = 7; P < 0.0001, mean ± SD) fitting very well with a causal mutation. This novel mutation was named FecX Bar (Barbarine prolific allele of the FecX/BMP15 gene) in accordance with the current names for existing fecundity genes. At the protein level, the FecX Bar mutation alters BMP15 by a non-conservative substitution (Ala > Cys) at position 101 followed by a frame shift coding for 112 alternative amino-acids terminated by a stop codon at position 113 of the alternative sequence (p.Ala101CysfsTer113, Fig. 2b). The additional alternative amino acids did not contain any putative conserved functional domains.
Sequencing of homozygous ewes carriers and non-carriers of the FecX Bar allele. a Sanger sequencing of the end of first exon of the ovine BMP15 gene. Nucleotide numbering and amino-acid translation are relative to the ATG start site. The FecX Bar variant haplotype (Bar/Bar) is indicated within green boxes and compared to wild-type haplotype (+/+). b Protein alignment between reference ovine BMP15 and Barbarine mutated BMP15A101CfsX113 translated from homozygous FecX Bar variant. The 112 alternative amino-acids generated by the frameshift (fs) are highlighted in green. Views were obtained from CLC MainWorkbench 7.6.4 (Qiagen Aarhus)
FecX Bar genotyping of Barbarine sheep populations
In order to search for the segregation of the FecX Bar allele in a larger population of Barbarine sheep, we developed an allele-specific PCR genotyping (Fig. 1). Firstly, the 25 sequenced ewes were analyzed by this PCR test indicating a 100% accuracy of the genotyping method. Thereafter, 17 servicing “W” rams were also genotyped by PCR and the genotype was 100% certified by Sanger sequencing. The FecX Bar mutation was searched by allele-specific PCR genotyping along with RFLP for FecX B and FecG H by in the actual “W” flock in 101 animals present in 2015 (74 ewes and 27 rams) and in 137 Barbarine animals (29 ewes and 108 rams) from several conventional flocks across Tunisia. FecX B and FecG H were absent from the 248 tested animals. For prolificacy trait, the low-high litter size cut-off classification was set at flock average LS = 1.12 to distinguish between individuals with simple litter size and individuals with typically more than one lamb per lambing. The frequency of the FecX Bar carrier females in the “W” flock reached 0.42 (39% of heterozygous and 3% of homozygous carrier) corresponding to 57% of heterozygous “W” ewes with a litter size exceeding 1.12 and a total of 86% of non-carrier homozygous ewes having a litter size less than 1.12 (Table 3). The value of GF by LS for heterozygous “W” showed that the Barbarine ewes, raised under low input production system, found difficulties to exteriorize its potential.
In the males of the “W” flock, the FecX Bar /Y genotype frequency reached 0.37 (Table 4). A total of 80% of non-carrier “W” males had a litter size more than 1.12, which could be an inheritance of the FecX Bar allele from the dam.
In contrast, the FecXBar allele was not found in the tested natural non-selected Barbarine females, except one male is carrier of this mutated allele. In conventional flocks, a total of 88% of females had a litter size less than 1.12.
In vivo effects of the FecX Bar mutation
Our primary sequencing analysis with selected “W” ewes indicated a positive relationship between FecX Bar mutation in BMP15 and ovulation rate in Barbarine breed. After genotyping of the entire “W” flock, it was possible to associate the genotype to LS and OR for a sample of ewes of the flock (Table 5). The standardized litter size performance (LS) of ewes with FecX + /FecX Bar genotype averaged 1.43 ± 0.51. This value tended to be higher than LS of non-carrier ewes (1.13 ± 0.32; P = 0.08). The same tendency was observed for OR trait (2.04 ± 0.75 vs. 1.37 ± 0.53, P = 0.08). By comparison, LS of wild-type ewes in conventional flocks was 1.08 ± 0.23 and thus not different from wild-type “W” females.
In parallel with the effect on OR and LS, it was interesting to investigate the streaky ovary phenotype repeatedly exhibited by adult females of the “W” flock coded as infertile and thereafter genotyped as homozygous carriers of the FecX Bar allele (Table 2). FecX Bar infertile ewes were characterized by an infantile genital tract and the ovaries did not carry any obvious follicular structures (Fig. 3). In contrast to non-carrier ovaries containing all follicle size classes and corpora lutea (Fig. 3a and c), histological studies of the FecX Bar/FecX Bar ovaries revealed only primordial and primary follicles (Fig. 3b). Like ewes homozygous for previously described BMP15 mutations, the cortical region of the FecX Bar/FecX Bar ovaries was densely colonized by primordial and primary follicles (Fig. 3d). Many of these primary follicles were abnormally constituted of large oocytes with thickened zonapellucida surrounded by disorganized granulosa cell layers (Fig. 3e). Oocyte-free follicular structures were also observed (Fig. 3e and f).
