Abstract
Background
Gene and genome duplication play important roles in the evolution of gene function. Compared to individual duplicated genes, gene clusters attract particular attention considering their frequent associations with innovation and adaptation. Here, we report for the first time the expansion of the apolipoprotein D (ApoD) ligand-transporter genes in a cluster manner specific to teleost fishes.
Results
Based on comparative genomic and transcriptomic analyses, protein 3D structure comparison, positive selection detection and breakpoints detection, the single ApoD gene in the ancestor expanded into two clusters following a dynamic evolutionary pattern in teleost fishes. Orthologous genes show conserved expression patterns, whereas lineage-specific duplicated genes show tissue-specific expression patterns and even evolve new gene expression profiles. Positive selection occurred in branches before and after gene duplication, especially for lineage-specific duplicated genes. Cluster analyses based on protein 3D structure comparisons, especially comparisons of the four loops at the opening side, show gene duplication-segregating patterns. Duplicated ApoD genes are predicted to be associated with forkhead transcription factors and MAPK genes. ApoD clusters are located next to the breakpoints of genome rearrangements.
Conclusions
Here, we report the expansion of ApoD genes specific to teleost fishes in a cluster manner for the first time. Neofunctionalization and subfunctionalization were observed at both the protein and expression levels after duplication. Evidence from different aspects—i.e., abnormal expression-induced disease in humans, fish-specific expansion, predicted associations with forkhead transcription factors and MAPK genes, specific expression patterns in tissues related to sexual selection and adaptation, duplicated genes under positive selection and their location next to the breakpoints of genome rearrangements—suggests the potentially advantageous roles of ApoD genes in teleost fishes. The cluster expansion of ApoD genes specific to teleost fishes provides thus an ideal evo-devo model for studying gene duplication, cluster maintenance and new gene function emergence.
Similar content being viewed by others
Background
Gene and genome duplication play important roles in evolution by providing new genetic materials [1]. The gene copies emerging from duplication events (including whole genome duplications (WGD)) can undergo different evolutionary fates, and a number of models have been proposed as to what can happen after duplication [2]. In many instances, one of the duplicates becomes silenced via the accumulation of deleterious mutations (i.e. pseudogenization or nonfunctionalization [1]). Alternatively, the original pre-duplication function can be subdivided between duplicates (i.e. subfunctionalization) [3], or one of the duplicates can gain a new function (i.e. neofunctionalization) [4]. Although the probability of accumulating beneficial substitutions is relatively low, there are, examples of neofunctionalization. For example, the duplication of dachshund in spiders and allies has been associated with the evolution of a novel leg segment [5]; the expansion of repetitive regions in a duplicated trypsinogen-like gene led to functional antifreeze glycoproteins in Antarctic notothenioid fish [6]; and the duplication of opsin genes is implicated in trichromatic vision in primates [7]. Another selective advantage of gene duplication can be attributed to increased numbers of gene copies, e.g. in the form of gene dosage effects [8, 9]. Multiple mechanisms can act together to shape different phases of gene evolution after duplication [10].
Functional changes after gene duplication can occur at the protein level [6, 11, 12]. For example, the physiological division of labour between the oxygen-carrier function of haemoglobin and the oxygen-storage function of myoglobin in vertebrates (subfunctionalization) [13] and the acquired enhanced digestive efficiencies of the duplicated gene encoding of pancreatic ribonuclease in leaf monkeys (neofunctionalization) [14]. However, the probability that functional mutations can occur in a coding region is relatively small due to pleiotropic constraints. Instead, changes at the expression level are more tolerable and can offer immediate phenotypic consequences [15, 16]. For example, complementary degenerative mutation in the regulatory regions of duplicated genes is a common mechanism for subfunctionalization [2, 17]. Many examples have provided evidence that duplicated genes acquiring new expression domains are linked to neofunctionalization (e.g., dac2, a novel leg segment in arachnids [5]; elnb, bulbus arteriosus in teleost fishes [18]; and fhl2b, egg-spots in cichlid fishes [19]).
In some cases, gene clusters resulting from gene duplication have attracted considerable attention, such as Hox gene clusters [20], globin gene clusters [21], paraHox gene clusters [22], MHC gene clusters [23] and opsin gene clusters [24]. Duplicated genes in clusters are usually related to innovation and adaptation [11, 24, 25], suggesting advantageous roles during evolution. The expansion of gene clusters can be traced back to WGD and tandem duplication [25, 26]. In addition to the two rounds of WGD that occurred before the split between cartilaginous and bony fish [27], the ancestor of teleost fishes experienced another round of WGD (teleost genome duplication, TGD) after divergence from non-teleost actinopterygians, including bichir, sturgeon, bowfin and spotted gar [28, 29]. This extra TGD provides an additional opportunity for gene family expansion in fishes [24, 30,31,32].
Genome rearrangements have been suggested to occur frequently in teleost fishes [33]. If genome rearrangements can capture locally adapted genes or antagonist sex-determining genes by reducing recombination, the rearranged genome can promote divergence and reproductive isolation [34, 35] and thus contribute to speciation and adaptation. Examples can be found in butterflies [36], mosquitoes [37] and fish [38]. This occurs especially when advantageous genes are located next to the breakpoints of a genome rearrangement due to the associated low recombination rates [39, 40]. Considering that the expansion of gene clusters is usually adaptive (as mentioned above) and is linked to genome instability [35, 41, 42], it will be interesting to investigate the roles of gene clusters located next to the breakpoints of genome rearrangements. However, related studies are sparse.
Here, we report for the first time the cluster expansion of apolipoprotein D (ApoD) in teleost fishes. The ApoD gene belongs to the lipocalin superfamily of lipid transport proteins [43, 44]. In humans, ApoD is suggested to function as a multi-ligand, multifunctional transporter (e.g., hormone and pheromone transporter) [44, 45], which is important in homeostasis and in the housekeeping of many organs [45]. It is expressed in multiple tissues, most notably in the brain and testis (see e.g. [44, 46, 47]) and is involved in the central and peripheral nervous systems [44]. However, no detailed analyses of the ApoD gene in fishes have been reported yet. Here, we investigate the evolutionary history of ApoD genes in fishes for the first time.
