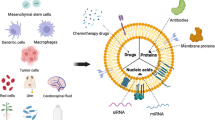
Abstract
Extracellular vesicles (EVs) have tremendous potential as nano/micron-sized drug delivery carriers. Their physical, chemical and biological characteristics distinguish them as unique carriers with specific pharmacokinetic, circulating metabolic, and biodistribution patterns in the delivery of therapeutic cargoes. They are critical mediators in the pathology of many diseases, including inflammatory diseases, fibrosis, and cancer, but they are also essential mediators in immunomodulation, cancer treatment, infectious defense, and tissue repair. In this review, we emphasize recent advances in oncology therapy using macrophage EVs, mesenchymal stem cell EVs, milk EVs, and plant EVs, as well as the advantages of EVs as delivery platforms and their prospective clinical applications and use.
Similar content being viewed by others
Introduction
Cancer is one of the most important burdens that humanity faces today. According to the World Health Organization, cancer is responsible for one out of every six fatalities worldwide. Despite increasing anticancer treatment efficacy, cancer mortality rates continue to increase worldwide (Maeda and Khatami 2018). Cancer's persistence is a significant impediment to its cure, and factors such as tumor recurrence, inappropriate physicochemical properties of anticancer drugs, and the related poor pharmacokinetic profile frequently affect treatment efficacy (Minchinton and Tannock 2006). Among these disadvantages, multidrug resistance (MDR) is one of the primary causes of poor efficacy, which is accomplished by decreasing cancer's responsiveness to common chemotherapeutic agents. Overexpression of efflux pumps (Giddings et al. 2021), hypoxic condition of the tumor microenvironment (TME) (Robey et al. 2005), epigenetic modifications (Assaraf et al. 2019) and the influence of cancer stem cells (Zhou et al. 2021) are the factors involved. Therefore, new therapeutic modalities are required to enhance the treatment of tumors, and EVs—an emerging nanomaterial—has a promising future as diagnostic and therapeutic carriers (Wang et al. 2021a; Zaimy et al. 2017; Dadwal et al. 2018; Jeswani et al. 2018; Feng et al. 2022).
EVs are small lipid membrane vesicles that are secreted into the extracellular space by almost all cell types. EVs play an essential role in information transport, transporting cargoes of packaged cellular proteins, nucleic acids, and metabolites from the parent cell to the recipient cell and mediating significant intercellular communication in physiological and pathological processes (Cianciaruso et al. 2019; Wang et al. 2020; Pu et al. 2021; Barone et al. 2022). Several isoforms of EVs regulate autocrine, paracrine, and endocrine functions (Record et al. 2014; Ela et al. 2013; Xu et al. 2013). EVs play a significant role in the progression of tumors. Both tumors and non-tumors secrete EVs, which can spread and impact different cell types at the initial tumor site as well as in distant organs. These EVs, which are frequently identified in the tissues and bodily fluids of cancer patients, are important mediators that mediate cellular communication between tumor cells and stromal cells in the local and distant metastatic microenvironment, coordinating multiple systemic pathophysiological processes (Becker et al. 2016). Thus, EVs can be used not only as diagnostic markers for tumors but also as anti-cancer therapeutic agents and anti-cancer vaccine delivery vehicles. As the function of EVs has been studied, there is increasing evidence that EVs can play an important part in disease progression (Feng et al. 2022; Wang et al. 2020, 2021b). Therefore, a comprehensive understanding of EVs and their role in disease pathology and treatment will provide us with novel approaches to the treatment of tumors in the coming clinical settings.
Extracellular vesicles
Pan and Johnstone described EVs as a heterogeneous collection of membrane structures containing intracellular molecules that are widely present in biological fluids (Street et al. 2012; Reinhardt et al. 2012; Taylor and Gerçel-Taylor 2005; Runz et al. 2007; Fernández-Llama et al. 2010; Chen et al. 2010; Admyre et al. 2007; Levänen et al. 2013; Pan and Johnstone 1983). Earlier research indicated that EV release was part of a processing mechanism for removing unwanted material from the cell. As research advanced, it was discovered that the release of EVs was also an important mediator of intercellular communication, involved in both normal physiological and pathological processes. EVs are classified into two types based on their size: 50–1000 nm vesicles produced by plasma membrane germination, which include exfoliated vesicles, microparticles and microvesicles (MVs), and apoptotic vesicles; and 40–160 nm sized exosomes from somatic cells (Kubo 2018).
In the cytoplasm, EVs are produced and degraded. Early endosomes are created via lipid raft-mediated endocytosis, while endosomes are formed through invagination of the plasma membrane. Early endosomes split into circulating endosomes after a portion of them fuses with endocytic vesicles (Morelli et al. 2004). The remaining early endosomes germinate inward and absorb nucleic acids, proteins and other substances to form intraluminal vesicles (ILVs), which gradually develop into late endosomes—the so-called multivesicular bodies (MVBs) (Stoorvogel et al. 1991). These MVBs can combine with lysosomes for degradation or with cell membranes to release exosomes into the extracellular environment (Grant and Donaldson 2009). Recent evidence suggests that Rab5–Rab7 GTPases are converted by SAND family proteins during the maturation of endosomes (Rink et al. 2005). The critical switch for Rab conversion is SAND-1/Mon1, which displaces RABX5 from the endosomal membrane, interrupts the positive feedback loop of RAB5 activation, and extends its recruitment by interacting with the HOPS complex, allowing RAB7 to be recruited at the same membrane (Poteryaev et al. 2010; Singh et al. 2014) (Fig. 1).
Advantages of EVs as a drug delivery system (DDS)
Size (Lerch et al. 2013), shape (Mitchell et al. 2021) and surface charge (Jeon et al. 2018) are presently known to influence the internalization of nanomaterials by cells. Furthermore, the stiffness of nano/micro materials, whether natural or engineered, is an essential factor in cellular phagocytosis (Dai et al. 2019). Exosomes have many unique characteristics, including good biocompatibility, low immunogenicity, minimized size, abundant surface proteins, and equipped molecular content, indicating their potential as excellent drug delivery carriers. EVs have a superior shuttling ability to cross biological barriers, and their specific homing affinity for their initial organ of origin helps to avoid liver traps (Qiao et al. 2020), and they can also effectively cross the blood–brain barrier (BBB) (Betzer et al. 2017; Shahjin et al. 2020) and the autophagy-lysosome pathway (Skotland et al. 2017; Polanco et al. 2021). Furthermore, EVs may remain in tumors for longer than other nanoparticles (Abello et al. 2019), have lower clearance rates (Belhadj et al. 2020), are more precisely localized, are more widely available, can be completely degraded and recovered in vivo, and have significant therapeutic effects as natural biologics rather than synthetic materials (Fu et al. 2021).