Histological sections of Barbarine sheep ovaries. Photomicrographs of histological section of the FecX Barnon-carrier (a and c) or homozygous carrier ovaries (b and d–f). a,c, Evidence of follicular growth with the presence of secondary and tertiary follicles in normal ovaries. b, FecX Bar/FecX Bar ovaries densely packed with primordial follicles in the cortex with no visible secondary or tertiary follicles. d, e, f, Ovarian cortex of FecX Bar/FecX Barovaries with primordial follicles and numerous abnormal follicular structures: primary-like follicles exhibiting enlarged oocytes with thickened zona pellucida surrounded by disorganized granulosa cell layers, and oocyte-free follicular structures
Discussion
Previous studies investigating inactivating mutations in BMP15 and/or GDF9 genes have shown that, while ewes heterozygous for the mutations have increased prolificacy, ewes homozygous for the mutation are sterile [6]. We had observed a similar phenotype, with both increased prolificacy and sterility in Tunisian Barbarian sheep of the “W” flock and thus investigated the presence of polymorphisms in BMP15 and GDF9 genes in this flock. Fitting well with the genetic determinism of this phenotype, previous preliminary studies using RFLP genotyping have evidenced the segregation of both BMP15/FecX B allele in high-prolific population of Barbarine ewe from conventional flocks [31] and GDF9/FecG H allele in both in conventional [32] and in the “W” flocks [33]. In the present study, none of the two alleles was found in the 248 Barbarine animals tested. Particularly for the “W” flock, this could be explained by a disturbed genetic management under the economic and political context in Tunisia during the last decade leading to elimination of important reproducers followed by a severe reduction of the flock size. This may have induced a bottleneck and the disappearance of some alleles.
However, a novel complex mutation in BMP15, FecX Barc.301–310 located at the end of the first exon, was observed to be present in the “W” flock, with ewes heterozygous for the mutation having increased ovulation rates and litter size whereas those homozygous for the mutation were sterile.
At the protein level, the FecX Bar mutation on DNA created a non-conservative substitution at position 101 followed by a frame shift coding for 112 alternative amino-acids. The new mutated protein sequence of 313 amino-acids is devoid of the BMP15 mature domain (starting at position 269 in the normal protein) and does not carry any new functional or putative protein domain. Consequently, BMP15 A101CfsX113 is supposed to be a non functional protein as already described for a similar mutation creating a frame shift (BMP15 W154NfsX55) in Rasa Aragonesa sheep [9, 10] or mutations creating premature stop codon (BMP15 Q291X and BMP15 Q239X) in Hanna and Cambridge sheep [7, 11]. Interestingly, all these latter mutations induced prolificacy at heterozygous state but sterility with streaky ovary phenotype when homozygous, resembling perfectly the phenotypes observed in Barbarine sheep of the “W” flock. Particularly, FecX Bar infertile ewes displayed an infantile genital tract and the ovaries did not carry any obvious follicular structures. As observed in Inverdale (FecX I), Hanna (FecX H) and Lacaune (FecX L) homozygous ewes, the cortical region of the FecX Bar/FecX Bar ovaries was densely colonized by primordial and primary follicles [7, 8, 39, 40]. All histological observations converge towards assuming a blockade of the primary to secondary follicle transition during folliculogenesis as an explanation of the infertility of the homozygous FecX Bar mutated Barbarine ewes.
By genotyping the entire “W” flock, we established that the FecX Bar allele segregates with a high frequency. Indeed, 42% of the female and 37% of the males were carrier of the FecX Bar allele perhaps indicating a particular selection pressure exerted on the FecX Bar allele to increase prolificacy of the “W” flock. In contrast, FecX Bar was not found in the tested non-selected Barbarine females from conventional flocks, except for one male. One would have expected more carriers since the “W” flock originates from this Barbarine population, but our DNA set from conventional flock may be too small and focused mainly on non prolific ewes. The presence of a ram carrying the FecX Bar mutation and belonging to a conventional flock could be the result of a progeny testing program for “W” rams conducted in 1999, where eight rams were transferred from INRAT to an OEP conventional flock [41]. A larger prospection into the Barbarine population, particularly on prolific females, would be necessary to find more FecX Bar carrier animals in order to conclude on the frequency of the mutation in conventional flocks.