Results
In silico screening and phylogenetic reconstruction of ApoD genes
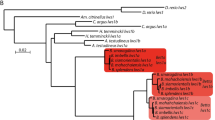
To investigate the expansion of ApoD genes, we performed phylogenetic reconstruction with high-quality assembly of genomes (Fig. 1a, b). There is one ApoD gene in coelacanth (Latimeria chalumnae), and two copies (A and B) in spotted gar (Lepisosteus oculatus) located in one cluster. Different numbers of ApoD genes are located in two clusters in different teleost fishes, i.e., two copies in cavefish (Astyanax mexicanus; A1 and A2) and in tetraodon (Tetraodon nigroviridis; B2a and A2); three copies in zebrafish (Danio rerio; A1, A2 and B2) and in cod (Gadus morhua; A1, A2 and B2b); four copies in platyfish (Xiphophorus maculatus; A1, A2, B2a and B2b); five copies in Amazon molly (Poecilia formosa; A1, A2, B2a, B2ba1, B2ba2) and in fugu (Takifugu rubripes; A1, A2, B1, B2a, B2b); six copies in medaka (Oryzias latipes; A1, A2m1, A2m2, A2m3, B2a, B2b); and eight copies in stickleback (Gasterosteus aculeatus; A1, A2s1, A2s2, B1, B2a, B2bs1, B2bs2, B2bs3) and in tilapia (Oreochromis niloticus; A1, A2t1, A2t2, A2t3, A2t4, B1, B2a, B2b). Noticeably, although the copy B2b in cod is clustered within the B2a clade based on a maximum likelihood (ML) tree, the bootstrap value is very low, which could be due to recent duplication (Fig. 1b). Considering its gene direction and syntenic nature relative to B2b genes in other species (Fig. 1a), we named it copy B2b. Sequence alignment for the tree construction can be found in https://doi.org/10.5061/dryad.39g63v2 [116].
a Cluster expansion of ApoD genes specific to teleost fishes after teleost genome duplication (TGD). Each arrow represents a single gene copy. Genes highlighted in red and orange represent paralogs derived from one common ancestor. Genes colored dark and light green represent paralogs derived from one common ancestor. Phylogeny reconstruction is based on a consensus fish phylogeny [24]. b Maximum likelihood phylogenetic tree reconstruction to infer gene duplication. Bootstrap values > 50% are marked on the branch. Lineage-specific duplication events are labelled. Note that although copy B2b of Gadus morhua is clustered within the B2a clade (labelled with a green star), its bootstrap value is low, which could be due to recent duplication. c The dynamic evolutionary pattern of ApoD genes across the phylogeny of teleost fishes. Highly variable copy numbers are detected in different lineages, especially in the Paracanthopterygii lineage. Stylephorus chordatus has lost all ApoD genes. Compared to copy A1, copy A2 exhibits more variable lineage-specific duplicates in different fishes, with the highest numbers appearing in tilapia (four copies). Copy B1 is absent in the whole clade of Gadiformes. Copy B2 only shows up in the species Danio rerio, Osmerus eperlanus and Parasudis fraserbrunneri. The co-existence of B2a and B2b is common in Percomorphaceae. The largest numbers of lineage-specific duplicated genes are found in tilapia (copy A2), medaka (copy A2) and stickleback (copy B2b) in Percomorphaceae
To further retrieve the evolutionary history of ApoD genes in fishes, we performed an in silico screen across the whole phylogeny of teleost fishes using draft genomes (Fig. 1c). No ApoD gene was detected in Stylephorus chordatus. Unlike copy A1, which shows no lineage-specific duplication, copy A2 exhibits variable lineage-specific copies in different fishes, with the highest number appearing in a cichlid fish, tilapia (four copies). Copy B1 is absent in the clade of Gadiformes but is kept in Acanthopterygii. Species in Danio rerio, Osmerus eperlanus and Parasudis fraserbrunneri possess copy B2. The co-existence of B2a and B2b is common in Percomorphaceae. The largest numbers of lineage-specific duplicated ApoD genes were found in tilapia (copy A2), medaka (copy A2) and stickleback (copy B2b) in Percomorphaceae. The predicted ApoD gene sequences can be found in https://doi.org/10.5061/dryad.39g63v2 [116].
To infer the relationship between ApoD gene duplication and TGD, syntenic analyses were conducted. These revealed that two paralogous regions in teleost fishes correspond to one ohnologous region in spotted gar and the chicken (Gallus gallus) (connected by the same colored lines in Fig. 2A). For example, the regions highlighted in yellow in linkage group (LG) 9 in spotted gar (left above), or on chicken chromosome (chr) 2 (bottom left) have paired paralogous regions in medaka. These are found on chr17, where the ApoD cluster I is located, and on chr20, where the ApoD cluster II is located. Similarly, the region highlighted in purple in LG3 of spotted gar and on chicken chr1 has paired paralogous regions (connected by purple lines) on chr17 and chr20 in medaka.
(a) Syntenic analyses of genome regions possessing ApoD clusters. The same color between spotted gar and the chicken represents orthologous chromosomes. Two paralogous duplicated segments in teleost fishes (chromosomes (chrs)/linkage groups (LGs) indicated in red) can be traced back to one corresponding orthologous region in spotted gar and the chicken (chrs/LGs labelled in black), linked by colorful lines. Arrows show the regions in which apolipoprotein D (ApoD) clusters are located in. (b) ApoD clusters next to the breakpoints of genome rearrangements before teleost genome duplication (TGD). The same color between spotted gar and the chicken represents orthologous chromosomes. The red arrows show breakpoints, and the black arrows show gene directions. Neighboring genes are named according to the Ensembl database. (c) Inversion with ApoD clusters next to the breakpoints occurred again in cichlid fishes. The haplochromine lineage, the most species-rich lineage of cichlid fishes, is labelled. (d) ApoD domains and protein-protein association predictions. a. Conserved domains include a single peptide of approx. 20 amino acids (AA) and a lipocalin domain of approx. 144 AA. b. Different paralogs exhibited differential associations. The common associations are with pla2g15, lcat and MAPK genes. One class (copies A2, B2a and B1) is associated with multiple forkhead transcription factors. The other class (copies A1 and B2b) lost this association. Instead, it is associated with the lipoprotein-related gene apoa1. The single ApoD gene in coelacanth possesses both associations
ApoD clusters next to the breakpoints of genome rearrangements
Syntenic analyses clearly show that more than one chromosome in spotted gar and the chicken are syntenic to the corresponding paralogous regions in which ApoD clusters are located in teleost fishes. For example, chromosomes (or LGs) containing ApoD clusters (chr17 and chr 20 in medaka, LG III and LG XXI in stickleback, LG9 and LG18 in tilapia, and chr2 and chr24 in zebrafish) are syntenic to chromosomes LG10, LG9, LG19, LG3 and LG14 in spotted gar and chr8, chr2, chr28, chr1 and chr9 in the chicken (Fig. 2A and B). Noticeably, ApoD clusters are located next to the breakpoints of genome rearrangements (Fig. 2A and B). The rearranged segments (approx. 80 kb to 200 kb syntenic with LG14 in spotted gar, Fig. 2B) include ApoD clusters and their neighboring genes (e.g., and2, samd7, sec62, nadkb, gpr160, skila, prkci and phc3 for Cluster I; otos, myeov2, and1, tmtopsb, tnk2a and tfr1b for Cluster II) (Fig. 2B). Analyses of available cichlid genomes further show that inversion of the segments (approx. 600 kb to 800 kb) with ApoD clusters as the breakpoints also occurred in species belonging to the most species-rich lineage of cichlid fishes, the haplochromine lineage, as supported by split-read analyses using Delly (Fig. 2C).