Our frequently used pharmaceutically active biomolecules include chemotherapeutic drugs, hormones, peptides, cytokines, proteins, and therapeutic oligonucleotides, and their physicochemical properties and route of administration have a significant impact on their biosafety and efficacy. EVs have a better safety profile, higher capacity, and the presence of small functional peptides on their surface for improved targeting as a medicinal carrier when compared to other carriers (Zhang et al. 2020). Furthermore, in clinical applications without delivery assistance, many conventional anticancer drugs, such as paclitaxel, doxorubicin, and curcumin, show low water solubility, short half-life, and poor stability, necessitating frequent high-dose administration. Loading the drug into EVs improves drug stability and bioavailability, maximizes retention in the target lesion, and greatly improves efficacy when compared to free delivery (Walker et al. 2019; Lin et al. 2019). Figure 2 shows the current common process of EVs as drug carriers against tumors.
In conclusion, as emerging nanotechnologies, the clinical application of EVs could provide a versatile delivery platform for next-generation therapies and usher in a new age of cell-free therapy. Needless to say, tailoring preventive or therapeutic interventions to those who will profit the most adds significant value to the development and practice of precision medicine.
Emerging EVs technology in tumor treatment
Role of EVs in tumor microenvironment (TME)
TME plays a crucial part in the development and prognosis of tumors. Extracellular matrix (ECM), stromal cells, various infiltrating immune cells, and cancer cells all work together to generate a dynamic, heterogeneous environment known as a solid tumor (Galli et al. 2020). Because of interactions between various components that resulted in ongoing epigenetic and functional plasticity, tumor and stromal cells have co-evolved. There are several methods to treat tumors by remodeling TME, and therapeutic approaches that target TME are a reliable strategy. This treatment can cross the physical barrier, but it is challenging to avoid causing excessive alterations to its supramolecular structure, which may lead to the spread of metastases in vivo (Chen et al. 2022a; Chiang and Lo 2014).
Due to the characteristics of EVs, this makes it crucial in TME. Tumor-derived EVs affect immune system homeostasis primarily by inducing immunosuppressive changes that shield the tumor. They can also stimulate epithelial cells to release factors involved in EMT, which results in the spread of tumor cells. Extracellular matrix remodeling and tumor invasion can also be caused by tumor-derived EVs (Becker et al. 2016). Therapeutic EVs that target TME have emerged in this context. When applied to tumor cells or solid tumors, EVs that can modify TME or EVs containing drugs have been found to have positive therapeutic effects. The impact of tumor-derived EVs and therapeutic EVs on the TME is depicted in Fig. 3.
Therapeutic extracellular vesicles (EVs) offer multiple advantages, such as enhancing the TME's immune system to inhibit tumor progression. They can efficiently deliver chemotherapeutic drugs into the TME, thereby increasing the therapeutic efficacy of these drugs. Moreover, they can regulate specific protein levels in the TME to suppress tumor progression and directly act on tumors to decelerate their growth. Conversely, tumor-derived EVs can stimulate the TME's immune system to promote tumor progression and alter factors within the TME that encourage tumor invasion and metastasis
Macrophage-derived EVs
Macrophages (Mφ) are highly heterogeneous immune cells that are widely involved in the pathological processes of various diseases and are closely linked to the tumor microenvironment and cancer cell metabolism. Macrophages are classified into two kinds based on their microenvironment: classically activated macrophages (CAMs, M1-type macrophages) and alternatively activated macrophages (AAMs, M2-type macrophages) (Mehla and Singh 2019; Mackaness 1962; Liu et al. 2020). In general, M1φ primarily functions to counteract early inflammation with danger signals, after which they tilt toward M2-like macrophages, whereas M2φ primarily functions as immunomodulators rather than as efficient "killers" (Reiner 2009). Mφ-EVs are important mediators not only in the pathology of various diseases (e.g., inflammatory diseases, fibrosis, and cancer), but also in immune regulation, cancer therapy, infectious defense, and tissue repair due to the nature of Mφ. Furthermore, M1-EVs can repolarize M2 tumor-associated macrophages (TAM) to M1φ, resulting in a pro-inflammatory microenvironment and enhancing the antitumor effects of checkpoint inhibitor treatment (Choo et al. 2018; Wang et al. 2021c).
There is evidence that loading drugs into macrophage-derived EVs to form EVs-drug complexes and then operating the complexes on tumor cells or tissues leads to improved tumor cell targeting, represents the fundamental building block for treating drug-resistant malignancies, and reduced tumor metastasis (Lou et al. 2022). Tian et al. (Tian et al. 2014) employed mouse immature dendritic cells (imDCs) to generate exosomes. ImDCs were engineered to express a well-characterized exosomal membrane protein (Lamp2b) coupled to an iRGD peptide specific for αv integrin (CRGDKGPDC). The results indicate that iRGD exosomes efficiently target and transport doxorubicin (DOX) to αv integrin-positive breast cancer cells in vitro. Wang et al. (Wang et al. 2019) found that loading paclitaxel (PTX) onto M1EVs to form a PTX-M1EVs complex and administering it to a mouse breast cancer model not only transported PTX into the tumor tissue but also increased the drug's anticancer effects. Zhao et al. (Zhao et al. 2021) found that loading gemcitabine (GEM) and deferasirox (DFX, which improves the therapeutic efficacy of GEM sensitivity) onto M1EVs formed a GEM-DFX-M1EVs complex, which greatly increased the beneficial impact on GEM-resistant PANC-1/GEM cells and 3D tumor spheroids. Wang et al. (2022) loaded the hydrophilic hypoxia-activating prodrug AQ4N (A) into the inner core of M1EVs after functionalizing the membranes with two hydrophobic agents (chemical excitation source CPPO(C) and photosensitizer Ce6(C)). And loaded the hydrophilic hypoxia-activating prodrug AQ4N (A) into the inner core of M1EVs to form the CCA- M1EVs complex, which was found to have a powerful therapeutic effect in Glioblastoma multiforme. Jorquera-Cordero et al. (2022) pretreated M1 with hyaluronic acid (HA) and the β-blocker carvedilol (CV) and then obtained the treated EVs of M1 The resulting MM1EVs, on which DOX was further loaded to form DOX-MM1EVs, were found to enhance the therapeutic effect of the complex against metastatic tumors and the antitumor effect of DOX was enhanced by the synergistic effect of HA and CV. Kim et al. (2016) extracted RAW 264.7 cell-derived exosomes (EXOs), loaded with the anticancer drug PTX to obtain EXOs-PTX complexes, and applied the complexes to a mouse lung metastatic cancer model, which showed high anticancer efficacy. Subsequently, Kim et al. (2018) further optimized this novel nanoformulation based on this by incorporating amino acetamide-polyethylene glycol (AA-PEG) carrier molecules to target the sigma receptors overexpressed by lung cancer cells. The resulting AA-PEG-EXOs-PTX complex with high loading capacity improved the tumor therapeutic efficacy. Li et al. (2020) developed an Mφ-EVs-encapsulated poly (lactic acid-ethanolic acid) nano platform for a DOX-targeting carrier in Triple-negative breast cancer (TNBC). The results showed that the nanocarrier significantly improved the efficiency of cellular drug cellular uptake, significantly increased tumor targeting efficacy, enhanced inhibition of tumor growth, and induced strong apoptosis of the tumor. Rayamajhi et al. (2019) used sEVs to hybridize with manufactured liposomes to create hybrid exosomes (HE) smaller than 200 nm in size. DOX was then loaded into HE and the drug-loaded HE was found to be more toxic to cancer cells. These findings point to Mφ-EVs as a promising drug delivery strategy for tumor therapy.