Findings of this study point out that the FecX Bar allele causes increase of OR by +0.7 ova and LS by +0.3 lambs at each parturition. It is equivalent or slightly lower than for other already known mutations in BMP15 acting on LS:FecX R increasing LS by +0.35 [10], and FecX I , FecX H and FecX O increasing LS by +0.6 [7, 14]. However, it should be noted that a few of the polyovulatory ewes present in the “W” flock were not carriers of the FecX Bar mutation. The factors increasing ovulation rate in these ewes are unknown, but could be related to other factors such as season, nutrition or as yet unidentified genetic factors [42]. Seasonal fluctuations were clearly observed in the “W” flock, as poly-ovulating ewes mated in spring/summer (May to July) had an OR of 1, whereas OR averaged 1.7–1.8 in autumn and winter [42].
Conclusions
Our results evidenced a new mutation in ovine BMP15 gene affecting the prolificacy and fertility of Barbarine ewes in the “W” flock selected for increased prolificacy. This new mutation named FecX Bar is to be added to the already-known 8 mutations in this major gene affecting prolificacy in sheep [13]. It is the first FecX mutation detected in the first exon of BMP15; all other mutations being in the second one.
This frame-shift mutation is supposed to be associated with the absence of BMP15 production by the oocyte, explaining the early blockage of folliculogenesis and the resulting streaky ovaries observed in infertile homozygous carrier females.
The presence at a high frequency of the FecX Bar carrier animals (females and males) in the “W” flock at INRAT represents an opportunity to disseminate this mutation in the conventional Barbarine population. Nevertheless, due to the sterility induced by crossing carrier animals, such genetic improvement will need strong and precise management. Moreover, the FecX Bar effect should also be analysis on other important traits such as lamb growth and survival, seasonality and global fertility to be fully usable in a genetic improvement program.
Abbreviations
- B4GALNT2:
-
Beta-1,4-N-acetyl-galactosaminyl transferase 2
- BMP15 :
-
Bone morphogenetic protein 15
- Fec:
-
Fecundity gene
- GDF9 :
-
Growth and differentiation factor 9
- LS:
-
Litter size
- OEP:
-
Office de l’Elevage et des Pâturages
- OR:
-
Ovulation rate
- OTD:
-
Office des Terres Domaniales
References
Bedhiaf-Romdhani S, Djemali M, Zaklouta M, Iniguez L. Monitoring crossbreeding trends in native Tunisian sheep breeds. Small Rumin Res. 2008;74:274–8.
Abdennebi L. Analyse des performances zootechniques de dix années d’élevage d’un troupeau ovin prolifique de la race Barbarine (Analysis of Zootechnic Performances Over 10 Years of a Prolific Sheep Flock of the Barbarine Breed). Master of Science, The National Institute of Agronomy of Tunisia. 1990.
Bedhiaf-Romdhani S, Abidi S, Atti N, Ben Salem H, Ben Salem M, Lassoued N, Othmane MH. Ruminant characterization and management for increased productivity: Half a century of scientific research. Annales de l’INRAT. 2013;86:93–138. ISSN 0365-4761. www.inrat.agrinet.tn
Lassoued N, Rekik M, Gonzalez-Bulnes A, Ben Salem I, Tounsi A. Prolific strains of Barbarine sheep are characterized by increased ovulation rate due to extended period of ovulatory follicle recruitment and co-dominance effects. Small Rumin Res. 2013;114:134–9.
Souza CJ, McNeilly AS, Benavides MV, Melo EO, Moraes JCF. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim Genet 2014;45(5):732-9.
Fabre S, Pierre A, Mulsant P, Bodin L, Di Pasquale E, Persani L, Monget P, Monniaux D. Regulation of ovulation rate in mammals: contribution of sheep genetic models. Reprod Biol Endocrinol. 2006;4:20.
Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta S, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–83.
Bodin L, Di Pasquale E, Fabre S, Bontoux M, Monget P, Persani L, Mulsant P. A novel mutation in the bone morphogenetic protein 15 gene causing defective protein secretion is associated with both increased ovulation rate and sterility in Lacaune sheep. Endocrinology. 2007;148:393–400.
Martinez-Royo A, Jurado JJ, Smulders JP, Marti JI, AlaBart JL, et al. A deletion in the bomorpho-genetic protein 15 gene causes sterility and increased prolificacy in Rasa Aragonesa sheep. Anim Genet. 2008;39:294–7.
Monteagudo LV, Ponz R, Tejedor MT, Lavina A, Sierra I. A 17 bp deletion in the bone morphogenetic protein 15 (BMP15) gene is associated to increased prolificacy in the Rasa Araganosa sheep breed. Anim Reprod Sci. 2009;110:139–46.
Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod. 2004;70:900–9.
Nicol L, Bishop SC, Pong-Wong R, Bendixen C, Holm LE, Rhind S, Mc Neilly SA. Homozygosity for a single base-pair mutation in the oocyte specific GDF9 gene results in sterility in Thoka sheep. Reproduction. 2009;138:921–33.
Sadighi M, Bodensteiner KJ, Beattie AE, Galloway SM. Genetic mapping of ovine growth differentiation factor 9 (GDF9) to sheep chromosome 5. Anim Genet. 2002;33:244–5.
Demars J, Fabre S, Sarry J, Rossetti R, Gilbert H, Persani L, Tosser-Klopp G, Mulsant P, Nowak Z, Drobik W, Martyniuk E, Bodin L. Genome-Wide Association Studies Identify Two Novel BMP15 Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep. PLoS Genet. 2013;9(4):e1003482.
Silva BD, Castro EA, Souza CJ, Paiva SR, Sartori R, Franco MM, Azevedo HC, Silva TASN, Vieira AMC, Neves JP, Melo EO. A new polymorphism in the Growth and Differentiation Factor 9 (GDF9) gene is associated with increased ovulation rate and prolificacy in homozygous sheep. Anim Genet. 2011;42:89–92.
Våge DI, Husdal M, Matthew PK, Klemetsdal G, Boman IA. A missense mutation in growth differentiation factor 9 (GDF9) is strongly associated with litter size in sheep. BMC Genet. 2013;14:1.
Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, Pisselet C, Riquet J, Mon-niaux D, Callebaut I, Cribiu E, Thimonieri J, Teyssieri J, Bodin L, Cognie Y, Chitour N, Elsen JM. Mutation in bone morphogenetic protein receptor-IB is associated with in-creased ovulation rate in Booroola Merino ewes. Proc Nat Acad Sci USA. 2001;98:5104–9.
Souza CJH, Mac Dougall C, Campbell BK, McNeilly AS, Baird DT. The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1B (BMPR-1B) gene. J Endocrinol. 2001;169:1–6.
Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan J, O’Connell AR, KP MN, Montgomery GW. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod. 2001;64:1225–35.
Drouilhet L, Mansanet C, Sarry J, Tabet K, Bardou P, Woloszyn F, Lluch J, Harichaux G, Viguie C, Monniaux D, Bodin L, Mulsant P, Fabre S. The Highly Prolific Pheno-type of Lacaune Sheep Is Associated with an Ectopic Expression of the B4GALNT2 Gene within the Ovary. PLoS Genet. 2013;9(9):e1003809.
Davis GH, Galloway SM, Ross IK, Gregan SM, Ward J, Nimbkar BV. DNA tests in prolific sheep from eight countries provide new evidence on origin of the Booroola (FecB) mutation. Biol Reprod. 2002;66(6):1869–74.
Davis GH. Major genes affecting ovulation rate in sheep. Genet Sel Evol. 2005;37(Suppl. 1):11–23.
Hua G, Yang L. A review of research progress of FecB gene in Chinese breeds of sheep. Anim Reprod Sci. 2009;116:1–9.
Zuo B, Qian H, Wang Z, Wang X, Nisa N, Bayier A, Ying S, Hu X, Gong C, Guo Z, Wang F. A study on BMPR-IB genes of Bayanbulak sheep. Asian Australas J Anim Sci. 2013;26(1):36–42.
Roy J, Polley S, De S, Mukherjee A, Batabyal S, Pan S, Brahma B, et al. Polymorphism of fecundity genes (FecB, FecX, and FecG) in the Indian Bonpala sheep. Anim Biotechnol. 2011;22(3):151–62.
Mahdavi M, Nanekarani S, Hosseini SD. Mutation in BMPR-IB gene is associated with litter size in Iranian Kalehkoohi sheep. Anim Reprod Sci. 2014;147:93–8.
Mullen MP, Hanrahan JP. Direct Evidence on the Contribution of a Missense Mutation in GDF9 to Variation in Ovulation Rate of Finn sheep. PLoS ONE. 2014;9(4):e95251.
Mullen MP, Hanrahan JP, Howard DJ, Powell R. Investigation of Prolific Sheep from UK and Ireland for Evidence on Origin of the Mutations in BMP15 (FecXG, FecXB) and GDF9 (FecGH) in Belclare and Cambridge Sheep. PLoS ONE. 2013;8(1):e53172.