Protein-protein association predictions and protein 3D structure comparisons
To assess the biological functions of different ApoD genes, protein-protein associations were predicted. Protein domain architecture analyses revealed that ApoD proteins in different fishes are composed of a signal peptide of approx. 20 amino acids and a lipocalin domain of approx. 144 amino acids (Fig. 2D). The common associations of ApoD genes are with the immunity-related gene pla2g15 [48, 49], the high-density lipoprotein biogenesis-related gene lcat [50], different copies of zg16 related to pathogenic fungi recognition [51] and MAPK genes [52, 53]. ApoD genes in teleost fishes can be subdivided into two classes based on the association predictions. ApoD genes from one class (copy A2, copy B2a and copy B1) are associated with forkhead transcription factors. ApoD genes from the other class (copy A1 and copy B2b) lost their associations with forkhead transcription factors but are associated with the lipoprotein-related gene apoa1 [54]. Noticeably, the only ApoD gene in coelacanth possesses associations with both forkhead transcription factors and apoa1. ApoD in coelacanth is also associated with genes encoding ligands that can activate the Notch signalling pathway (jag1, jag2) [55], and the gene belongs to the annexin family (anxaII) (Fig. 2D). Noticeably, more members of forkhead transcription factors and MAPK genes are associated with ApoD genes after duplication. Unlike in other fishes, copy A1 in zebrafish is associated with bone resorption-related duplicated genes (ostf1a, ostf1b, ostf1c) [56], the cell growth and division-related gene ppp2cb [57], the neurodevelopment-related gene rab3gap2 [58] and the guanylate-binding gene gbp1 [59] (Fig. 2D).
Homology protein structure modelling of different ApoD genes shows a conserved structure, including a cup-like central part made up of eight antiparallel β-sheet strands and two ends connected by loops (a wide opening side formed by loops 1, 3, 5 and 7 and a narrow closed bottom formed by loops 2, 4 and 6; Fig. 3a). Interestingly, unlike the very conserved cup-like central part, the loops are highly variable (Fig. 3a). Cluster analyses based on the whole protein 3D structure can clearly segregate different duplicates. This is especially true of lineage-specific duplicated genes that are clustered together. Copy B1s is clustered nested within the copy A2 clade (Fig. 3b). The same analyses focused on only the four loops (loops 1, 3, 5 and 7) at the opening side show a similar segregation pattern (Fig. 3c). However, cluster analyses focused only on the three loops (loops 2, 4 and 6) at the bottom side did not show a gene duplication-segregation pattern (Fig. 3d). Details concerning the PDB files and the cluster results can be found in https://doi.org/10.5061/dryad.39g63v2 [116].
a Protein 3D structure modelling. Different ApoDs among species exhibit a conserved 3D structure, including a cup-like central part made up of eight antiparallel β-sheet strands and two ends connected by loops (a wide opening part formed by loops 1, 3, 5 and 7 and a narrow closed bottom formed by loops 2, 4 and 6). Unlike the very conserved cup-like central part, the loops at the two ends are highly variable. Note that most sites under positive selection are located on the loops or on the connections between the loops and the cup-like central part. b Cluster analyses based on the whole protein 3D structure. Cluster analyses can clearly segregate different ApoD duplicates, including lineage-specific duplicated genes. c Cluster analyses based on loops 1, 3, 5 and 7 at the opening side. Cluster analyses can segregate different ApoD duplicates. d Cluster analyses based on loops 2, 4 and 6 at the bottom. This cluster analyses cannot segregate different duplicates
Positive selection detection of ApoD genes
Positive selection detection using a branch-site model within codeml in PAML revealed that positive selection occurred in branches before and after duplication, for example, the branch of copy A2 in zebrafish; the ancestral branch of copies B2a and B2b in fugu, medaka and platyfish; and, especially, in lineage-specific duplicated genes, such as in stickleback, cichlid fishes and Amazon molly (Fig. 4 and https://doi.org/10.5061/dryad.39g63v2 [116]). Positive selection sites under Bayes empirical Bayes (BEB) with a posterior probability > 95% are shown in Fig. 5 and https://doi.org/10.5061/dryad.39g63v2 [116]. Noticeably, most sites under positive selection are located on the highly variable loops or on the connections between loops and the cup-like central part at the opening side (loops 3 and 5; Figs. 3a and 5). Multiple tests of positive selection on all possible foreground branches followed by Bonferroni correction is a relatively conservative strategy. If we only focus on one prior hypothesis on a particular branch without doing multiple test corrections, we will find more branches with dN/dS values significantly larger than 1 (p < 0.05; https://doi.org/10.5061/dryad.39g63v2 [116]). However, we chose to use our strategy to make the analyses more rigorous. Even with this strict but comprehensive strategy, our results still show that positive selection occurred in branches before and after gene duplication, especially for lineage-specific duplicated genes. Details about the codon alignment for the PAML analyses can be found in https://doi.org/10.5061/dryad.39g63v2 [116].
Positive selection detection using a branch-site model within codeml in PAML. Many duplicated ApoD genes are under positive selection, with a value of ω significantly larger than 1 after Bonferroni correction, especially for lineage-specific duplicated genes. Note that if the whole clade is designated as the foreground branch when detecting positive selection, each branch of this clade will be labelled if its ω value is significantly larger than 1. The unrooted tree was used for PAML analyses, and the rooted tree here is only for presentation. More details can be found in https://doi.org/10.5061/dryad.39g63v2 [116]
Amino acid alignment of ApoD genes among species with protein 3D structure annotation. The purple arrow represents the β-strand, and the yellow arrow represents the loop. Sites under positive selection under Bayes empirical Bayes (BEB) with a posterior probability > 95% are labelled with a red rectangle. Most sites under positive selection are distributed on the loops or on the connections between the loop and the β-strand. Note that the positions of the sites under positive selection are based on the codon alignment used for PAML analyses (https://doi.org/10.5061/dryad.39g63v2 [116]) instead of on the amino acid alignment here, but the amino acids that under positive selection are the same in Fig. 5 and https://doi.org/10.5061/dryad.39g63v2 [116]
Gene expression profile detection of ApoD genes in different species
Based on the available raw transcriptomic data analyses and the results from quantitative polymerase chain reaction (qPCR), the gene expression profiles of ApoD genes were detected in different species (Fig. 6). Copy A1 is mainly expressed in the skin and the eye. Noticeably, copy A1 is also highly expressed in the novel anal fin pigmentation patterns of the cichlid fish, Astatotilapia burtoni, which is consistent with the findings of our previous study [60]. Copy A2 shows redundant expression patterns in the gills, eye, skin and gonads in zebrafish and cavefish, but its orthologous A2 genes, including lineage-specific duplicated genes in the species of Acanthomorphata tested herein (cod, stickleback, medaka, tilapia and A. burtoni) are expressed in the gills and in the related apparatus, the lower pharyngeal jaw. Copy B2 in zebrafish shows redundant expression profiles (in the skin and gonads) overlapping with the profiles of copies A1 and A2. Copies B2a and B2b show specific expression patterns in different fishes of Acanthomorphata tested herein. For example, copy B2b is expressed in the ovary and in the liver in cod, whereas B2a and B2bs are mainly expressed in the spleen and in the liver of the stickleback. The expression of B2a and B2b was not detected in adult medaka, tilapia and A. burtoni. Interestingly, based on the available transcriptomic data for the different developmental stages of the gonads in tilapia, we found that B2a and B2b are highly specifically expressed at the early developmental stage of gonad tissues (5 days after hatching, 5dah; Fig. 6). Copy B1 is highly specifically expressed in the liver in tilapia and in A. burtoni but not in stickleback in which B1 is inverted (Fig. 1a). Noticeably, no expression profile was detected for copy B in spotted gar, at least, not in the tissues we tested. Instead, expression in tissues including the skin, eye, gill, liver, testis and brain were detected for copy A in spotted gar. Details can be found in Fig. 6 and https://doi.org/10.5061/dryad.39g63v2 [116].