Allogeneic EVs obtained from immune cells or tumors act on tumors to stimulate antigen presentation and T cells in vivo, resulting in the improved antitumor activity of T cells (Xu et al. 2020; Shi et al. 2020), making Mφ-EVs a viable option for tumor vaccines. Certain macrophage-released factors (e.g., insulin-like growth factor, IGF-1) can play an important role in vaccine-induced immune responses by regulating immune cell homeostasis (Yoon et al. 2017). M1EVs can also cause a shift in TAMs from anti-inflammatory M2-like to pro-inflammatory M1-like. Seena et al. (Jean-Toussaint et al. 2021; McDonald et al. 2014) stimulated RAW 264.7 cells with lipopolysaccharide (LPS), collected their secreted sEVs, and prophylactically acted on neuronal cells and animal models, observing the downregulation of pro-inflammatory target genes, which aided in the reduction of chronic pain. Cheng et al. (2017) prophylactically administered a peptide vaccine combined with M1EVs subcutaneously in a melanoma animal model and induced a stronger and more sustained immune response, showing the peptide vaccine induced a stronger and longer-lasting immune response in a melanoma animal model, demonstrating stronger anti-tumor effects. Tumor vaccines can overcome tumor-induced immunosuppression, enhance immunogenicity, activate the patient's immune system, and induce cellular and humoral immune responses in the body. It is worth mentioning that tumor vaccines usually need to be used in combination with other therapies or adjuvants to improve the extent, breadth, quality, and longevity of the vaccine immune response (Finn 2008). The roles of Mφ-derived EVs in tumors are summarized in Table 1.
As mentioned above, Macrophage-derived EVs show great promise for applications that can easily evade immunological surveillance, have the ability to enhance or suppress immune activity, and may somewhat reduce some of the risks associated with whole cell therapy (Schulert and Grom 2015). In addition, compared to Mφ, EVs are nanoscale, can remain in circulation for a long time and cross the biological barrier, enough to provide tumor suppression with the same targeting and intrinsic function. All described strategies demonstrate the design of Mφ-EVs through different technical approaches to further enhance their potential application in cancer therapy. As we had hypothesized, these nanoparticles exhibit excellent adaptability to various modification processes, thus showing great versatility. However, after realizing the pathogenicity of Mφ-EVs in disease, further study is necessary to figure out how to appropriately regulate their release or their embedded content to prevent the development and progression of disease. Moreover, macrophages are able to react differently to various microenvironments, a characteristic that seems to be important for their potent therapeutic effects in disease. However, unlike whole-cell therapy, Mφ-EVs alone do not have similar effects, necessitating precise Mφ-EV property modulation for various diseases or conditions prior to intervention. Since reprogramming or reconstituting Mφ-EVs for disease treatment seems promising and the low yield and/or limited functionality of Mφ-EVs using conventional methods, there is an urgent need for new isolation or manufacturing methods that enable higher yield/functionality of EVs. Overall, the potential of macrophage-derived EVs for immunomodulation and disease intervention is quite promising.
Mesenchymal stem cells-derived EVs
MSCs, discovered about 40 years ago (Friedenstein et al. 1970), are self-renewing cells found in various tissues (Murphy et al. 2013). They can be isolated from the umbilical cord, cord blood, adipose, placenta, dental pulp, and spinal cord tissues (Rezaie et al. 2022). MSCs are pluripotent cells that can differentiate into adipocytes, myocytes, osteocytes, and chondrocytes (Zuk et al. 2001; Minguell et al. 2001; Nombela-Arrieta et al. 2011). MSCs have a unique immunophenotypic capacity, tissue repair capacity, immunomodulatory capacity, and homing capacity to cross multiple biological barriers (Wang et al. 2014a; Ankrum et al. 2014). Furthermore, based on the paracrine fusion of MSCs with target cells, their mechanism of action was first shown to be enhanced by the cooperation of soluble bioactive factors, such as cytokines and growth factors. However, shortly thereafter, it was found that the amplification of their mechanism of action in the context of secretion was dependent on their EVs (Meldolesi 2022).
MSCs-EVs have been shown to play an important role in cancer development, but their role in tumors remains controversial due to their heterogeneity or the status of the cancers they affect. EVs derived from MSCs isolated from human bone marrow (BM) increase the expression of VEGF in gastric cancer cells, promoting the growth of gastric cancer in vivo (Zhu et al. 2012). BM-MSCs-EVs from patients with multiple myeloma (MM) contains higher levels of oncogenic proteins, cytokines, and adhesion factors, all promoting tumor growth (Roccaro et al. 2013). BM-MSCs-EVs were also found to inhibit proliferation and promote apoptosis in hepatocellular carcinoma, Kaposi's sarcoma, and ovarian tumor cell lines (Bruno et al. 2013). EVs derived from MSCs isolated from human umbilical cords (UC) were found to induce drug resistance in gastric cancer cells through activation of the CaM-Ks/Raf/MEK/ERK pathway in both in vivo and in vitro experiments (Ji et al. 2015). EVs derived from human umbilical cord Wharton's jelly MSCs (hWJ-MSCs) may reverse the development of bladder cancer cells by downregulating phosphorylation of Akt protein kinase and upregulating cleaved caspase-3 (Wu et al. 2013). EVs derived from adipose tissue (AT)-derived MSCs were shown to reduce Bcl-xL activity, promote apoptosis through the caspase-3/7 pathway, and inhibit prostate cancer by delivering miR-145 (Takahara et al. 2016). BM-MSCs-EVs and UC-MSCs-EVs reduced tumor cell proliferation and induced apoptosis in cancer cells in the U87MG glioblastoma cell line, whereas AT-MSCs-EVs promoted cancer cell proliferation (Fattore et al. 2015). As MSC culture conditions may affect the overall characteristics of the secreted vesicles, The inconsistency between these results suggests that more studies are needed to develop a personalized MSC culture condition.