Chu MX, Liu ZH, Jiao CL, He YQ, Fang L, Ye SC, Chen GH, Wang JY. Mutations in BMPR-IB and BMP-15 genes are associated with litter size in Small Tailed Han Sheep (Ovisaries). J Anim Sci. 2007;85:598–603.
Vacca GM, Dhaouadia A, Rekik M, Carcangiu V, Pazzola M, Dettori MI. Prolificacy genotypes at BMPR 1B, BMP15 and GDF9 genes in North African sheep breeds. Small Rumin Res. 2010;88(1):67–71.
Jemmali B, Bedhiaf S, Djemali M. Screening FecXB mutation of the BMP15 gene in Tunisian Barbarine sheep. In : Proceedings of the 9th WCGALP. 2010.
Jemmali B, Romdhani S, Djemali M. Frequency of prolificacy genes in the Tunisian Barbarine sheep. France: Journées 3R (Rencontres Recherches Ruminants); 2011. p. 422.
Bedhiaf-Romdhani S, Jemmali B, Ben SY. Novel phenotype management using molecular characterization in prolific sheep ‘W’ line in Tunisia. EAAP scientific committee: Wageningen Academic Publishers; 2016.
Montgomery GW, Size JA. Extraction of DNA from sheep white blood cells. N Z J Agric Res. 1990;33:437–41.
Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Downers Grove: Johnson Printers; 1992. p. 453–4.
Monniaux D, Caraty A, Clément F, Dalbiès-Tran R, Dupont TJ, Fabre S, Gérard N, Mermillod P, Monget P, Uzbekova S. Développement folliculaire ovarien et ovulation chez les mammifères. Inra Prod Anim. 2009;22(2):59–76.
Statistical Analysis Systems Institute. SAS user’s guide, version 9.00. SAS Institute Inc., Cary, NC, USA. 2002.
Bedhiaf-Romdhani S, Djemali M. New genetic parameters to exploit genetic variability in low input production systems. Livest Sci. 2006;99:119–23.
Braw-Tal R, McNatty KP, Smith P, Heath DA, Hudson NL, Phillips DJ, McLeod BJ, Davis GH. Ovaries of ewes homozygous for the X-linked Inverdale gene (FecXI) are devoid of secondary and tertiary follicles but contain many abnormal structures. Biol Reprod. 1993;49:895–907.
McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL. Oocyte expressed genes affecting ovulation rate. Mol Cell Endocrinol. 2005;234:57–66. Review
Bedhiaf-Romdhani S, Djemali M, Ben Gara A, Rekik B, Ben Hamouda M, Aloulou R, Rekik M, Bouix J, Clement V, Bibe B, François D, Jemmali B, Ben Sassi M. Genetic characterization and management for better valorization of two lines of Barbarine sheep breed selected for two distinct objectives: Prolificacy and Growth. Annales de l’INRAT. 2016;89:154–57. www.inrat.agrinet.tn.
Toosi BM, Seekallu SV, Rawlings NC. Effects of the rate and duration of physiological increases in serum FSHconcentrations on emergence of follicular waves in cyclic ewes. Biol Reprod. 2010;83(4):648–55.
Acknowledgements
The contribution of Office de l’Elevage et des Pâturages (OEP) and Office des Terres Domaniales (OTD) of Tunisia to this work by allowing access to flocks and sheep is gratefully acknowledged. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Funding
This work was supported by grant from the National Institute of Agronomic Research of Tunisia, Ministry of Higher Education and Scientific Research of Tunisia and the French “Agence Nationale de la Recherche” (ANR 2010 BLAN 1608 01, MONOPOLY).
Availability of data and materials
All related data are available within the manuscript.
Authors’ contributions
Conceived and designed the experiments: NL, SBR, SF, MR. Performed the experiments: SF, FW, ZK, NL, SBR, MA, AR, MR. Analyzed the data: SBR. Contributed reagents/materials/analysis tools: NL, SBR, SF, FW, AM, AR. Wrote the paper: SF, ZK, NL, SBR, MR. All authors have read and approved the final manuscript.
Competing interests
The authors have declared that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The experimental procedure was conducted and approved by the Agricultural and Scientific Research Government Committees (CPERA-Tunisia) in accordance with the guidelines for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Both OEP and OTD, owners of the sheep flocks, gave permission to be included in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lassoued, N., Benkhlil, Z., Woloszyn, F. et al. FecX Bar a Novel BMP15 mutation responsible for prolificacy and female sterility in Tunisian Barbarine Sheep. BMC Genet 18, 43 (2017). https://doi.org/10.1186/s12863-017-0510-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-017-0510-x