Gene expression profiles of different ApoD genes. a Expression patterns of ApoD genes in different fishes. Each block represents a single gene copy. The phylogeny reconstruction was based on the consensus fish phylogeny from a previous study [24]. b Detailed gene expression profiles of ApoD genes among fishes. Red, tissues with a high expression level; dark grey, data unavailable (either because the tissue does not exist in the species or is undetected in this study); light grey, tissues with a low expression level. LPJ, the lower pharyngeal jaw in cichlid fishes. TGD, teleost genome duplication. c Specific expressions of copy B2a and copy B2b at early developmental stages of the gonads in tilapia. Analyses are based on the available transcriptomic data from a previous study [117]. RPKM, reads per kilobase per million mapped reads; dah, days after hatching
Discussion
Dynamic evolution of ApoD genes in teleost fishes
Here, we report the cluster expansion of ApoD genes specific to teleost fishes for the first time. Phylogenetic reconstruction and syntenic analyses clearly show the expansion of ApoD genes into two clusters with lineage-specific tandem duplications after TGD. The interplay between genome duplication and tandem duplication to prompt the expansion of the gene family has already been shown in a few studies [61,62,63]. It has been suggested that the fixation of duplications is much more common in genome regions in which the rates of mutations are elevated due to the presence of already-fixed duplication, which is the so-called “snow-ball” effect [64]. Both genome duplication and tandem duplication can produce raw genetic materials to fuel the diversification of teleost fishes at later stages, e.g., morphological complexity and ecological niche expansion, which is compatible with the “time lag model” [65]. Neofunctionalization and subfunctionalization after duplication are important steps to realize this diversification, and both can be detected in ApoD duplicates.
The functional divergence of ApoD genes occurred at the protein level. On one hand, based on the protein-protein association predictions, although common associations with MAPK genes are shared among ApoD genes, subdivided associations with forkhead transcription factors and apoa1 were detected in different paralogous ApoD genes, indicating subfunctionalization. Noticeably, more members of forkhead transcription factors and MAPK genes are associated with ApoD genes after duplication. In this case, neofunctionalization could also occur but was not limited to the dosage effects. On the other hand, divergence was also detected at the protein-structure level. Protein structures are often related to functional divergence [66,67,68,69]. New folds can even evolve novel functions [70, 71]. Even mutations of a few residues can induce structural changes [72, 73]. Therefore, structure-based inference is important for understanding molecular function. Our protein 3D structure modelling shows a conserved backbone conformation, in spite of sequence divergence. Indeed, this is a feature of the lipocalin family [67], to which ApoDs belong [43, 44, 74]. The cup-like central part is used to transport large varieties of ligands, such as hormones and pheromones [74]. Interestingly, cluster analyses based on the whole protein structure or on only the four loops at the opening side can segregate different duplicates, although not for copy B1. Considering that copy B1 has been lost multiple times, its function might not be essential and could share functions with other copies; thus, it is not surprising if it is clustered together within the copy A2 clade. The cluster results of two other individual genes (copy B2 of zebrafish and B2b of medaka) do not affect the general segregation pattern. However, unlike the four loops at the opening side, cluster analyses based on only the three loops (loops 2, 4 and 6) at the bottom side did not show a clear segregation pattern. It has been suggested that the loops of lipocalin proteins can affect ligand binding specificities, which are similar to the binding modes of antibodies [75], and mediate protein-protein interactions [67]. Their segregation with different duplicates might indicate that functional divergence occurred at the loops at the opening side. The evidence that most amino acids under positive selection are located on the loops or connections between loops and strands further indicates the potentially important roles of these loops during evolution. Actually, reshaping different parts of the protein structure is an efficient way to produce functional divergence at the protein level within a short time [74], and this could be one of the ways by which divergence at the protein level occurred in ApoDs.
The functional divergence of ApoD genes also occurred at the expression level. Different ApoD genes in zebrafish and cavefish exhibited redundant expression profiles but became more specific as the numbers of tandem duplicates increased, indicating subfunctionalization. Noticeably, new expression profiles were detected in duplicated paralogs, e.g., copy A1 in novel anal fin pigmentation patterns and copy A2s in the lower pharyngeal jaw in A. burtoni, which belongs to the most species-rich cichlid fish lineage, the haplochromine lineage. These two traits are key innovations associated with adaptive radiation in cichlid fishes [76]. With expression changes, duplication can be an important source for the emergence of novelty [77], especially if they are adaptive. The relaxed selection pressure induced by the changing expression profiles can even further prompt the accumulation of mutations at the protein level.
Potential advantageous roles of ApoD genes in teleost fishes
The ApoD gene has been thoroughly studied in human and mice, and its abnormal expression was reported to be related to human diseases, such as Parkinson’s disease and Alzheimer’s disease [78,79,80]. Interestingly, as our study revealed here, ApoD dynamically expanded in fishes into two clusters on different chromosomes. Furthermore, these expanded duplicates are transcriptionally active. For example, they are highly expressed and are restricted to different tissues related to sexual selection (skin [81], eye [82, 83], gonads, and anal fin pigmentation patterns [60]) and adaptation (gills [84,85,86,87], spleen [88, 89], and lower pharyngeal jaw [76, 90]). These tissues are also derived from the neural crest, which is a key innovation in vertebrates [76, 91,92,93]. Additionally, many duplicated ApoD genes are under positive selection, especially for lineage-specific duplicated genes. Combined with their associations with MAPK genes and forkhead transcription factors and their functions as pheromone and hormone transporters, we report evidence that suggests the potential importance of ApoD genes in fishes.
Noticeably, two clusters exhibited distinct expression patterns. ApoD genes in cluster I in most fishes are expressed in tissues related to sexual selection (skin, eye, gonad and anal fin pigmentation), but in cluster II, ApoD genes are mainly expressed in tissues related to adaptation (gills and lower pharyngeal jaw). Interestingly, both clusters were maintained with their neighboring genes and are located next to the breakpoints of genome rearrangements during evolution. An inversion of a chromosomal section containing ApoD clusters next to breakpoints occurred again in the haplochromine lineage, the most species-rich lineage of cichlid fishes [94]. If genome rearrangements can capture locally adaptive genes or genes related to sexual antagonism, it could accelerate divergence by reducing recombination rates, thus prompting speciation and adaptation and even the emergence of neo-sex chromosomes [95]. Given that many ApoD genes are under positive selection, their location next to the breakpoints of genome rearrangements gives them more opportunities to be involved in speciation and adaptation, which deserves further investigation.