MSCs also have great potential as drug carriers. Del Fattore et al. (2015) delivered vincristine (VCR) to UC-MSCs-EVs in a glioblastoma cell line and found increased cytotoxicity to cancer cells compared to free drug and unmodified EVs. Pascucci et al. (2014) pretreated MSCs with PTX and found that MSCs released increased MVs and expressed strong antiproliferative activity on the human pancreatic cancer cell line CFPAC-1. Munoz et al. (2013) discovered that MSC-derived exosomes loaded with anti-miR-9 reversed the expression of multidrug transporter proteins in drug-resistant glioblastoma multiforme cells and reversed chemoresistance. Katakowski et al. (2013) injected MSCs-EVs expressing miR-146b into a rat brain tumor model and found that tumor cell growth, migration, and invasion were inhibited, greatly reducing the development of glioma xenografts. Shimbo et al. (2014) delivered MSCs-EVs encapsulated with miR-143 to osteosarcoma cells, which significantly reduced the migration of osteosarcoma cells. Lou et al. (2015) transfected miR-122 into AT-MSCs and isolated EVs delivered miR-122 into hepatocellular tumor cells, which enhanced the sensitivity of tumor cells to chemotherapeutic agents in vitro and in vivo through the alternation of gene expression and tumor proliferation. O'Brien et al. (2018) subjected MSCs-EVs encapsulated with miR-379 to breast cancer and discovered that breast cancer progression was significantly inhibited in vivo. Li and Wu (2023) delivered MSCs-EVs encapsulated with miR-598 to straight non-small cell lung cancer (NSCLC) and showed that proliferation and migration of NSCLC cells were inhibited in vivo and in vitro. In a mouse model of pancreatic cancer, Kamerkar et al. (2017) found that electroporated MSCs exosomes with siRNAs targeting oncogenic KRAS inhibited cancer progression and significantly improved overall survival. Bliss et al. (2016) found that breast cancer cells prompted MSCs to secrete exosomes with the characteristics of specific miRNAs, such as miR222/223, thereby reducing the therapeutic effect. Based on this discovery, MSCs encapsulated with antagomiR-222/223 were designed and applied to breast cancer cells to put them into dormancy, resulting in improved chemotherapy sensitivity and survival. The roles of MSCs-derived EVs in tumors are summarized in Table 2.
In conclusion, these findings demonstrate that MSCs-EVs has the potential to be effective in drug and/or nucleic acid delivery. Moreover, MSCs are a promising nanocarrier due to their superiority in large-scale EV production, clinical applicability (Yeo et al. 2013), and their safety profile. However, since the role of MSCs is still controversial and different MSCs may produce different EVs that are different in size and function, we need to select the appropriate MSCs for different tumors. In addition, cancer stem cells (CSCs) are also widely present in the ecological niche and are closely related to cancer progression (Kim et al. 2021; Pan et al. 2017), so targeting CSCs-EVs while searching for a new therapeutic modality would be an innovative approach.
Milk-derived EVs
Milk is a complex fluid with multiple immunoreactive capabilities of cells and soluble proteins that provide immunity and influence the immune system (Cacho and Lawrence 2017), and milk-derived EVs (MEVs) have also been shown to have the ability to influence the immune response (Admyre et al. 2007; Sun et al. 2013; Nordgren et al. 2019). Furthermore, EVs from different dairy products have similar bidirectional immunomodulatory effects as raw milk EVs, not only promoting normal macrophage proliferation, increasing NO and cytokine (IL-1β, IL-6, and TNF-α) levels, and regulating immune-related miRNAs, but also inhibiting the LPS-induced TLR4/NF-κB pathway and inflammatory cytokines (Li et al. 2022).
Milk-derived EVs were found to reduce primary tumor growth in mouse models of colon and breast cancer, but milk-derived EVs accelerated tumor metastasis in mouse models of breast and pancreatic cancer, suggesting that milk-derived EVs (at reasonable concentrations or for reasonable periods) can be used for tumor therapy judiciously (Samuel et al. 2021). Curcumin has limited pharmaceutical use despite its many bioactive and therapeutic properties due to its poor water solubility, stability, and low systemic bioavailability. Curcumin loading onto milk EVs acting on a human epithelial home rectal adenocarcinoma cell line (Caco-2) revealed that encapsulating curcumin into EVs improved its stability, solubility, and bioavailability (Vashisht et al. 2017). He et al. (2022) addressed the problem of immature nanofabrication and inadequate bioavailability of chiral nanostructures, and the chiral peptide Au(I) infinite covalent polymer (DPAICP) was camouflaged using MEVs feasible camouflage technology to construct a complex with good drug properties in the B16F10 homozygous malignant melanoma model, LLC Lewis orthotopic transplantation lung cancer model and patient-derived orthotopic xenograft (PDOX) colon cancer mouse model to restore the p53 signaling pathway for cancer treatment. In addition, oral administration of the complex further activated T cells and enhanced the effect of anti-PD1 immunotherapy. Multimodal image-guided photothermal therapy (PTT) has great potential for cancer treatment due to its low side effects and efficacy. Jing et al. (2023) designed a therapeutic nanoprobe for positron emission tomography/computed tomography/near-infrared fluorescence (PET/CT/NIRF) imaging and image-guided photothermal therapy (PTT) for colon cancer by taking the anti-inflammatory ability and tumor-sparing ability of goat milk-derived EVs (GEVs). Compared with indocyanine green (ICG) mediated PTT, PTT mediated by GEVs/ICG nanoprobes triggered stronger anti-tumor immune and inflammatory responses. In addition, nanoprobes can attenuate PTT-induced inflammation due to the anti-inflammatory efficacy of GMVs. The roles of milk-derived EVs in tumors are summarized in Table 3.
Milk can be a source for harvesting large amounts of EVs due to its biocompatibility and cost-effective potential, and MEVs have great potential as drug carriers for hydrophilic and lipophilic drugs, including chemotherapeutic drugs. This nanodevice technology could overcome the limitations associated with low oral bioavailability of chemopreventive and chemotherapeutic drugs and reduce the total administered dose, thereby minimizing or eliminating the toxicity generally associated with high doses. MEVs formulation of drugs not only improves biological efficacy but targeting tumors with ligand-functionalized MEVs can further improve specificity and eliminate off-target side effects of the drug. The multiple properties of MEVs, such as physical and biological stability, tolerability, scalability of manufacturing processes, versatility in the drugs they can carry, and the ability to functionalize with ligands, make them ideal candidates for drug delivery with a wide range of therapeutic uses. However, further studies are needed to rule out the potential toxicity of the long-term use of MEVs.
Plant-derived EVs
Plant-derived EVs (PDEVs) are biologically and morphologically similar to mammalian-derived EVs (MDEVs) (An et al. 2007) but have different nucleic acid, lipid, and protein contents compared to mammalian EVs (Zhang et al. 2016a; Alfieri et al. 2021), and sometimes contain plant-specific specific chemical components associated with plants (Ji et al. 2016; Cui et al. 2020). PDEVs can be isolated from the sap of plants (Zhao et al. 2018; Pocsfalvi et al. 2018; Xiao et al. 2018) and the pulp or roots of plants (Teng et al. 2018; Wang et al. 2013; Zhuang et al. 2015). In addition, EVs can be isolated from the seeds of plants (Regente et al. 2009) and dried plant material (Woith and Melzig 2019). PDEVs are highly stable under various environmental conditions, are enriched with enzymes essential for cell wall remodeling and proteins with antifungal and antibacterial properties (Canal and Pinedo 2018; Liu et al. 2021; Regente et al. 2017; Rutter and Innes 2018), and are resistant to degradation by certain digestive enzymes (Akuma et al. 2019). The suitability of PDEVs as alternatives to MDEVs is currently being investigated, allowing researchers to circumvent the technical limitations of MDEVs. In terms of their large-scale production, PDEVs have great potential for applications in disease treatment and in the development of nanocarrier DDS capable of administering various doses due to their physiological, chemical, and biological properties (Zhang et al. 2016a; Yang et al. 2018; Rome 2019; Jimenez-Jimenez et al. 2019). Due to these beneficial properties, PDEVs outperform mammalian EVs in terms of therapeutic efficacy.
Phytochemicals are effective in inhibiting tumor proliferation (Abu-Dahab et al. 2014; Fiore et al. 2006), and PDEVs, to some extent, share this property. It has been shown that PDEVs can alter immune regulation in terms of the tumor microenvironment, including macrophages (Wang et al. 2014b; Mu et al. 2014), and that PDEVS play an important role in the treatment of inflammation (Zhuang et al. 2015; Zhang et al. 2017) and tumors (Robertis et al. 2020; Chen et al. 2022b). A population of nanovesicles isolated from Citrus fruit exhibited a strong inhibitory effect on chronic granulocytic leukemia (CML) by inhibiting angiogenesis and TRAIL-mediated apoptosis (Raimondo et al. 2015). Lemon-derived EVs (LDEVs) elevates the level of GADD45a, leading to S-phase arrest and apoptosis in gastric cancer cells, thereby inhibiting gastric cancer progression (Yang et al. 2020). Apple-derived NPs (APNPs) affect intestinal transport proteins as well as human Caco-2 cells (Fujita et al. 2018). EVs derived from hairy root (HR) cultures obtained from Salvia dominica exhibit selective and potent pro-apoptotic activity against pancreatic and breast cancer cells (Boccia et al. 2022). Cannabidiol-derived EVs (CBDEVs) caused G0/G1 phase arrest in the hepatocellular carcinoma cell cycle and significantly induced cell death in the hepatocellular carcinoma cell lines HepG2 and Huh-7 cells by activating the mitochondria-dependent apoptotic signaling pathway (Tajik et al. 2022). EVs derived from Dendropanax morbifera and Pinus densiflora have cytotoxic effects on breast and skin tumor cells, and the combination of both can increase the toxic effects to some extent (Kim et al. 2020). Tea leaf-derived EVs (TLEVs) can increase ROS levels in breast cancer cell lines, trigger mitochondrial damage in tumor cells and block the cell cycle, causing tumor cell apoptosis and thus slowing tumor growth (Chen et al. 2023). Ginseng-derived nanoparticles (GDNPs) can alter TLR4 and MyD88 signaling thereby altering M2 macrophage polarization, thereby inhibiting melanoma growth (Cao et al. 2019).
The specificity of plant-derived exosomes given by specific orientations and their ability to manipulate genes for treatment, transfer hydrophobic drugs, and escape immune attacks, make them suitable for collaboration for future medical applications in DDS (Cong et al. 2022). Drug-targeting systems using PDEVs as carriers enable efficient targeted drug delivery. Exogenous bovine serum albumin (BSA) and heat shock protein (HSP70) were delivered to human peripheral blood mononuclear cells and colon cancer cells after loading on grapefruit-derived extracellular vesicles (GEEVs), revealing that protein-loaded PDEVs significantly improved exogenous protein uptake by human cells compared to the same proteins without EVs (Garaeva et al. 2021). Ginger-derived EVs (GDEVs) and folic acid (FA), a small molecule ligand that can bring nanoparticles into cells within 15–30 min via a receptor-mediated endocytic pathway. A complex is formed, exogenous surviving protein siRNA is loaded onto the complex, and the siRNA-FA-3WJ-GDEVs complex is intravenously delivered to the tumor. Inhibition of tumor growth was observed on a xenograft model, promoting apoptosis of tumor tissue cells and significantly reducing tumor weight (Li et al. 2018). It was also found that GDENs lacking FA modification was much far likely to be taken up by cancer cells in vivo compared to FA-modified GDENs (Li et al. 2018). Besides, PDEVs can alleviate intestinal inflammation and have great potential in the treatment of inflammatory bowel disease (IBD) and colitis-associated cancer (CAC). DOX was loaded on FA-modified ginger-derived nanocarriers (GDNVs) to form DOX-FA-GDNVs complexes for targeted delivery to Colon-26 tumors. The results showed enhanced inhibition of tumor growth compared to the free drug (Zhang et al. 2016b). The roles of plant-derived EVs in tumors are summarized in Table 4.
PDNVs can deliver nucleic acids and drugs, are more biocompatible than synthetic carriers and can be mass-produced, making them attractive for pharmaceutical applications in the medical field. However, the limitations of PDEVs are their unknown mechanism of uptake action and the many biomolecules that may cause side effects due to the versatility of these components. Little is known about plant-derived EVs and their function, origin, formation, and secretion have not been thoroughly studied (An et al. 2007; Borges and Martienssen 2015). Although it has been established that PDEVs are first transported to the liver and other organs after uptake (Gioia et al. 2020; Deng et al. 2017), the mechanisms of vesicular uptake of plant-derived EVs by mammalian cells are clathrin-mediated endocytosis, phagocytosis, macropinocytosis, and plasma or endosomal membrane fusion of EVs (Zhuang et al. 2015). However, the findings of these studies only explain a portion of the pathways by which PDEVs enter mammalian cells, and much more research is needed to better understand the therapeutic potential of PDEVs. Another unanswered question is whether any fraction derived from plants can be considered equivalent. We don't know if nanoparticles from roots, stems/stalks, or leaves make a difference, or if they do, so this is an issue that deserves attention in future studies. The pharmacokinetics, pharmacodynamics and safety of PDEVs in a wide range of animal disease models also need to be explored further. Overall, although phytochemicals are effective in inhibiting tumor proliferation, the application of PDNVs in these areas is still empirical and further experiments on the stability and function of PDEVs in various settings are still needed, and this information may help to better understand the future application of PDEVs in therapy.
Summary
In summary, extracellular vesicles (EVs) possess a range of unique properties and play crucial roles in various biological processes such as angiogenesis, apoptosis, cellular homeostasis maintenance, antigen delivery, inflammatory responses, and intercellular signaling. These attributes render them highly promising for oncological disease applications. Their multiple unique properties make them particularly advantageous for tumor diagnosis and treatment. Nevertheless, it is important to note that these very properties also present challenges for EV application. Among the EVs currently under study, macrophage-derived EVs can evade immune surveillance and exhibit potent immune activity, making them highly effective in tumor therapy. However, their low yield and limited functionality render them unsuitable for large-scale production. While mesenchymal stem cell (MSC)-derived EVs, milk-derived EVs, and plant-derived EVs can be produced on a larger scale and possess distinct properties, they are not yet well understood, and outcomes vary depending on the source. In conclusion, despite their vast potential in oncology therapy, EVs face several challenges that need to be addressed. Technological advancements are expected to resolve these issues in the future. A comprehensive overview of the advantages and disadvantages of EVs in the context of tumor therapy development is provided in Table 5.