Conclusions
Here, we report the cluster expansion of ApoD genes in a cluster manner specific to teleost fishes for the first time. Different types of evidence based on computational evolutionary analyses strongly suggest the potentially advantageous roles of ApoD genes in fishes. An in-depth functional characterization of ApoD genes could help consolidate a model for the study of subfunctionalization and neofunctionalization. Moreover, finding the regulatory mechanisms behind the cluster expansion of ApoD genes and the reason why the ApoD gene expansion was specific to fishes remain open questions. As more fish genomes with high assembly quality become available, especially those of closely related species such as cichlid fishes, the roles of ApoD genes in fish speciation and adaptation can be further investigated. Above all, the ApoD clusters reported here provide an ideal evo-devo model for studying gene duplication, cluster maintenance, and the gene regulatory mechanism and their roles in speciation and adaptation.
Methods
In silico screening and phylogenetic reconstruction to infer gene duplication
To retrieve ApoD gene duplication in teleost fishes, we first extracted orthologs and paralogs in fishes with available genome data from Ensembl (Release 84) [96] and the NCBI database (https://www.ncbi.nlm.nih.gov/genome/). To confirm gene copy numbers, all orthologs and paralogs were used as queries in a tblastx search against the corresponding genomes. For all unannotated positive hits, a region spanning approx. 2 kb was extracted, and open reading frames (ORF) were predicted using Augustus (http://augustus.gobics.de/) [97]. A BLAST search was then performed to compare the predicted coding sequences with the existing transcriptome database to retrieve the corresponding cDNA sequences. The cDNAs were then re-mapped to the corresponding predicted genes to re-check the predicted exon-intron boundaries. Coding sequences of genes from humans and spotted gar, as well as those of neighboring genes, were used to perform a tblastx search against the genomes of lamprey (Lampetra fluviatilis) and amphioxus (Branchiostoma belcheri). To infer gene duplication, an ML analysis was performed using ApoD genes retrieved from available assembled genomes in RAxML v8.2.10 [98] with the GTR + G model and the ‘-f a’ option, which generates the optimal tree and conducts 10,000 rapid bootstrap searches.
To further retrieve ApoDs from the draft genomes of other fishes across the whole phylogeny [23], all sequences retrieved above were used as queries in a tblastx search using a threshold e value of 0.001. The hit scaffolds were retrieved, and genes within the scaffolds were predicted using Augustus. The predicted ApoDs were then translated and re-aligned with known ApoD ORFs to further confirm the exon-intron boundaries. All predicted orthologs and paralogs were used as a query again in a tblastx search against the corresponding genome data until no more ApoD genes were predicted.
Syntenic analyses and inversion detection
To further confirm the relationship between ApoD gene duplication and TGD, gene regions adjacent to the duplicates and to the outgroup species that did not experience TGD (including spotted gar and the chicken) were retrieved. To this end, a window of 5 Mb around the ApoD clusters in teleost fishes, as well as the corresponding chromosomes in spotted gar and the chicken, were retrieved from the Ensemble and NCBI databases. Syntenic analyses were performed with SyMap [99]. To further detect the structural variation around the ApoD genes in cichlid fishes, we retrieved the available cichlid genome raw data from [100]. The programme Delly [101], based on paired-end split-reads analyses, was used to detect the inversion and its corresponding breakpoints of the segments that contain the ApoD clusters in cichlid fishes, with tilapia as the reference.
Protein-protein interaction prediction, protein 3D structure modelling and comparisons
To predict the biological functions of ApoD genes, the protein domains of ApoD genes and protein-protein interactions were predicted with the Simple Modular Architecture Research Tool (SMART) [102, 103] and the stringdb database http://string-db.org/ [104]. To determine whether there were divergences at the protein level for different ApoDs, protein 3D structures were simulated with Swiss-model [105,106,107] using the human ApoD crystal protein structure (PDB ID 2hzr) as the template. The results were further visualized, evaluated and analyzed with Swiss-PdbViewer [108]. To compare the protein structures, we first extracted and converted the corresponding information in the PDB file using Swiss-PdbViewer. Protein 3D structure comparisons were then conducted using Vorolign [109], which can compare closely related protein structures even when structurally flexible regions exist [109]. The protein 3D structure comparisons and cluster analyses were conducted using the ProCKSI-server (http://www.procksi.net/). Clustering results were further visualized using Figtree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree). Copy B2ba2 of Amazon molly was not included in the cluster analyses due to its relatively short sequence. The PDB files were used as input files. For the global protein structure comparisons, PDB files were extracted directly from the simulation results of Swiss-model [105,106,107]. To only obtain PDB files of the loop region, we revised the PDB files manually to get rid of the cup-like central part. To consider the highly variable connections between the loops and the cup-like central part (Fig. 3a), we also included the structures of two more residues next to the loops. The resulting PDB files were further checked using Swiss-PdbViewer.
Positive selection detection
To examine whether ApoD duplicates underwent adaptive sequence evolution, a branch-site model was used to test positive selection affecting a few sites along the target lineages (called foreground branches) in codeml within PAML [110, 111]. The rates of non-synonymous to synonymous substitutions (ω or dN/dS) with a priori partitions for foreground branches (PAML manual) were calculated. Considering the very dynamic evolutionary pattern of ApoD genes, we tested for positive selection in every branch in each species. In this case, we designated each branch as the foreground branch to run the branch-site model multiple times. Noticeably, there are two different ways to designate the foreground branch in the branch-site model. One way is to designate only the branch as the foreground branch. The other way is to designate the whole clade (all the branches within the clade including the ancestral branch) as the foreground branch. We included both perspectives in our data analyses (https://doi.org/10.5061/dryad.39g63v2 [116]). If the whole clade was under positive selection, we labelled all the branches within this clade, including the ancestral branch, with ω > 1 in the results (Fig. 4).
All model comparisons in PAML were performed with fixed branch lengths (fix_blength = 2) derived under the M0 model in PAML. Alignment gaps and ambiguity characters were removed (Cleandata = 1). A likelihood ratio test was used to test for statistical significance. In addition, Bonferroni correction was conducted for multiple-test correction [112]. The BEB was used to identify sites that are under positive selection. Sites under positive selection under BEB with a posterior probability > 0.95 are given in Fig. 5 and https://doi.org/10.5061/dryad.39g63v2 [116].
Gene expression profile analyses
To determine the expression profiles of ApoD duplicates in different fishes, raw available transcriptomic data from spotted gar, zebrafish, cavefish, cod, medaka, tilapia and A. burtoni were retrieved from NCBI https://www.ncbi.nlm.nih.gov/ (https://doi.org/10.5061/dryad.39g63v2 [116]). Raw reads were mapped to the corresponding cDNAs (http://www.ensembl.org/) to calculate the RPKM (reads per kilobase per million mapped reads) value. qPCR was used to detect the expression profiles of different ApoD duplicates in the tissues not included in the available transcriptomes (https://doi.org/10.5061/dryad.39g63v2 [116]). Fish samples for qPCR include A. burtoni (provided by Prof. Walter Salzburger, University of Basel, Switzerland), stickleback (collected from Nideraach and Romanshorn in Switzerland by Dr. Dario Moser), zebrafish (provided by Prof. Markus Affolter, Biozentrum, University of Basel, Switzerland), tilapia (provided by Prof. Deshou Wang, Southwest University, Chongqing, China) and medaka (provided by Prof. Jing Wei, Southwest University, Chongqing, China). Prior to tissue dissection, specimens were euthanized with MS 222 (Sigma-Aldrich, USA) following an approved procedure (permit nr. 2317 issued by the cantonal veterinary office, Switzerland; Guidelines for the Care and Use of Laboratory Animals prescribed by the Regulation of Animal Experimentation of Chongqing, China). RNA isolation was performed according to the TRIzol protocol (Invitrogen, USA). DNase treatment was performed with the DNA-free™ Kit (Ambion, Life Technologies, USA). The RNA quantity and quality were determined with a NanoDrop1000 spectrophotometer (Thermo Scientific, USA). cDNA was produced using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, USA). The housekeeping gene elongation factor 1 alpha (elfa1) [113], ubiquitin (ubc) [114] and ribosomal protein L7 (rpl7) [115] were used as endogenous controls. qPCR was performed on a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Life Technologies) using the SYBR Green Master Mix (Roche, Switzerland) with an annealing temperature of 58 °C and following the manufacturer’s protocols. Primers are available in https://doi.org/10.5061/dryad.39g63v2 [116].
Abbreviations
- ApoD:
-
Apolipoprotein D
- BEB:
-
Bayes empirical Bayes
- chr:
-
Chromosome
- elfa1:
-
Elongation factor 1 alpha
- LG:
-
Linkage group
- ML:
-
Maximum likelihood
- ORF:
-
Open reading frame
- qPCR:
-
Quantitative polymerase chain reaction
- RPKM:
-
Reads per kilobase per million mapped reads
- rpl7:
-
Ribosomal protein L7
- SMART:
-
Simple Modular Architecture Research Tool
- TGD:
-
Teleost genome duplication
- ubc:
-
Ubiquitin
- WGD:
-
Whole genome duplication
References
Ohno S. Evolution by gene duplication. Berlin, Heidelberg: Springer; 1970.
Innan H, Kondrashov F. The evolution of gene duplications: classifying and distinguishing between models. Nat Rev Genet. 2010;11:97–108.
Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–45.
Rastogi S, Liberles DA. Subfunctionalization of duplicated genes as a transition state to neofunctionalization. BMC Evol Biol. 2005;5:28.
Turetzek N, Pechmann M, Schomburg C, Schneider J, Prpic N-M. Neofunctionalization of a duplicate dachshund gene underlies the evolution of a novel leg segment in arachnids. Mol Biol Evol. 2016;33:109–21.
Chen L, DeVries AL, Cheng C-HC. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci. 1997;94:3811–6.
Dulai KS, von Dornum M, Mollon JD, Hunt DM. The evolution of trichromatic color vision by opsin gene duplication in New World and Old World Primates. Genome Res. 1999;9:629–38.
Tang Y-C, Amon A. Gene copy-number alterations: a cost-benefit analysis. Cell. 2013;152:394–405.
Lin Z, Li W-H. Expansion of hexose transporter genes was associated with the evolution of aerobic fermentation in yeasts. Mol Biol Evol. 2011;28:131–42.
Glasauer SMK, Neuhauss SCF. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Gen Genomics. 2014;289:1045–60.
Baalsrud HT, Voje KL, Tørresen OK, Solbakken MH, Matschiner M, Malmstrøm M, et al. Evolution of hemoglobin genes in codfishes influenced by ocean depth. Sci Rep. 2017;7:7956.
Zhang J, Zhang Y, Rosenberg HF. Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat Genet. 2002;30:411–5.
Storz JF, Opazo JC, Hoffmann FG. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol Phylogenet Evol. 2013;66:469–78.
Zhang J. Parallel adaptive origins of digestive RNases in Asian and African leaf monkeys. Nat Genet. 2006;38:819–23.
Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–16.
Carroll SB. Evolution at two levels: on genes and form. PLoS Biol. 2005;3:e245.
Jiménez-Delgado S, Pascual-Anaya J, Garcia-Fernàndez J. Implications of duplicated cis-regulatory elements in the evolution of metazoans: the DDI model or how simplicity begets novelty. Brief Funct Genomic Proteomic. 2009;8:266–75.
Moriyama Y, Ito F, Takeda H, Yano T, Okabe M, Kuraku S, et al. Evolution of the fish heart by sub/neofunctionalization of an elastin gene. Nat Commun. 2016;7:10397.
Santos ME, Braasch I, Boileau N, Meyer BS, Sauteur L, Böhne A, et al. The evolution of cichlid fish egg-spots is linked with a cis-regulatory change. Nat Commun. 2014;5:5149.
Carrasco AE, McGinnis W, Gehring WJ, De Robertis EM. Cloning of an X. laevis gene expressed during early embryogenesis coding for a peptide region homologous to Drosophila homeotic genes. Cell. 1984;37:409–14.
Proudfoot NJ, Shander MH, Manley JL, Gefter ML, Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980;209:1329–36.
Brooke NM, Garcia-Fernàndez J, Holland PWH. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature. 1998;392:920–2.
Malmstrøm M, Matschiner M, Tørresen OK, Star B, Snipen LG, Hansen TF, et al. Evolution of the immune system influences speciation rates in teleost fishes. Nat Genet. 2016;48:1204–10.
Cortesi F, Musilová Z, Stieb SM, Hart NS, Siebeck UE, Malmstrøm M, et al. Ancestral duplications and highly dynamic opsin gene evolution in percomorph fishes. Proc Natl Acad Sci. 2015;112:1493–8.
Garcia-Fernàndez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6:881–92.
Hardison RC. Evolution of hemoglobin and its genes. Cold Spring Harb Perspect Med. 2012;2:a011627.
Robinson-Rechavi M, Boussau B, Laudet V. Phylogenetic dating and characterization of gene duplications in vertebrates: the cartilaginous fish reference. Mol Biol Evol. 2004;21:580–6.
Sato Y, Nishida M. Teleost fish with specific genome duplication as unique models of vertebrate evolution. Environ Biol Fish. 2010;88:169–88.
Braasch I, Gehrke AR, Smith JJ, Kawasaki K, Manousaki T, Pasquier J, et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 2016;48:427–37.
Brunet FG, Volff J-N, Schartl M. Whole genome duplications shaped the receptor tyrosine kinase repertoire of jawed vertebrates. Genome Biol Evol. 2016;8:1600–13.
Gillis WQ, St John J, Bowerman B, Schneider SQ. Whole genome duplications and expansion of the vertebrate GATA transcription factor gene family. BMC Evol Biol. 2009;9:207.
Voldoire E, Brunet F, Naville M, Volff J-N, Galiana D. Expansion by whole genome duplication and evolution of the sox gene family in teleost fish. Vaudry H, editor. PLoS One. 2017;12:e0180936.
Sémon M, Wolfe KH. Rearrangement rate following the whole-genome duplication in teleosts. Mol Biol Evol. 2007;24:860–7.
Kirkpatrick M, Barton N. Chromosome inversions, local adaptation and speciation. Genetics. 2006;173:419–34.