Discussion and prospects
Cancer is a significant burden in all countries, and it is rising with economic growth. However, there are currently no effective therapeutic approaches. Intercellular communication is critical for maintaining homeostasis in vivo in single cells and multicellular organs, and EVs contain a variety of molecules of communication substances with multiple benefits that have a significant impact on cancer cell communication with local cells and distant organs, participating in many physiological and pathological pathways. This makes it possible to explore new approaches through EVs for safe and effective treatment. The ability of Mφ-EVs to exploit the characteristics of donor cells, such as convergent chemotaxis toward inflammatory tissues and tumor sites via macrophagic features, helps to achieve personalized and effective anti-cancer therapy. In addition to this, allogeneic exosomes derived from macrophages or tumors allow us to harvest tumor vaccines. MSCs are easily obtainable, and MSC-EVs have properties such as low immunogenicity and high biocompatibility, as well as the ability to be efficiently modified to selectively reach target tissues. MEVs preserve the bioactive molecules sequestered in milk, inherent properties that allow them to survive in harsh, degradable environments and to be distributed in peripheral tissues. This is encouraging evidence that the various cargoes carried by MEVs are epigenetically regulated in the receptor. Medicinal plants contain specific phytochemicals, proteins, and nucleic acids, making the study of PDEVs especially appealing given the variety and abundance of medicinal plants, as well as the advantage of being consistently isolated in large quantities. When these emerging EV technologies are combined, they will play a critical role in future oncology treatments. It's worth noting that one study discovered that delivering mouse self-derived EVs loaded with exogenous siRNA to the mouse brain was significantly effective in the treatment of Alzheimer's disease (Alvarez-Erviti et al. 2011). Is it possible to achieve promising results in tumor treatment by collecting and modifying self-derived EVs from patients? Is it also possible to combine the use of the aforementioned EVs with unexpected results? In conclusion, with appropriate modifications, these nanovesicles can be used for tumor therapy and antitumor drug development.
Emerging EVs technologies offer new therapeutic ideas for the treatment of cancer through metabolic reprogramming techniques. However, these nanotechnologies are increasingly being recognized as a complex, tightly regulated phenomenon that affects and/or is influenced by various characteristics of tumor cells and TMEs. Notably, we need to consider not only the complex regulation of metabolism by internal factors but also regulation by external factors, which frequently affect the efficacy of the treatment. Furthermore, We need to further optimize the standardized methods for large-scale isolation and purification because the current conventional methods of EV extraction and purification are ineffective. Finding the best source of EVs is important considering there is instability in the EV source and different EVs contain different cargoes. We need to identify methods that can quickly and accurately quantify and characterize EVs as preserving EVs is a challenging process. We also need to conduct more in-depth studies on the pharmacokinetics of EVs in vivo, safe and efficient methods of EVs delivery to the body or targeted therapeutic sites, and optimal doses for treating various diseases. The impact of genetic engineering techniques on EVs remain largely unknown, nanotechnology for cancer has not yet matured, and many studies have not yet been converted from experimental to clinical stages. Clarification of these key concerns would make EVs a more fantastic novel cell-free therapeutic strategy for the clinical treatment of tumors, factors that have largely impeded further biomedical applications. Certainly, EVs have a promising future. New techniques for EV isolation and purification are under study, high quality and easy storage technologies will emerge, the understanding and application of EVs will become clearer, and the application of nanotechnology will become more proficient and will provide more practical options for future exosome applications. In conclusion, the emerging EVs technology is a promising means of treating oncology, and we need a deeper understanding in order to address the challenges and opportunities presented by the rapidly evolving cancer nanotechnology and biology.
Availability of data and materials
Not applicable.
References
Abello J, Nguyen TDT, Marasini R, Aryal S, Weiss ML (2019) Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 9(8):2325–2345
Abu-Dahab R, Abdallah MR, Kasabri V, Mhaidat NM, Afifi FU (2014) Mechanistic studies of antiproliferative effects of Salvia triloba and Salvia dominica (Lamiaceae) on breast cancer cell lines (MCF7 and T47D). Z Naturforsch C J Biosci 69(11–12):443–451
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M et al (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179(3):1969–1978
Akuma P, Okagu OD, Udenigwe CC (2019) Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front Sustain Food Syst 3(23)
Alfieri M, Leone A, Ambrosone A (2021) Plant-derived nano and microvesicles for human health and therapeutic potential in nanomedicine. Pharmaceutics 13(4):498
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
An Q, van Bel AJ, Hückelhoven R (2007) Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav 2(1):4–7
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32(3):252–260
Assaraf YG, Brozovic A, Gonçalves AC, Jurkovicova D, Linē A, Machuqueiro M et al (2019) The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat 46:100645
Barone A, d’Avanzo N, Cristiano MC, Paolino D, Fresta M (2022) Macrophage-derived extracellular vesicles: a promising tool for personalized cancer therapy. Biomedicines. 10(6):1252
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D (2016) Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30(6):836–848
Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X et al (2020) A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles 9(1):1806444
Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G et al (2017) In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 11(11):10883–10893
Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K et al (2016) Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res 76(19):5832–5844
Boccia E, Alfieri M, Belvedere R, Santoro V, Colella M, Del Gaudio P et al (2022) Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun Biol 5(1):848
Borges F, Martienssen RA (2015) The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol 16(12):727–741
Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G (2013) Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 22(5):758–771
Cacho NT, Lawrence RM (2017) Innate immunity and breast milk. Front Immunol 8:584
Cao M, Yan H, Han X, Weng L, Wei Q, Sun X et al (2019) Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer 7(1):326
Chen C, Skog J, Hsu CH, Lessard RT, Balaj L, Wurdinger T et al (2010) Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10(4):505–511
Chen W, Yuan Y, Li C, Mao H, Liu B, Jiang X (2022a) Modulating tumor extracellular matrix by simultaneous inhibition of two cancer cell receptors. Adv Mater 34(10):e2109376
Chen Q, Li Q, Liang Y, Zu M, Chen N, Canup BSB et al (2022b) Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B 12(2):907–923
Chen Q, Zu M, Gong H, Ma Y, Sun J, Ran S et al (2023) Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnol 21(1):6
Cheng L, Wang Y, Huang L (2017) Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther 25(7):1665–1675
Chiang YT, Lo CL (2014) pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials 35(20):5414–5424
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR et al (2018) M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano 12(9):8977–8993
Cianciaruso C, Beltraminelli T, Duval F, Nassiri S, Hamelin R, Mozes A et al (2019) Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep 27(10):3062–3080
Cong M, Tan S, Li S, Gao L, Huang L, Zhang HG et al (2022) Technology insight: plant-derived vesicles—how far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev 182:114108
Cui Y, Gao J, He Y, Jiang L (2020) Plant extracellular vesicles. Protoplasma 257(1):3–12
Dadwal A, Baldi A, Kumar NR (2018) Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol 46(sup2):295–305
Dai Z, Yu M, Yi X, Wu Z, Tian F, Miao Y et al (2019) Chain-length- and saturation-tuned mechanics of fluid nanovesicles direct tumor delivery. ACS Nano 13(7):7676–7689
de la Canal L, Pinedo M (2018) Extracellular vesicles: a missing component in plant cell wall remodeling. J Exp Bot 69(20):4655–4658
De Robertis M, Sarra A, D’Oria V, Mura F, Bordi F, Postorino P et al (2020) Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules 10(5):742
Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L et al (2015) Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther 15(4):495–504
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M et al (2017) Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther 25(7):1641–1654
Di Gioia S, Hossain MN, Conese M (2020) Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med 15(1):1096–1122
Ela S, Mäger I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347–357
Feng S, Lou K, Zou X, Zou J, Zhang G (2022) The potential role of exosomal proteins in prostate cancer. Front Oncol 12:873296
Fernández-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA (2010) Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 77(8):736–742
Finn OJ (2008) Cancer immunology. N Engl J Med 358(25):2704–2715
Fiore G, Nencini C, Cavallo F, Capasso A, Bader A, Giorgi G et al (2006) In vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother Res 20(8):701–703
Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3(4):393–403
Fu J, Wang D, Mei D, Zhang H, Wang Z, He B et al (2015) Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Control Release 204:11–19
Fu P, Zhang J, Li H, Mak M, Xu W, Tao Z (2021) Extracellular vesicles as delivery systems at nano-/micro-scale. Adv Drug Deliv Rev 179:113910
Fujita D, Arai T, Komori H, Shirasaki Y, Wakayama T, Nakanishi T et al (2018) Apple-derived nanoparticles modulate expression of organic-anion-transporting polypeptide (OATP) 2B1 in Caco-2 cells. Mol Pharm 15(12):5772–5780
Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A (2020) Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res 39(1):89
Garaeva L, Kamyshinsky R, Kil Y, Varfolomeeva E, Verlov N, Komarova E et al (2021) Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep 11(1):6489
Giddings EL, Champagne DP, Wu MH, Laffin JM, Thornton TM, Valenca-Pereira F et al (2021) Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat Commun 12(1):2804
Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10(9):597–608
He W, Zhang Z, Yang W, Zheng X, You W, Yao Y et al (2022) Turing milk into pro-apoptotic oral nanotherapeutic: de novo bionic chiral-peptide supramolecule for cancer targeted and immunological therapy. Theranostics 12(5):2322–2334
Jean-Toussaint R, Lin Z, Tian Y, Gupta R, Pande R, Luo X et al (2021) Therapeutic and prophylactic effects of macrophage-derived small extracellular vesicles in the attenuation of inflammatory pain. Brain Behav Immun 94:210–224
Jeon S, Clavadetscher J, Lee DK, Chankeshwara SV, Bradley M, Cho WS (2018) Surface charge-dependent cellular uptake of polystyrene nanoparticles. Nanomaterials 8(12):1028
Jeswani G, Paul SD, Jha AK (2018) Advances in the delivery of cancer therapeutics: a comprehensive review. Curr Drug Deliv 15(1):21–36
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y et al (2015) Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 14(15):2473–2483
Ji J, Sundquist J, Sundquist K (2016) Gender-specific incidence of autoimmune diseases from national registers. J Autoimmun 69:102–106
Jimenez-Jimenez S, Hashimoto K, Santana O, Aguirre J, Kuchitsu K, Cárdenas L (2019) Emerging roles of tetraspanins in plant inter-cellular and inter-kingdom communication. Plant Signal Behav 14(4):e1581559
Jing B, Gao Y, Guo F, Jiang D, Guo R, Wang J et al (2023) Engineering goat milk-derived extracellular vesicles for multiple bioimaging-guided and photothermal-enhanced therapy of colon cancer. Biomater Sci 11:1408–1421
Jorquera-Cordero C, Lara P, Cruz LJ, Schomann T, van Hofslot A, de Carvalho TG et al (2022) Extracellular vesicles from M1-polarized macrophages combined with hyaluronic acid and a β-blocker potentiate doxorubicin’s antitumor activity by downregulating tumor-associated macrophages in breast cancer. Pharmaceutics 14(5):1068
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA et al (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546(7659):498–503
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O et al (2013) Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335(1):201–204
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL et al (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12(3):655–664
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL et al (2018) Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine 14(1):195–204
Kim K, Yoo HJ, Jung JH, Lee R, Hyun JK, Park JH et al (2020) Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J Funct Biomater. 11(2):22
Kim DK, Kim YN, Kim YE, Lee SY, Shin MJ, Do EK et al (2021) TRIB2 stimulates cancer stem-like properties through activating the AKT-GSK3β-β-catenin signaling axis. Mol Cells 44(7):481–492
Kubo H (2018) Extracellular vesicles in lung disease. Chest 153(1):210–216
Lerch S, Dass M, Musyanovych A, Landfester K, Mailänder V (2013) Polymeric nanoparticles of different sizes overcome the cell membrane barrier. Eur J Pharm Biopharm 84(2):265–274
Levänen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL et al (2013) Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol 131(3):894–903
Li X, Wu F (2023) Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov 9(1):3
Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P (2018) Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep 8(1):14644
Li S, Wu Y, Ding F, Yang J, Li J, Gao X et al (2020) Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 12(19):10854–10862
Li Y, Zhang X, Zhang C, Yang J, Chi H, Li A et al (2022) Comparative study on the immunomodulatory function of extracellular vesicles from different dairy products. Food Funct 13(5):2504–2514
Lin Q, Qu M, Zhou B, Patra HK, Sun Z, Luo Q et al (2019) Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release 311–312:104–116
Liu J, Wan M, Lyon CJ, Hu TY (2020) Nanomedicine therapies modulating macrophage dysfunction: a potential strategy to attenuate cytokine storms in severe infections. Theranostics 10(21):9591–9600
Liu G, Kang G, Wang S, Huang Y, Cai Q (2021) Extracellular vesicles: emerging players in plant defense against pathogens. Front Plant Sci 12:757925
Lou G, Song X, Yang F, Wu S, Wang J, Chen Z et al (2015) Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 8:122
Lou K, Feng S, Luo H, Zou J, Zhang G, Zou X (2022) Extracellular vesicles derived from macrophages: current applications and prospects in tumors. Front Bioeng Biotechnol 10:1097074
Mackaness GB (1962) Cellular resistance to infection. J Exp Med 116(3):381–406
Maeda H, Khatami M (2018) Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med 7(1):11
McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R et al (2014) Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 155(8):1527–1539
Mehla K, Singh PK (2019) Metabolic regulation of macrophage polarization in cancer. Trends Cancer 5(12):822–834
Meldolesi J (2022) Cancer stem cells and their vesicles, together with other stem and non-stem cells, govern critical cancer processes: perspectives for medical development. Int J Mol Sci 23(2):625
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6(8):583–592
Minguell JJ, Erices A, Conget P (2001) Mesenchymal stem cells. Exp Biol Med 226(6):507–520
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20(2):101–124
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD et al (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104(10):3257–3266
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B et al (2014) Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res 58(7):1561–1573
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P (2013) Delivery of functional Anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids 2(10):e126
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45(11):e54
Nombela-Arrieta C, Ritz J, Silberstein LE (2011) The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12(2):126–131
Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, Romberger DJ (2019) Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J Nutr Biochem 64:110–120
O’Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C et al (2018) Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 37(16):2137–2149
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33(3):967–978
Pan T, Xu J, Zhu Y (2017) Self-renewal molecular mechanisms of colorectal cancer stem cells. Int J Mol Med 39(1):9–20
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E et al (2014) Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 192:262–270
Pocsfalvi G, Turiák L, Ambrosone A, Del Gaudio P, Puska G, Fiume I et al (2018) Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol 229:111–121
Polanco JC, Hand GR, Briner A, Li C, Götz J (2021) Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol 141(2):235–256