Farré M, Micheletti D, Ruiz-Herrera A. Recombination rates and genomic shuffling in human and chimpanzee - a new twist in the chromosomal speciation theory. Mol Biol Evol. 2013;30:853–64.
Joron M, Frezal L, Jones RT, Chamberlain NL, Lee SF, Haag CR, et al. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature. 2011;477:203–6.
Ayala D, Fontaine MC, Cohuet A, Fontenille D, Vitalis R, Simard F. Chromosomal inversions, natural selection and adaptation in the malaria vector Anopheles funestus. Mol Biol Evol. 2011;28:745–58.
Barth JMI, Berg PR, Jonsson PR, Bonanomi S, Corell H, Hemmer-Hansen J, et al. Genome architecture enables local adaptation of Atlantic cod despite high connectivity. Mol Ecol. 2017;26:4452–66.
Stevison LS, Hoehn KB, Noor MAF. Effects of inversions on within- and between-species recombination and divergence. Genome Biol. Evol. 2011;3:830–41.
Corbett-Detig RB. Selection on inversion breakpoints favors proximity to pairing sensitive sites in Drosophila melanogaster. Genetics. 2016;204:259–65.
Otto SP. The evolutionary consequences of polyploidy. Cell. 2007;131:452–62.
Hufton AL, Groth D, Vingron M, Lehrach H, Poustka AJ, Panopoulou G. Early vertebrate whole genome duplications were predated by a period of intense genome rearrangement. Genome Res. 2008;18:1582–91.
Ayrault Jarrier M, Levy G, Polonovski J. Etude des alpha-lipoprot’eines s’eriques humaines par. Bull Soc Chim Biol (Paris). 1963;45:703–13.
Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, et al. Apolipoprotein D. Biochim Biophys Acta - Protein Struct Mol Enzymol. 2000;1482:185–98.
Weech P, Provost P, Tremblay N, Camato R, Milne R, Marcel Y, et al. Apolipoprotein D - An atypical apolipoprotein. Prog Lipid Res. 1991;30:259–66.
Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, et al. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986;261:16535–9.
Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res. 1991;32:1959–70.
Gilleron M, Lepore M, Layre E, Cala-De Paepe D, Mebarek N, Shayman JA, et al. Lysosomal lipases PLRP2 and LPLA2 process mycobacterial multi-acylated lipids and generate T cell stimulatory antigens. Cell Chem Biol. 2016;23:1147–56.
Bailey SD, Xie C, Do R, Montpetit A, Diaz R, Mohan V, et al. Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the diabetes reduction assessment with ramipril and rosiglitazone medication (DREAM) study. Diabetes Care. 2010;33:2250–3.
Fotakis P, Kuivenhoven JA, Dafnis E, Kardassis D, Zannis VI. The effect of natural LCAT mutations on the biogenesis of HDL. Biochemistry. 2015;54:3348–59.
Tateno H, Yabe R, Sato T, Shibazaki A, Shikanai T, Gonoi T, et al. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology. 2012;22:210–20.
Plestant C, Anton ES. Scaling the MAPK signaling threshold during CNS patterning. Dev Cell. 2013;25:221–2.
Shvartsman SY, Coppey M, Berezhkovskii AM. MAPK signaling in equations and embryos. Fly (Austin). 2009;3:62–7.
Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: a molecule of diverse function. Indian J Clin Biochem. 2016;31:253–9.
Fiaschetti G, Schroeder C, Castelletti D, Arcaro A, Westermann F, Baumgartner M, et al. NOTCH ligands JAG1 and JAG2 as critical pro-survival factors in childhood medulloblastoma. Acta Neuropathol Commun. 2014;2:39.
Reddy S, Devlin R, Menaa C, Nishimura R, Choi SJ, Dallas M, et al. Isolation and characterization of a cDNA clone encoding a novel peptide (OSF) that enhances osteoclast formation and bone resorption. J Cell Physiol. 1998;177:636–45.
Wong C-H, Fung Y-WW, Ng EK-O, Lee SM-Y, Waye MM-Y, Tsui SK-W. LIM domain protein FHL1B interacts with PP2A catalytic β subunit - a novel cell cycle regulatory pathway. FEBS Lett. 2010;584:4511–6.
Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev. 2008;58:236–46.
Pandita E, Rajan S, Rahman S, Mullick R, Das S, Sau AK. Tetrameric assembly of hGBP1 is crucial for both stimulated GMP formation and antiviral activity. Biochem J. 2016;473:1745–57.
Gu L, Xia C. Revelation of the genetic basis for convergent innovative anal fin pigmentation patterns in cichlid fishes. bioRxiv. 2017. https://doi.org/10.1101/165217.
Hofberger JA, Nsibo DL, Govers F, Bouwmeester K, Schranz ME. A complex interplay of tandem- and whole-genome duplication drives expansion of the L-type lectin receptor kinase gene family in the brassicaceae. Genome Biol Evol. 2015;7:720–34.
Bellieny-Rabelo D, Oliveira AEA, Venancio TM. Impact of whole-genome and tandem duplications in the expansion and functional diversification of the F-box family in legumes (Fabaceae). PLoS One. 2013;8:e55127.
Hammoudi V, Vlachakis G, Schranz ME, van den Burg HA. Whole-genome duplications followed by tandem duplications drive diversification of the protein modifier SUMO in angiosperms. New Phytol. 2016;211:172–85.
Kondrashov FA, Kondrashov AS. Role of selection in fixation of gene duplications. J Theor Biol. 2006;239:141–51.
Schranz ME, Mohammadin S, Edger PP. Ancient whole genome duplications, novelty and diversification: the WGD radiation lag-time model. Curr Opin Plant Biol. 2012;15:147–53.
Engel A, Gaub HE. Structure and mechanics of membrane proteins. Annu Rev Biochem. 2008;77:127–48.
Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318:1–14.
McLachlan AD. Protein Structure and Function. Annu Rev Phys Chem. 1972;23:165–92.
Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981;290:449–54.
Friedman JM. Structure, dynamics, and reactivity in hemoglobin. Science. 1985;228:1273–80.
Ewart KV, Lin Q, Hew CL. Structure, function and evolution of antifreeze proteins. Cell Mol Life Sci C. 1999;55:271–83.
Arévalo-Pinzón G, Curtidor H, Muñoz M, Patarroyo MA, Bermudez A, Patarroyo ME. A single amino acid change in the plasmodium falciparum RH5 (PfRH5) human RBC binding sequence modifies its structure and determines species-specific binding activity. Vaccine. 2012;30:637–46.
Schaefer C, Rost B. Predict impact of single amino acid change upon protein structure. BMC Genomics. 2012;13:S4.
Flower DR. Multiple molecular recognition properties of the lipocalin protein family. J Mol Recognit. 1995;8:185–95.
Skerra A. Engineered protein scaffolds for molecular recognition. J Mol Recognit. 2000;13:167–87.
Salzburger W. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol Ecol. 2009;18:169–85.
Rogers RL, Shao L, Thornton KR. Tandem duplications lead to novel expression patterns through exon shuffling in Drosophila yakuba. Begun DJ, editor. PLOS Genet. 2017;13:e1006795.
Chen Y, Jia L, Wei C, Wang F, Lv H, Jia J. Association between polymorphisms in the apolipoprotein D gene and sporadic Alzheimer’s disease. Brain Res. 2008;1233:196–202.
Helisalmi S, Hiltunen M, Vepsäläinen S, Iivonen S, Corder EH, Lehtovirta M, et al. Genetic variation in apolipoprotein D and Alzheimer’s disease. J Neurol. 2004;251:951–7.
Waldner A, Dassati S, Redl B, Smania N, Gandolfi M. Apolipoprotein D concentration in human plasma during aging and in Parkinson’s disease: a cross-sectional study. Parkinsons Dis. 2018;2018:1–7.
Wellenreuther M, Svensson EI, Hansson B. Sexual selection and genetic colour polymorphisms in animals. Mol Ecol. 2014;23:5398–414.
Carleton KL, Parry JWL, Bowmaker JK, Hunt DM, Seehausen O. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol Ecol. 2005;14:4341–53.
Flamarique IN, Bergstrom C, Cheng CL, Reimchen TE. Role of the iridescent eye in stickleback female mate choice. J Exp Biol. 2013;216:2806–12.
Bystriansky JS, Schulte PM. Changes in gill H+-ATPase and Na+/K+-ATPase expression and activity during freshwater acclimation of Atlantic salmon (Salmo salar). J Exp Biol. 2011;214:2435–42.
McCormick SD, Bradshaw D. Hormonal control of salt and water balance in vertebrates. Gen Comp Endocrinol. 2006;147:3–8.
Sakamoto T. Growth hormone and prolactin in environmental adaptation. Zool Sci. 2003;20:1497–8.
Foskett JK, Bern HA, Machen TE, Conner M. Chloride cells and the hormonal control of teleost fish osmoregulation. J Exp Biol. 1983;106:255–81.
Papetti C, Harms L, Windisch HS, Frickenhaus S, Sandersfeld T, Jürgens J, et al. A first insight into the spleen transcriptome of the notothenioid fish Lepidonotothen nudifrons: resource description and functional overview. Mar Genomics. 2015;24:237–9.
Huang Y, Chain FJJ, Panchal M, Eizaguirre C, Kalbe M, Lenz TL, et al. Transcriptome profiling of immune tissues reveals habitat-specific gene expression between lake and river sticklebacks. Mol Ecol. 2016;25:943–58.
Muschick M, Indermaur A, Salzburger W. Convergent evolution within an adaptive radiation of cichlid fishes. Curr Biol. 2012;22:2362–8.
Green SA, Simoes-Costa M, Bronner ME. Evolution of vertebrates as viewed from the crest. Nature. 2015;520:474–82.
Barlow-Anacker AJ, Fu M, Erickson CS, Bertocchini F, Gosain A. Neural crest cells contribute an astrocyte-like glial population to the spleen. Sci Rep. 2017;7:45645.
Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–17.
Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5:17.
Charlesworth D. Evolution of recombination rates between sex chromosomes. Philos Trans R Soc B Biol Sci. 2017;372:20160456.
Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic Acids Res. 2014;42:D749–55.
Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–44.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3.
Soderlund C, Bomhoff M, Nelson W. SyMAP v3.4: a turnkey synteny system with application to plant genomes. Nucleic Acids Res. 2011;39:e68.
Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513:375–81.
Rausch T, Zichner T, Schlattl A, Stutz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28:i333–9.
Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43:D257–60.
Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46:D493–6.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–8.
Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2008;4:1–13.
Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201.
Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30:S162–73.
Birzele F, Gewehr JE, Csaba G, Zimmer R. Vorolign-fast structural alignment using Voronoi contacts. Bioinformatics. 2007;23:e205–11.
Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–91.
Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–6.
Anisimova M, Yang Z. Multiple hypothesis testing to detect lineages under positive selection that affects only a few sites. Mol Biol Evol. 2007;24:1219–28.
McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol. 2008;9:102.
Hibbeler S, Scharsack JP, Becker S. Housekeeping genes for quantitative expression studies in the three-spined stickleback Gasterosteus aculeatus. BMC Mol Biol. 2008;9:18.
Zhang Z, Hu J. Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci. 2007;95:356–68.
Gu L, Xia C. Data from: cluster expansion of apolipoprotein D (ApoD) genes in teleost fishes. Dryad Digital Repository. 2018. https://doi.org/10.5061/dryad.39g63v2.
Tao W, Sun L, Shi H, Cheng Y, Jiang D, Fu B, et al. Integrated analysis of miRNA and mRNA expression profiles in tilapia gonads at an early stage of sex differentiation. BMC Genomics. 2016;17:328.
Acknowledgements
We thank Prof. Walter Salzburger for his valuable suggestions and also Prof. Deshou Wang for his help with the sampling of tilapia and for the laboratory support. We particularly thank Prof. Ziheng Yang for his valuable suggestions about positive selection detection using PAML. Many thanks to the kind computing support from Prof. Zhengwang Zhang. We appreciate the valuable suggestions and comments from the editor, Rafael Zardoya, and two anonymous reviewers very much. Many thanks also to Dario Moser, Heinz-Georg Belting, Jing Wei and Hua Ruan for their help with fish sampling. Many thanks for the help with fish dissection provided by Yang Zhao, Fabrizia Ronco, Attila Rüegg, Adrian Indermaur, Xianbo Zhang and He Ma. Many thanks to Michael Matschiner, Lukas Zimmermann, Peter Fields, Yuchen Sun, Yanyan Xu, Zihui Zhang and De Chen for their help and discussions. We also would like to express our thanks to Prof. Xiangjiang Zhan for the comments on the rebuttal letter. Many thanks to Prof. Anders Moller, Prof. Yang Liu, Dr. Jennifer McDowall, Dr. Juan Felipe Ortiz, Dr. Stephanie Birnbaum and Elsevier Language Editing Service for the corrections of English language.
Funding
The design of the study, samples and data collection, data analyses, manuscript writing and publication were supported by grants from the Doctoral Scholarship of University of Basel, Switzerland; the Postdoctoral Foundation from Southwest University, China; the China Postdoctoral Science Foundation (2018 M633311); the Chongqing Special Postdoctoral Science Foundation (XmT2018019) and the National Natural Science Foundation of China (31802283) to LG.
Availability of data and materials
The datasets supporting the results of this article are available in the Dryad repository, https://doi.org/10.5061/dryad.39g63v2 [116].
Author information
Authors and Affiliations
Contributions
LG discovered the expansion of the ApoD gene cluster, conceived and designed the experiments, performed data analyses and wrote the manuscript. LG and CX carried out protein 3D structure comparisons and cluster analyses. Both authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal experiments reported in this study have been approved by the cantonal veterinary office in Basel under permit number 2317, Switzerland; Guidelines for Care and Use of Laboratory Animals prescribed by the Regulation of Animal Experimentation of Chongqing, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gu, L., Xia, C. Cluster expansion of apolipoprotein D (ApoD) genes in teleost fishes. BMC Evol Biol 19, 9 (2019). https://doi.org/10.1186/s12862-018-1323-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-018-1323-x