Poteryaev D, Datta S, Ackema K, Zerial M, Spang A (2010) Identification of the switch in early-to-late endosome transition. Cell 141(3):497–508
Pu J, Xu Z, Nian J, Fang Q, Yang M, Huang Y et al (2021) M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discov 7(1):182
Qiao L, Hu S, Huang K, Su T, Li Z, Vandergriff A et al (2020) Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 10(8):3474–3487
Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L et al (2015) Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 6(23):19514–19527
Rayamajhi S, Nguyen TDT, Marasini R, Aryal S (2019) Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater 94:482–494
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841(1):108–120
Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L (2009) Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett 583(20):3363–3366
Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L (2017) Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot 68(20):5485–5495
Reiner NE (2009) Methods in molecular biology. Macrophages and dendritic cells. Methods and protocols. Preface. Methods Mol Biol 531:v–vi
Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE (2012) Bovine milk exosome proteome. J Proteomics 75(5):1486–1492
Rezaie J, Nejati V, Mahmoodi M, Ahmadi M (2022) Mesenchymal stem cells derived extracellular vesicles: a promising nanomedicine for drug delivery system. Biochem Pharmacol 203:115167
Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122(5):735–749
Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ (2005) Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 7(4):324–330
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M et al (2013) BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest 123(4):1542–1555
Rome S (2019) Biological properties of plant-derived extracellular vesicles. Food Funct 10(2):529–538
Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D et al (2007) Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol 107(3):563–571
Rutter BD, Innes RW (2018) Extracellular vesicles as key mediators of plant-microbe interactions. Curr Opin Plant Biol 44:16–22
Samuel M, Fonseka P, Sanwlani R, Gangoda L, Chee SH, Keerthikumar S et al (2021) Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat Commun 12(1):3950
Schulert GS, Grom AA (2015) Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 66:145–159
Shahjin F, Chand S, Yelamanchili SV (2020) Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol 15(3):443–458
Shi X, Sun J, Li H, Lin H, Xie W, Li J et al (2020) Antitumor efficacy of interferon-γ-modified exosomal vaccine in prostate cancer. Prostate 80(11):811–823
Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S et al (2014) Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 445(2):381–387
Singh MK, Krüger F, Beckmann H, Brumm S, Vermeer JEM, Munnik T et al (2014) Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr Biol 24(12):1383–1389
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41
Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL (1991) Late endosomes derive from early endosomes by maturation. Cell 65(3):417–427
Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS et al (2012) Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10:5
Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L (2013) Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell 4(3):197–210
Tajik T, Baghaei K, Moghadam VE, Farrokhi N, Salami SA (2022) Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomed Pharmacother 152:113209
Takahara K, Inamoto T, Ibuki N, Uchimoto T, Saito K, Takai T et al (2016) 245 MicroRNA-145 mediates the inhibitory effect of adipose-derived stem cells on androgen-independent prostate cancer. Eur Urol Suppl 15(3):e245
Taylor DD, Gerçel-Taylor C (2005) Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 92(2):305–311
Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A et al (2018) Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 24(5):637–652
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ et al (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7):2383–2390
Vashisht M, Rani P, Onteru SK, Singh D (2017) Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol 183(3):993–1007
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K et al (2019) Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9(26):8001–8017
Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L et al (2013) Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun 4:1867
Wang Y, Chen X, Cao W, Shi Y (2014a) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15(11):1009–1016
Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q et al (2014b) Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther 22(3):522–534
Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H et al (2019) Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 9(6):1714–1727
Wang Y, Zhao M, Liu S, Guo J, Lu Y, Cheng J et al (2020) Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis 11(10):924
Wang Y, Li S, Wang X, Chen Q, He Z, Luo C et al (2021a) Smart transformable nanomedicines for cancer therapy. Biomaterials 271:120737
Wang Y, Li C, Zhao R, Qiu Z, Shen C, Wang Z et al (2021b) CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics 11(13):6315–6333
Wang D, Xue M, Chen J, Chen H, Liu J, Li Q et al (2021c) Macrophage-derived implantable vaccine prevents postsurgical tumor recurrence. Biomaterials 278:121161
Wang X, Ding H, Li Z, Peng Y, Tan H, Wang C et al (2022) Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct Target Ther 7(1):74
Woith E, Melzig MF (2019) Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Int J Mol Sci 20(2):357
Wu S, Ju GQ, Du T, Zhu YJ, Liu GH (2013) Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE 8(4):e61366
Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y et al (2018) Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 6:e5186
Xu L, Yang BF, Ai J (2013) MicroRNA transport: a new way in cell communication. J Cell Physiol 228(8):1713–1719
Xu Z, Zeng S, Gong Z, Yan Y (2020) Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer 19(1):160
Yang C, Zhang M, Merlin D (2018) Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B 6(9):1312–1321
Yang M, Liu X, Luo Q, Xu L, Chen F (2020) An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol 18(1):100
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ et al (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65(3):336–341
Yoon IS, Park H, Kwak HW, Woo Jung Y, Nam JH (2017) Macrophage-derived insulin-like growth factor-1 affects influenza vaccine efficacy through the regulation of immune cell homeostasis. Vaccine 35(36):4687–4694
Zaimy MA, Saffarzadeh N, Mohammadi A, Pourghadamyari H, Izadi P, Sarli A et al (2017) New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther 24(6):233–243
Zhang M, Viennois E, Xu C, Merlin D (2016a) Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 4(2):e1134415
Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E et al (2016b) Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther 24(10):1783–1796
Zhang M, Wang X, Han MK, Collins JF, Merlin D (2017) Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 12(16):1927–1943
Zhang C, Shang Y, Chen X, Midgley AC, Wang Z, Zhu D et al (2020) Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano 14(9):12133–12147
Zhao Z, Yu S, Li M, Gui X, Li P (2018) Isolation of exosome-like nanoparticles and analysis of microRNAs derived from coconut water based on small RNA high-throughput sequencing. J Agric Food Chem 66(11):2749–2757
Zhao Y, Zheng Y, Zhu Y, Zhang Y, Zhu H, Liu T (2021) M1 Macrophage-derived exosomes loaded with gemcitabine and deferasirox against chemoresistant pancreatic cancer. Pharmaceutics 13(9):1493
Zhou HM, Zhang JG, Zhang X, Li Q (2021) Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Target Ther 6(1):62
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y et al (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315(1):28–37
Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D et al (2015) Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles 4:28713
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Acknowledgements
All figures are created with BioRender.com.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82260141); the Natural Science Foundation of Jiangxi Province (Nos. 20202BABL206031; 20224BAB216024) and the Medjaden Young Scientist Fund (MJR20201127).
Author information
Authors and Affiliations
Contributions
HL conceived the manuscript, HL searched for publications and draft the manuscript. HL and JJ edited tables and figures. JJ, KL, HH, SF, FZ and JZ reviewed the manuscript and polished the grammar. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, H., Jin, J., Jin, J. et al. Emerging applications of extracellular vesicles in tumor therapy. Cancer Nano 14, 63 (2023). https://doi.org/10.1186/s12645-023-00217-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12645-023-00217-3